Abstract
Purpose:
A major determinant of survival in patients with congenital diaphragmatic hernia (CDH) is severity of pulmonary hypoplasia. This study addresses the comparative effectiveness of prenatal methods of lung assessment in predicting mortality, extracorporeal membrane oxygenation (ECMO), and ventilator-dependency.
Methods:
We retrospectively reviewed all patients born with isolated CDH between 2004–2008. Lung-to-head ratio (LHR) and observed-to-expected LHR (OELHR) were obtained from prenatal ultrasounds. Percent-predicted lung volume (PPLV) was obtained from fetal MRI (fMRI). Postnatal data included in-hospital mortality, need for ECMO, and ventilator-dependency at day-of-life 30.
Results:
Thirty-seven patients underwent 81 prenatal ultrasounds, while 26 of this sub-cohort underwent fMRI. Gestational age during imaging study was associated with LHR (p=0.02), but not OELHR (p=0.12) or PPLV (p=0.72). PPLV, min-LHR, and min-OELHR were each associated with mortality (p=0.03, p=0.02, p=0.01), ECMO (p<0.01, p<0.01, p=0.03), and ventilator-dependency (p<0.01, p<0.01, p=0.02). For each outcome, PPLV was a more discriminative measure, based on Akaike’s information criterion. Using longitudinal analysis techniques for patients with multiple ultrasounds, OELHR remained associated with mortality (p=0.04), ECMO (p=0.03), and ventilator-dependency (p=0.02), while LHR was associated with ECMO (p=0.01) and ventilator-dependency (p=0.02) but not mortality (p=0.06).
Conclusion:
When assessing fetuses with CDH, OELHR and PPLV may be most helpful for counseling regarding postnatal outcomes.
Index Words: Congenital Diaphragmatic Hernia
Congenital diaphragmatic hernia (CDH) is a defect occurring in one of 2,200 live births, which impacts lung parenchymal and vascular development. Mortality ranges from 30% overall1 to 20% at tertiary care centers experienced in the treatment of CDH patients2,3. Even among survivors, morbidity remains high4 due to the pulmonary hypoplasia and pulmonary hypertension associated with CDH5–7. The degree of prenatal pulmonary hypoplasia has been found to be a useful prognostic index for persistent pulmonary hypertension of the newborn8 and mortality9–11.
The most common method of prenatal lung assessment is the lung-to-head ratio (LHR) as measured by ultrasound, given its widespread availability12. The observed-to-expected LHR (OELHR) was later developed in response to the finding of variation in LHR with estimated gestational age (EGA) at the time of ultrasound.11,13 More recently, percent-predicted lung volume (PPLV) as measured by fetal MRI (fMRI) has become available to evaluate lung size in three dimensions.14
The purpose of this study was to compare the effectiveness of LHR, OELHR, and PPLV in predicting mortality, need for extracorporeal membrane oxygenation (ECMO), and ventilator-dependency among patients born with CDH.
METHODS
Study base
After receiving institutional review board approval, we retrospectively reviewed the medical records of all patients born with CDH at a single tertiary care institution between January 2004 and December 2008. Inclusion criteria consisted of diagnosis with isolated CDH. Patients were excluded from the study due to lack of available prenatal ultrasound. Variables collected included prenatal imaging studies, perinatal data, ventilator data, and clinical outcomes. To quantify the severity of CDH in our patients, each patient’s predicted probability of survival was calculated using the CDH Study Group equation:
where exp is the exponential function, BW is birth weight, and Apgar5 is the Apgar score at five minutes of life.15
Lung size
Measures of lung size included LHR, OELHR, and PPLV. Prenatal ultrasounds were retrieved to determine LHR and OELHR. LHR was assessed by measurement of the contralateral lung in two-dimensions at the level of the atria and division by the head circumference.11,13 We computed the EGA-normalized measure of LHR, OELHR, by the following equation:16
Next, fMRI lung volumes were calculated from consecutive sections in two out of three imaging planes. An expected lung volume was calculated for each patient based on the patient’s EGA using the equation developed by Osada et al.:17
PPLV was then derived from the equation:18
Endpoints
The primary outcome was in-hospital mortality. The secondary outcomes were need for ECMO during hospital course and ventilator-dependency at day of life (DOL) 30.
Statistical analysis
We tabulated patient characteristics for the overall cohort. Percentages were based on available data. Next, we assessed variation with EGA among the measures of prenatal lung size. Because several patients had more than one prenatal ultrasound performed, we used univariable generalized linear mixed modeling (GLMM) to assess variation of LHR and OELHR with EGA. GLMM is a longitudinal analysis random-effects technique that accounts for patient-specific repeated measures. Because no patient had more than one fMRI, simple linear regression modeling was used to evaluate the association of PPLV with EGA.
We then used two different analyses to address the association between mortality, need for ECMO, and ventilator-dependency and prenatal lung size as measured by each of the three imaging techniques. First, the minima values of LHR (min-LHR) and OELHR (min-OELHR) were used for patients with multiple prenatal ultrasound studies. Simple logistic regression was used to model the association of min-LHR, min-OELHR, and PPLV with mortality, need for ECMO, and ventilator-dependency. Because each measure of lung size was a ratio, the distributions were non-normal and thus the log-transformation was employed to stabilize the variance of each. The three models were compared using Akaike’s information criterion (AIC), with lower AIC indicating better fit. They were visually compared using receiver operating characteristic (ROC) curves, with higher area under the curve indicating better fit. Second, to further quantify the effect on mortality and ventilator-dependency of LHR and OELHR without requiring a summary statistic (i.e. minima values), simple GLMM was again utilized. Patients with missing data were excluded from each respective univariable and multivariable analysis. A p value < 0.05 was considered statistically significant. All data were analyzed using SAS version 9.3 (Cary, NC).
RESULTS
Baseline patient characteristics
Of the 74 patients born with isolated CDH between 2004 and 2008, thirty-seven patients had undergone pre-natal ultrasound imaging and met inclusion and exclusion criteria. Twenty-six patients also underwent an fMRI study. Baseline characteristics and outcomes are tabulated in Table 1. Patients were followed to hospital discharge with a median follow-up time of 40 days (26 – 87). 62.2% of patients were male. Median EGA at birth was 38.0 weeks (interquartile range [IQR] = 36.0 – 39.0). In-hospital mortality for the overall cohort was 21.6%. The median predicted survival of the overall cohort based on the CDH study group equation was 64.9% (IQR = 40.4 – 80.0). 45.9% of patients underwent one or more courses of ECMO. At DOL30, 43.7% of patients were dependent on a ventilator for respiration.
Table 1.
Patient characteristics of 37 patients born with CDH.
| Variable | n (%) |
|---|---|
| Male sex | 23 (62.2) |
| Left-sided defect | 24 (64.9) |
| Intra-thoracic liver | 18 (48.6) |
| Need for ECMO | 17 (45.9) |
| Ventilator-dependent (DOL30) | 14 (43.7) |
| In-hospital mortality | 8 (21.6) |
| Variable | median (IQR) |
| Predicted survival, % | 64.9 (40.4 – 80.0) |
| Lung size | |
| Min. LHR | 1.3 (0.9 – 1.8) |
| Min. OELHR | 0.4 (0.3 – 0.7) |
| PPLV | 0.5 (0.3 – 0.7) |
| EGA, weeks | 38.0 (36.0 – 39.0) |
| Birth weight, kg | 3.1 (2.7–3.3) |
| 5-minute Apgar score | 7.0 (5.0 – 8.0) |
CDH, congenital diaphragmatic hernia; DOL, day of life; ECMO, extracorporeal membrane oxygenation; EGA, estimated gestational age at birth; IQR, interquartile range; kg, kilogram; LHR, lung-to-head ratio; OELHR, observed-to-expected LHR; PPLV, %- predicted lung volume; SEM, standard error of the mean; TV/kg, tidal volume per kilogram
Consistency across EGA
For the 81 prenatal ultrasounds performed, the median EGA during study was 30.2 weeks (range, 18.1 – 38.5 weeks). The median min-LHR and min-OELHR were 1.34 (IQR = 0.9 – 1.8) and 0.4 (IQR = 0.3 – 0.7), respectively. For the 25 fMRIs performed, the median EGA during study was 24.3 weeks (range, 20.0 – 38.0). The median PPLV was 0.5 (IQR = 0.3 – 0.7). EGA was found to be associated with LHR (p = 0.02), but not OELHR (p = 0.12) or PPLV (p = 0.72), as illustrated in Figures 1A–C.
Figure 1A–C.
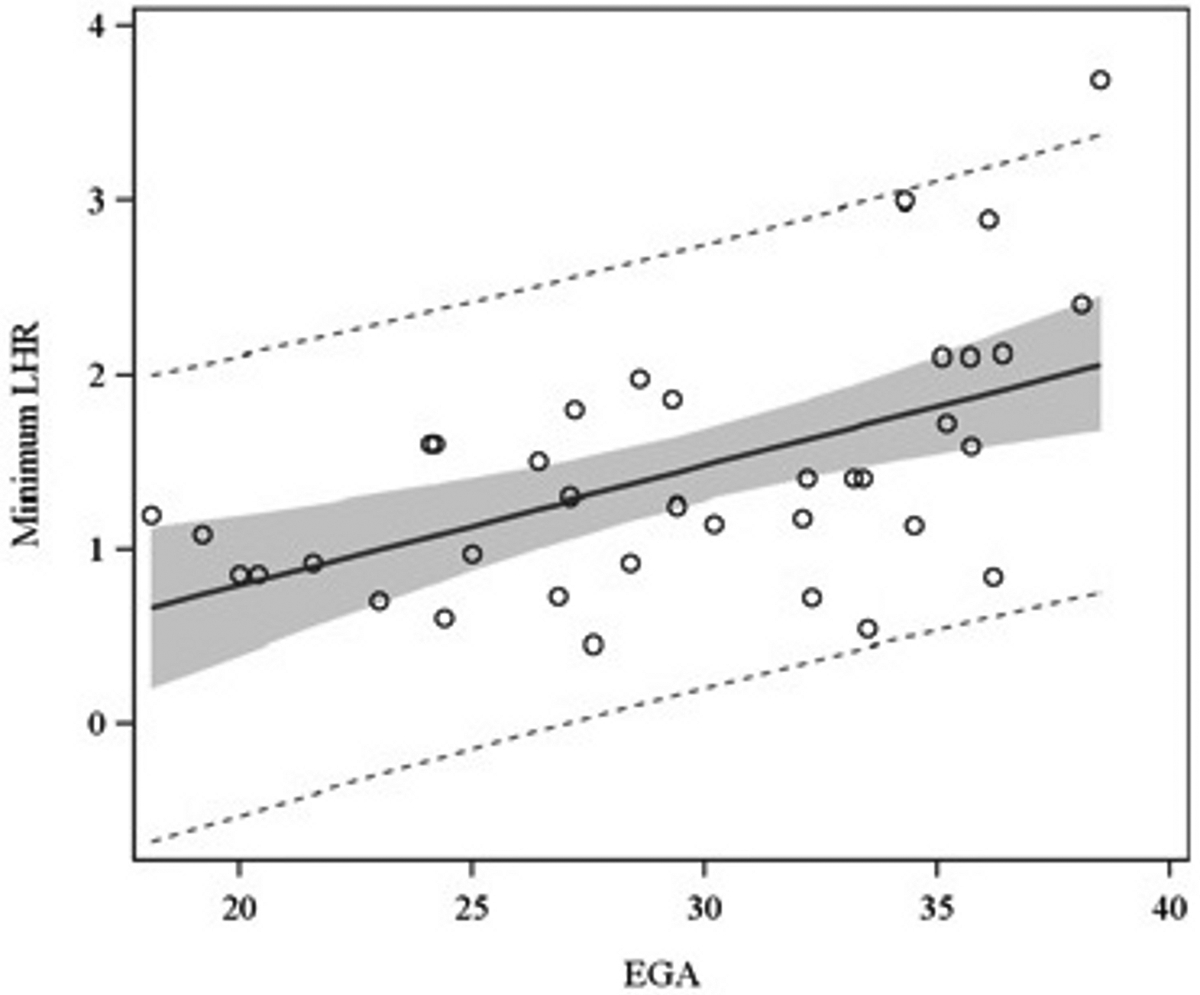
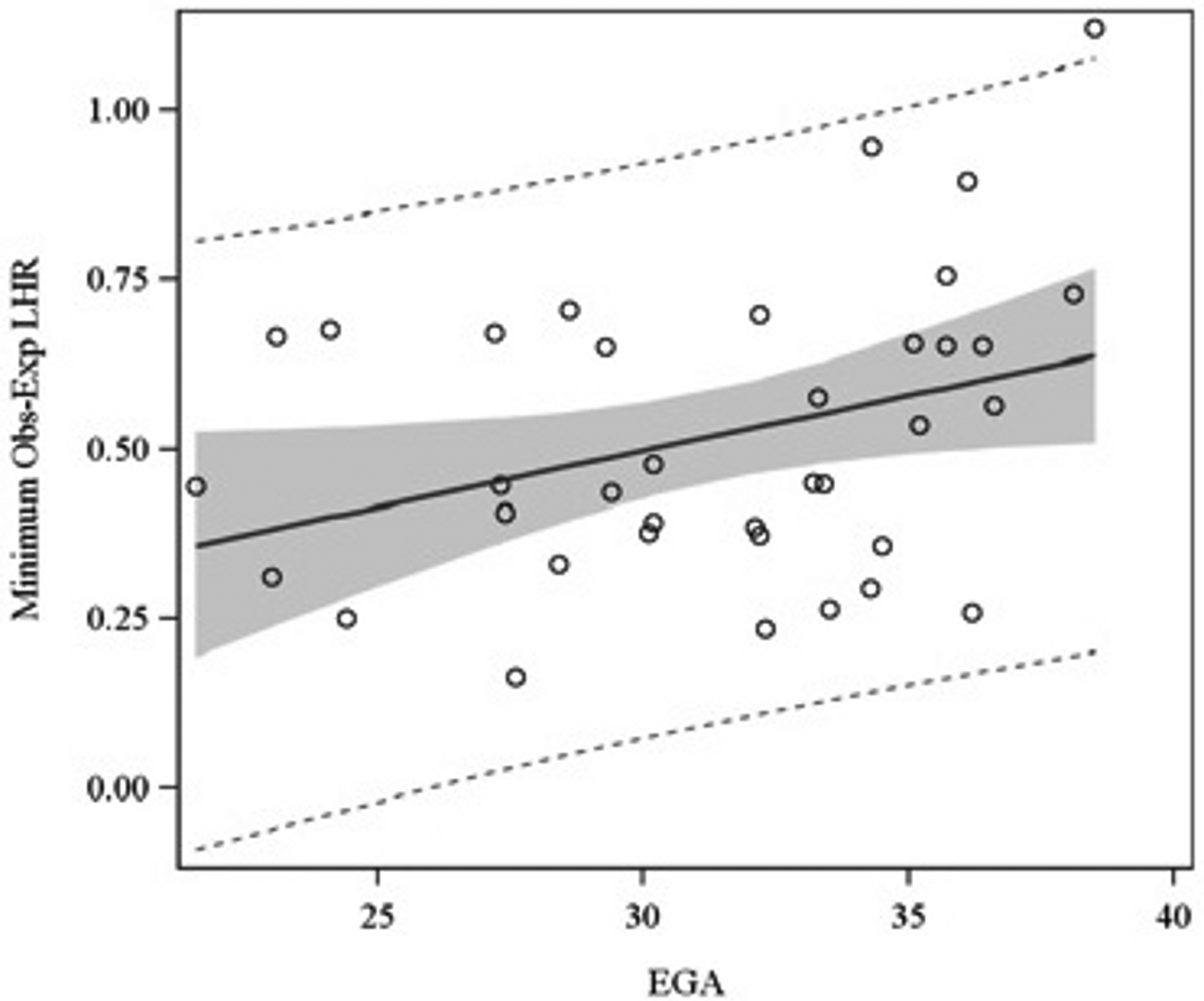
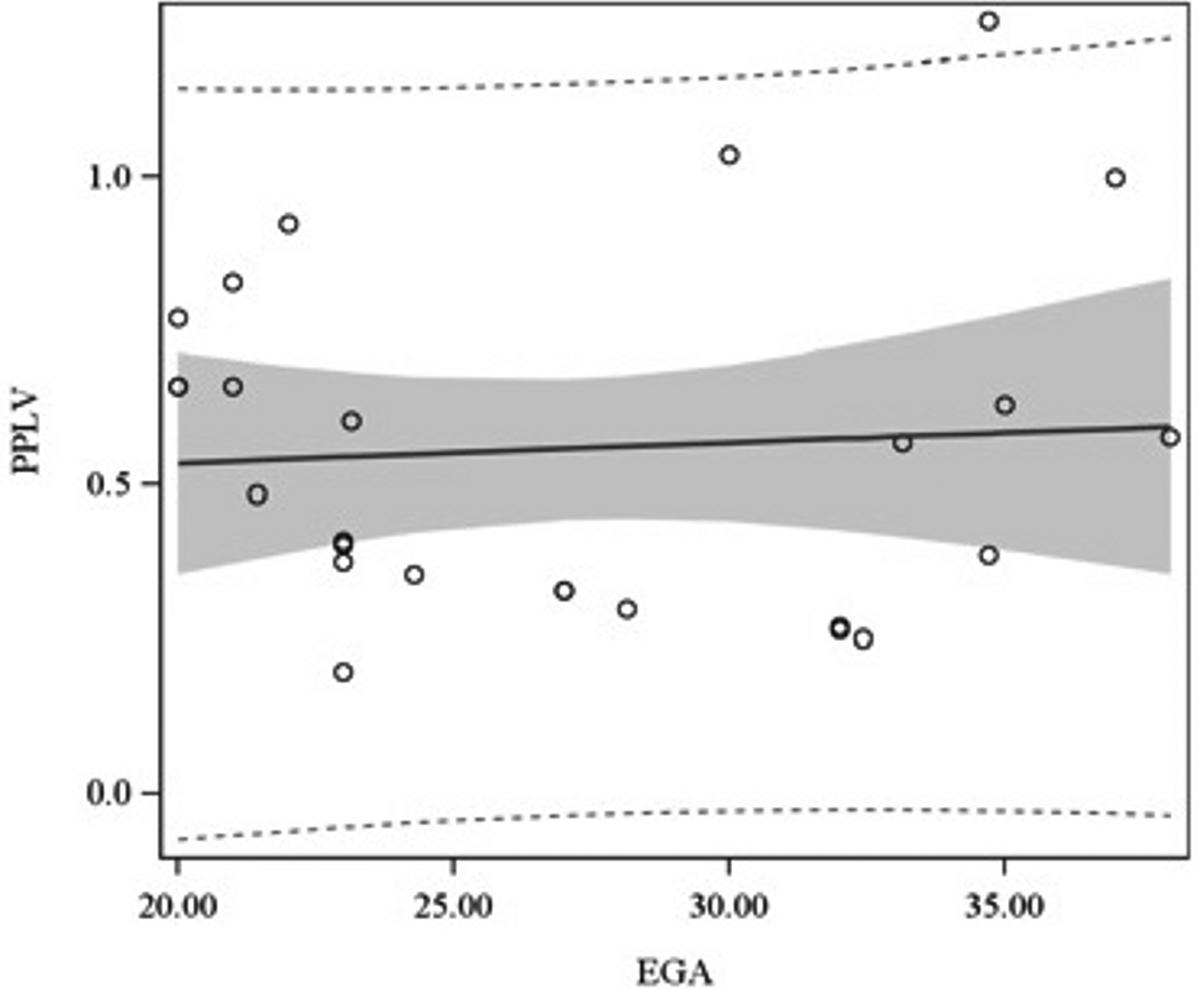
Association of LHR (Figure 1A), OELHR (Figure 1B), and PPLV (Figure 1C) with EGA. EGA, estimated gestational age; LHR, lung-to-head ratio; OELHR, observed-to-expected lung-to-head ratio; PPLV, percent-predicted lung volume.
Primary outcome: mortality
In-hospital mortality for the overall cohort was 21.6%. We compared the association with mortality of min-LHR, min-OELHR, and PPLV, using simple logistic regression in order to develop ROC curves for each of the three measures. As reported in Table 2, all three measurements were significantly associated with mortality. PPLV had the smallest AIC, indicating superior discrimination. Of the measures, PPLV had the highest area under the ROC curve, followed by OELHR, and then LHR, as displayed in Figure 2. Next, we assessed the association between the ultrasound measures of lung size and mortality, without utilizing the minima values. Using a longitudinal analysis technique for repeated measures (GLMM) of all 81 ultrasounds that controlled for individual-patient effects, OELHR was found to be associated with mortality (p = 0.04), while LHR was not (p = 0.06).
Table 2.
Association of pre-natal measures of lung size with mortality.
| Variable | n | Odds ratio | 95% Confidence interval | P | AIC |
|---|---|---|---|---|---|
| Min. LHR | 37 | 0.062 | 0.006 – 0.606 | 0.02 | 35.6 |
| Min. OELHR | 37 | 0.033 | 0.002 – 0.472 | 0.01 | 35.6 |
| PPLV | 26 | 0.061 | 0.005 – 0.807 | 0.03 | 27.8 |
AIC, Akaike’s information criterion, LHR, lung-to-head ratio; OR, odds ratio; PPLV, percent-predicted lung volume
Figure 2.
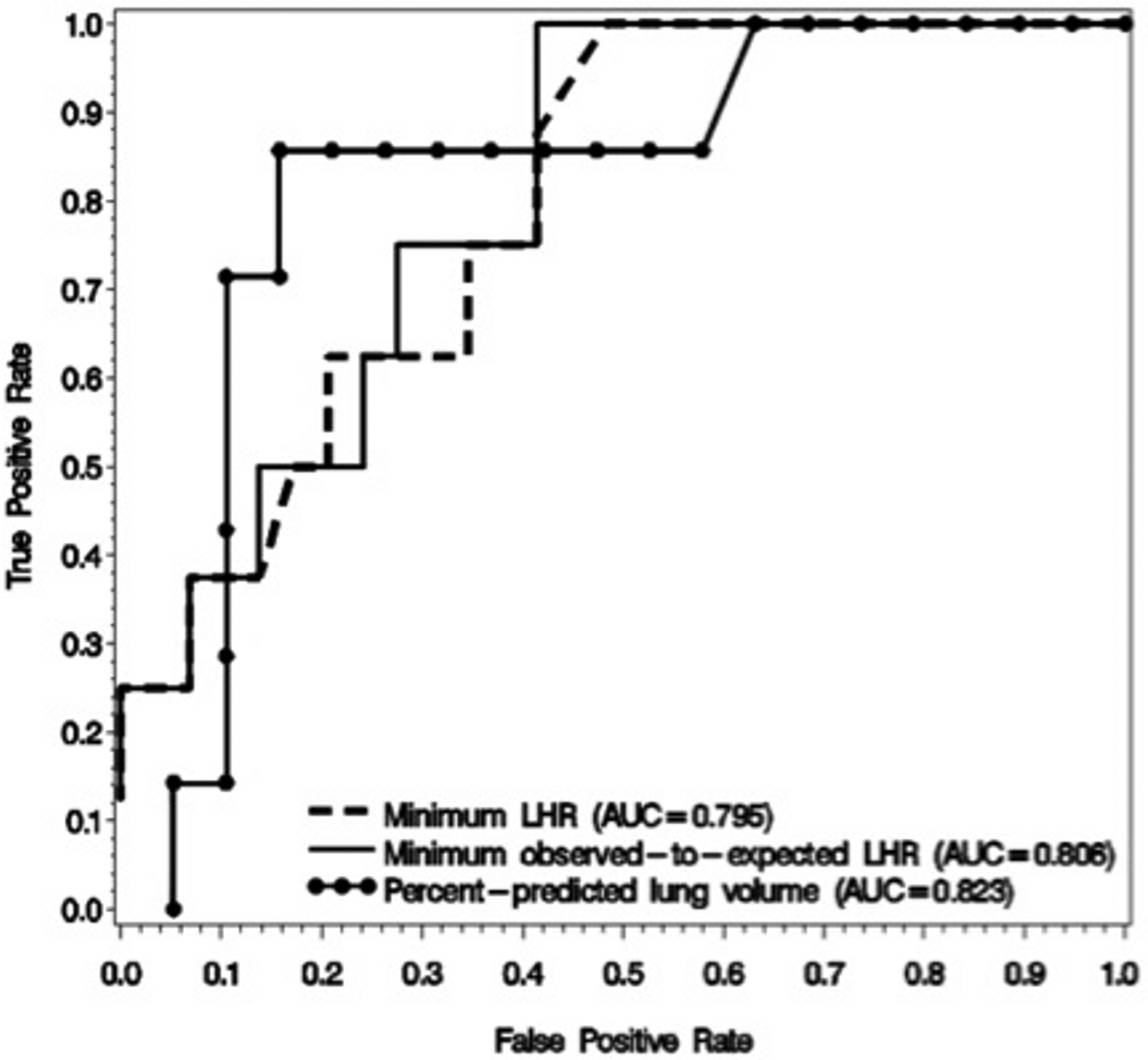
Receiver operating characteristic curves of LHR, OELHR, and PPLV for the primary outcome of mortality. AUC, area under the curve; LHR, lung-to-head ratio; PPLV, percent-predicted lung volume.
Secondary outcomes: need for ECMO and ventilator-dependency
Among all patients, 45.9% of patients underwent one or more courses of ECMO. The three measurements of lung size, min-LHR (p < 0.01), min-OELHR (p < 0.01), and PPLV (p = 0.03) were each associated with need for ECMO, as reported in Table 3. The AIC of PPLV was the lowest, indicating this model had the best fit of the three. Again, using GLMM to analyze all 81 ultrasounds, both LHR (p = 0.01) and OELHR (p = 0.03) were associated with need for ECMO.
Table 3.
Association of pre-natal measures of lung size with need for ECMO.
| Variable | n | Odds ratio | 95% Confidence interval | P | AIC |
|---|---|---|---|---|---|
| Min. LHR | 37 | 0.06 | 0.01 – 0.41 | <0.01 | 39.9 |
| Min. OELHR | 37 | 0.002 | <0.001 – 0.188 | <0.01 | 44.7 |
| PPLV | 26 | 0.01 | <0.001 – 0.641 | 0.03 | 33.3 |
AIC, Akaike’s information criterion; ECMO, extra-corporeal membrane oxygenation; LHR, lung-to-head ratio; OR, odds ratio; PPLV, percent-predicted lung volume
At DOL30, 43.7% of all patients were dependent on a ventilator for respiration. The three measurements of lung size, min. LHR (p < 0.01), min. OELHR (p < 0.01), and PPLV (p = 0.02) were each associated with ventilator dependency at DOL30, as reported in Table 4. The AIC of PPLV was the lowest, indicating this model had the best fit of the three. Finally, using GLMM to analyze all 81 ultrasounds, both LHR (p = 0.02) and OELHR (p = 0.02) were associated with ventilator-dependency at DOL30.
Table 4.
Association of pre-natal measures of lung size with ventilator-dependency.
| Variable | n | Odds ratio | 95% Confidence interval | P | AIC |
|---|---|---|---|---|---|
| Min. LHR | 32 | 0.035 | 0.003 – 0.387 | <0.01 | 45.9 |
| Min. OELHR | 32 | 0.019 | 0.001 – 0.308 | <0.01 | 45.9 |
| PPLV | 23 | 0.045 | 0.003 – 0.660 | 0.02 | 27.6 |
AIC, Akaike’s information criterion, LHR, lung-to-head ratio; OR, odds ratio; PPLV, percent-predicted lung volume
DISCUSSION
When assessing fetuses with CDH, our findings showed that min-LHR, min-OELHR, and PPLV were each independently associated with mortality, need for ECMO, and ventilator-dependency. Only OELHR and PPLV were associated with mortality without using minima values (i.e. using all ultrasounds performed, rather than min-LHR and min-OELHR). While LHR varied with EGA, PPLV and OELHR were both found to be independent of EGA. The three-dimensional modality, PPLV, was found to be slightly more discriminative for the outcomes of need for ECMO, ventilator-dependency, and mortality, compared with LHR and OELHR.
Similar to previous reports, we found that OELHR does not vary with EGA, in contrast to LHR.12,19,20 In the present study we found that PPLV likewise did not vary with EGA. LHR has been shown to be most reliably associated with mortality when measured on prenatal ultrasound performed between 24 – 26 weeks gestation.10 Outside of this range however, the association between LHR and mortality is controversial,10,21 because LHR varies with EGA.22 This is likely due to the fact that early in pregnancy (among non-CDH fetuses), the lateral and vertical growth of the developing pulmonary parenchyma are roughly equivalent, while later in pregnancy there is a greater increase in vertical, as compared with lateral growth.23 The contralateral lung is measured in the transverse two-dimensional plane to calculate LHR, thus lateral growth affects the measure.11 Furthermore, research suggests that for CDH fetuses, the contralateral lung compression due to the herniation is more severe in the lateral than in the vertical direction.23 These findings suggest that the ratio measures of lung size (OELHR and PPLV) are indicators of post-natal outcome that are valid across a wide spectrum of prenatal development.
In our study, OELHR and PPLV were independently associated with mortality, while LHR was not. The min-LHR value was also found to be associated with mortality, but the need to obtain LHRs at several different EGA time points and record the minimum value is more cumbersome and, thus, potentially less clinically useful. While several studies have shown a correlation between LHR and mortality, most restrict the timeframe of measurement to a tight window within which LHR may be more valid. For example, a recent meta-analysis reported that prior to 32 weeks EGA, LHR < 1.0 was associated with mortality.9
There are similar existing knowledge gaps in the literature regarding the newer measures of lung size, OELHR and PPLV. The association between OELHR and mortality has typically been shown among cohorts with relatively high mortality rates. The present study addressed the utility of the measures of lung size among lower mortality cohorts at a tertiary care center experienced in the care of patients with CDH. Although the CDH study group equation predicted a survival of only 65% in our cohort, our actual survival was 79.4%. Jani et al. in their 2007 retrospective analysis showed OELHR from 18 – 38 weeks of gestation to be validly associated with mortality.24 However, the mortality rate in the report was relatively high at 37.3%, which may better power the analysis. A more recent report from the same group, in which overall mortality was 36%, showed PPLV to be more strongly associated with post-natal survival, as compared with LHR.25 The mortality rate in our study was 21.6%, potentially making this sample a more realistic assessment of the utility of the measures of lung size. After adjusting for liver position, a 2012 retrospective study by Schaible et al. found an association between mortality and combined OELHR and PPLV, however did not assess the utility of these measures of lung size as independent markers.26 In a 2009 study with a 21% mortality rate comparable to that of the present analysis, Kilian et al. found PPLV, LHR, and OELHR in order of strength of association to be significant predictors of survival.27 The present study found OELHR to be a superior predictor of survivor compared to LHR.
In infants with CDH, ECMO is the gold standard of many therapies directed toward alleviating pulmonary hypertension. ECMO allows the lungs to “rest,” avoiding barotrauma, while pulmonary arterial reactivity diminishes.28–30 In our cohort, nearly half of patients (46%) underwent one or more courses of ECMO. During the study period, we routinely followed a strategy of “gentle ventilation” for patients with CDH.31 Our institutional criteria for ECMO entails failure of the “gentle ventilation” strategy and our overall ECMO utilization is approximately 40%. In this particular cohort, we had a disproportionately high percentage of patients with right-sided CDH (35%). We have previously shown that patients with right-sided CDH require higher ECMO utilization at our institution.32 Each of the measures of prenatal lung size (min-LHR, min-OELHR, LHR, OELHR, and PPLV) were associated with need for ECMO. This finding corroborates existing literature which suggests a relationship between lung size and need for ECMO.26
Given that pulmonary dysfunction is the leading cause of death in CDH, ventilator dependency is a critical outcome. Furthermore, in patients with CDH, persistent ventilator dependency is often taken as an indicator of chronic lung disease.26 In our study, nearly half of patients (44%) relied on ventilator support at DOL30. We found all measures of prenatal lung size (min-LHR, min-OELHR, LHR, OELHR, and PPLV) to be associated with ventilator-dependency at DOL30. In a conflicting 2005 retrospective analysis, Heling et al. did not find an association between LHR and ventilator parameters, however the study was underpowered to detect a difference with a sample size of seventeen patients.33 Schaible et al. in their 2012 retrospective analysis showed OELHR and PPLV to be superior to OELHR alone in prediction of ventilator-dependency, but did not assess whether the measures of prenatal lung size were significantly associated with the outcome.26
Limitations
There were several limitations to the present study. This was a retrospective analysis, which carries an inherent risk of bias. Variables that were not collected for the purpose of clinical management were unavailable. Furthermore, as a study of a congenital defect, those patients who did not survive to birth were not able to be included in the analysis. Perhaps most importantly, prenatal imaging studies were not available for all patients. Similarly, the prenatal measures were not assessed at the same EGA in all patients. While this variability in timing may have introduced statistical noise, the wide range of times at which prenatal studies were obtained may provide a more realistic estimation of clinical application and may improve the study’s generalizability. We chose to assess three common and feasible measures of lung size, LHR, OELHR, and PPLV. The gold standard measure of pulmonary hypoplasia, post-mortem lung-to-body weight,34 was not assessable. Other studies have employed pulmonary vascularization as a marker of severity of pulmonary disease,35,36 which was likewise not available for our cohort.
Given that PPLV and OELHR were independent of EGA and associated with mortality, need for ECMO, and ventilator dependency, these measures were found to be superior to LHR. When assessing fetuses with CDH, OELHR using ultrasound or PPLV utilizing fetal MRI may be most helpful for counseling regarding postnatal outcomes, including mortality, need for ECMO, and ventilator-dependency.
Sources of funding:
ALM is the recipient of an NIH: National Heart, Lung, and Blood Institutes T35 grant.
Footnotes
Disclosures: None of the authors disclosed any relevant financial relationships.
Institutional Review Board: approved, #HUM00028066
REFERENCES
- 1.Lally KP, Lally PA, Lasky RE, et al. : Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120(3):e651–657. [DOI] [PubMed] [Google Scholar]
- 2.Boloker J, Bateman DA, Wung J-T, et al. : Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair.[see comment]. Journal of Pediatric Surgery. 2002;37(3):357–366. [DOI] [PubMed] [Google Scholar]
- 3.Javid PJ, Jaksic T, Skarsgard ED, et al. : Survival rate in congenital diaphragmatic hernia: the experience of the Canadian Neonatal Network. Journal of Pediatric Surgery. 2004;39(5):657–660. [DOI] [PubMed] [Google Scholar]
- 4.Cortes RA, Keller RL, Townsend T, et al. : Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg. 2005;40(1):36–45; discussion 45–36. [DOI] [PubMed] [Google Scholar]
- 5.Chiu P, Hedrick HL: Postnatal management and long-term outcome for survivors with congenital diaphragmatic hernia. Prenat Diagn. 2008;28(7):592–603. [DOI] [PubMed] [Google Scholar]
- 6.Peetsold MG, Heij HA, Kneepkens CM, et al. : The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatr Surg Int. 2009;25(1):1–17. [DOI] [PubMed] [Google Scholar]
- 7.Pober BR, Lin A, Russell M, et al. : Infants with Bochdalek diaphragmatic hernia: sibling precurrence and monozygotic twin discordance in a hospital-based malformation surveillance program. Am J Med Genet A. 2005;138A(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Buys Roessingh AS, Dinh-Xuan AT: Congenital diaphragmatic hernia: current status and review of the literature. Eur J Pediatr. 2009;168(4):393–406. [DOI] [PubMed] [Google Scholar]
- 9.Knox E, Lissauer D, Khan K, et al. : Prenatal detection of pulmonary hypoplasia in fetuses with congenital diaphragmatic hernia: a systematic review and meta-analysis of diagnostic studies. J Matern Fetal Neonatal Med. 2010;23(7):579–588. [DOI] [PubMed] [Google Scholar]
- 10.Yang SH, Nobuhara KK, Keller RL, et al. : Reliability of the lung-to-head ratio as a predictor of outcome in fetuses with isolated left congenital diaphragmatic hernia at gestation outside 24–26 weeks. Am J Obstet Gynecol. 2007;197(1):30 e31–37. [DOI] [PubMed] [Google Scholar]
- 11.Metkus AP, Filly RA, Stringer MD, et al. : Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996;31(1):148–151; discussion 151–142. [DOI] [PubMed] [Google Scholar]
- 12.Claus F, Sandaite I, DeKoninck P, et al. : Prenatal anatomical imaging in fetuses with congenital diaphragmatic hernia. Fetal Diagn Ther. 2011;29(1):88–100. [DOI] [PubMed] [Google Scholar]
- 13.Lipshutz GS, Albanese CT, Feldstein VA, et al. : Prospective analysis of lung-to-head ratio predicts survival for patients with prenatally diagnosed congenital diaphragmatic hernia. J Pediatr Surg. 1997;32(11):1634–1636. [DOI] [PubMed] [Google Scholar]
- 14.Victoria T, Bebbington MW, Danzer E, et al. : Use of magnetic resonance imaging in prenatal prognosis of the fetus with isolated left congenital diaphragmatic hernia. Prenat Diagn. 2012:1–9. [DOI] [PubMed] [Google Scholar]
- 15.The Congenital Diaphragmatic Hernia Study Group: Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. Journal of Pediatric Surgery. 2001;36(1):141–145. [DOI] [PubMed] [Google Scholar]
- 16.Peralta CF, Cavoretto P, Csapo B, et al. : Assessment of lung area in normal fetuses at 12–32 weeks. Ultrasound Obstet Gynecol. 2005;26(7):718–724. [DOI] [PubMed] [Google Scholar]
- 17.Osada H, Kaku K, Masuda K, et al. : Quantitative and qualitative evaluations of fetal lung with MR imaging. Radiology. 2004;231(3):887–892. [DOI] [PubMed] [Google Scholar]
- 18.Barnewolt CE, Kunisaki SM, Fauza DO, et al. : Percent predicted lung volumes as measured on fetal magnetic resonance imaging: a useful biometric parameter for risk stratification in congenital diaphragmatic hernia. J Pediatr Surg. 2007;42(1):193–197. [DOI] [PubMed] [Google Scholar]
- 19.Jani JC, Benachi A, Nicolaides KH, et al. : Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33(1):64–69. [DOI] [PubMed] [Google Scholar]
- 20.Jani J, Nicolaides KH, Benachi A, et al. : Timing of lung size assessment in the prediction of survival in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;31(1):37–40. [DOI] [PubMed] [Google Scholar]
- 21.Laudy JA, Van Gucht M, Van Dooren MF, et al. : Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters. Prenat Diagn. 2003;23(8):634–639. [DOI] [PubMed] [Google Scholar]
- 22.Ba’ath ME, Jesudason EC, Losty PD: How useful is the lung-to-head ratio in predicting outcome in the fetus with congenital diaphragmatic hernia? A systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2007;30(6):897–906. [DOI] [PubMed] [Google Scholar]
- 23.Jani J, Peralta CF, Van Schoubroeck D, et al. : Relationship between lung-to-head ratio and lung volume in normal fetuses and fetuses with diaphragmatic hernia. Ultrasound in Obstetrics & Gynecology. 2006;27(5):545–550. [DOI] [PubMed] [Google Scholar]
- 24.Jani J, Nicolaides KH, Keller RL, et al. : Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. 2007;30(1):67–71. [DOI] [PubMed] [Google Scholar]
- 25.Jani J, Cannie M, Sonigo P, et al. : Value of prenatal magnetic resonance imaging in the prediction of postnatal outcome in fetuses with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2008;32(6):793–799. [DOI] [PubMed] [Google Scholar]
- 26.Schaible T, Busing KA, Felix JF, et al. : Prediction of chronic lung disease, survival and need for ECMO therapy in infants with congenital diaphragmatic hernia: additional value of fetal MRI measurements? Eur J Radiol. 2012;81(5):1076–1082. [DOI] [PubMed] [Google Scholar]
- 27.Kilian AK, Schaible T, Hofmann V, et al. : Congenital diaphragmatic hernia: predictive value of MRI relative lung-to-head ratio compared with MRI fetal lung volume and sonographic lung-to-head ratio. AJR Am J Roentgenol. 2009;192(1):153–158. [DOI] [PubMed] [Google Scholar]
- 28.Kim ES, Stolar CJ: ECMO in the newborn. Am J Perinatol. 2000;17(7):345–356. [DOI] [PubMed] [Google Scholar]
- 29.Lally KP: The Role of ECMO in Neonates with CDH. AAP Grand Rounds. 1999;2(3):28-. [Google Scholar]
- 30.Rothenbach P, Lange P, Powell D: The use of extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Seminars in Perinatology. 2005;29(1):40–44. [DOI] [PubMed] [Google Scholar]
- 31.Garcia A, Stolar CJ: Congenital diaphragmatic hernia and protective ventilation strategies in pediatric surgery. Surg Clin North Am. 2012;92(3):659–668, ix. [DOI] [PubMed] [Google Scholar]
- 32.Bryner BS, Kim AC, Khouri JS, et al. : Right-sided congenital diaphragmatic hernia: high utilization of extracorporeal membrane oxygenation and high survival. J Pediatr Surg. 2009;44(5):883–887. [DOI] [PubMed] [Google Scholar]
- 33.Heling KS, Wauer RR, Hammer H, et al. : Reliability of the lung-to-head ratio in predicting outcome and neonatal ventilation parameters in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2005;25(2):112–118. [DOI] [PubMed] [Google Scholar]
- 34.Cannie M, Jani J, Meersschaert J, et al. : Prenatal prediction of survival in isolated diaphragmatic hernia using observed to expected total fetal lung volume determined by magnetic resonance imaging based on either gestational age or fetal body volume. Ultrasound Obstet Gynecol. 2008;32(5):633–639. [DOI] [PubMed] [Google Scholar]
- 35.Ruano R, Aubry MC, Barthe B, et al. : Quantitative analysis of fetal pulmonary vasculature by 3-dimensional power Doppler ultrasonography in isolated congenital diaphragmatic hernia. Am J Obstet Gynecol. 2006;195(6):1720–1728. [DOI] [PubMed] [Google Scholar]
- 36.Ruano R, Takashi E, da Silva MM, et al. : Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound Obstet Gynecol. 2012;39(1):42–49. [DOI] [PubMed] [Google Scholar]


