Abstract
Background:
Splenic infarction (SI) is a rare clinical entity seldom encountered in veterinary medicine. Its most frequent causes include thromboembolic status, splenomegaly, and cardiac disease. Although thrombotic elements from the circulation provide the most common context for thromboembolic SIs, immune-mediated hemolytic anemia (IMHA) has not been reported as an underlying disease in canine SI.
Case description:
A 2-year-old, female spayed Dachshund, was referred with vomiting, hematochezia, and brown colored urine over the preceding 4 days. Physical examination revealed abnormalities including generalized weakness, jaundice, and splenomegaly; blood work showed pancytopenia and hyperbilirubinemia. Erythrocyte agglutination, polychromasia, and spherocytes on a peripheral blood smear were observed and IMHA concurrent with thrombocytopenia was diagnosed.
Findings/treatment and outcome:
Although erythrocyte agglutination and leukopenia disappeared after treatment, anemia and thrombocytopenia were unresponsive to oral immunosuppressive drugs and repeated transfusions. Further abdominal ultrasound identified an occlusive splenic vein thrombus. Splenic histopathology found marked multifocal to coalescing necrosis, and hemorrhage consistent with multiple SI. Symptoms resolved following splenectomy combined with 1 month of immunosuppressive medication, and the dog was healthy on follow-up evaluation after 2 years.
Conclusion:
Immune-mediated hemolytic anemia is an incompletely characterized cause of SI. This report establishes a potential and novel causal role for IMHA in canine SI. We believe it to be the first case report of SI in a dog with refractory IMHA and thrombocytopenia, successfully managed by splenectomy combined with short-term immunosuppressive therapy.
Key Words: Hemolytic anemia, Splenic infarction, Thrombocytopenia, Thromboembolism, Venous thrombosis
Introduction
Splenic infarction (SI) results from diseases that cause hypercoagulable states, splenomegaly, and cardiac disease (Hardie et al., 1995 ▶; Spangler and Kass, 1999 ▶). In dogs, it occurs in only 1 to 2% of all splenic lesions (Hardie et al., 1995 ▶). Diseases underlying SI in dogs include gastric dilation and volvulus, hyper-adrenocorticism, myeloid metaplasia, myocardial infarction, and disseminated intravascular coagulation (Hardie et al., 1995 ▶; Spangler and Kass, 1999 ▶).
Immune-mediated hemolytic anemia (IMHA) is common in dogs, sometimes inducing hypercoagulable states and splenomegaly. In human medicine, the most common causes of SI are hematologic diseases, including autoimmune hemolytic anemia (AIHA) (Nores et al., 1998 ▶). Splenic infarction in human patients with AIHA is also uncommon but not unknown (Park et al., 2011 ▶; Usküdar et al., 2012 ▶). To our knowledge, SI associated with IMHA has not been reported in canine patients. Here, we report such a case, in which SI was secondary to thrombosis of the splenic vein. Our report aims to inform both diagnosis and treatment of this life-threatening pathology in dogs, and to contribute a comprehensive description of its clinical signs.
Case description
A 2-year-old spayed female Dachshund presented with vomiting, hematochezia, and brown colored urine over 4 days. Upon arrival, the dog had hyperthermia (39.3°C), normal heart rate (156 beats/min), and tachypnea (60 breaths/min); physical examination revealed generalized jaundice. The complete blood count revealed pancytopenia [white blood cell (WBC) 3.85 × 109/L, reference interval (RI) 5.05-16.76 × 109/L; hematocrit 10.4%, RI 40-55%; platelet count 34 × 103/µL, RI 200-500 × 103/µL]. We observed moderate numbers of spherocytes (4-6 per high-power field), and polychromasia and anisocytosis. A saline blood agglutination test was positive. The serum biochemical profile showed hyperbilirubinemia (2.0 mg/dL, RI 0-0.9 mg/dL), hypophosphatemia (2.1 mg/dL, RI 2.5-6.8 mg/dL), hypocalcemia (6.5 mg/dL, RI 7.9-12 mg/dL), and hypoproteinemia (4.9 mg/dL, RI 5.2-8.2 mg/dL). Electrolyte analysis showed hyponatremia (143 mmol/L, RI 144-160 mmol/L), hypochloremia (103 mmol/L, RI 109-122 mmol/L), and hypokalemia (2.5 mmol/L, RI 3.5-5.8 mmol/L). D-dimer (0.1 mg/dL, RI 0-0.3 mg/dL) and coagulation tests including prothrombin time and partial thromboplastin time were normal. Commercial enzyme-linked immunosorbent assay (ELISA) (SNAP 4DX, IDEXX Laboratories, Seoul, Korea) and a polymerase chain reaction (PCR) panel for tick-vector borne diseases (IDEXX Laboratories, Seoul, Korea) were negative, ruling out infectious diseases as a cause of the anemia. Thoracic and abdominal radiography revealed no remarkable abnormalities other than splenomegaly. Based on these findings, we diagnosed IMHA and thrombocytopenia concurrent with splenomegaly.
The patient was given a blood transfusion for symptom amelioration and therapy with prednisolone (1 mg/kg per os (PO) twice daily, Yuhan Co., Ltd., Seoul, Korea), azathioprine (2 mg/kg PO once daily, Celltrion Pharm, Chungbuk, Korea), famotidine (0.5 mg/kg PO twice daily, Ilhwa Pharm, Gangwon, Korea), and cephalexin (30 mg/kg PO twice daily, Boryung Pharm, Kyungki, Korea) was initiated. Although the leukopenia resolved and erythrocyte agglutination disappeared a few days after therapy initiation, other hematologic abnormalities including anemia and thrombocytopenia proved refractory to the oral immunosuppressive drugs and repeated blood transfusions.
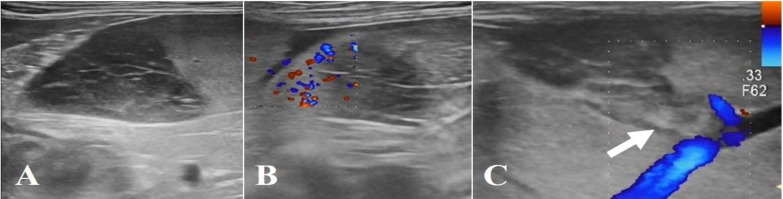
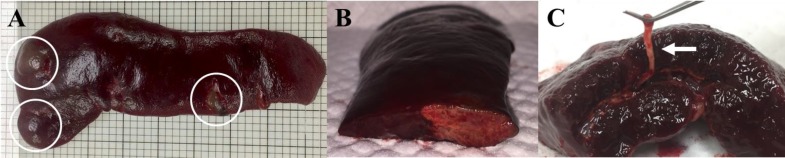
On the 4th day of hospitalization, we performed abdominal Doppler ultrasound to identify possible causes of progressive anemia and thrombocytopenia. This revealed hypoechoic, heterogenous, poorly demarcated, and irregular shaped structures with no blood flow on E-flow in the splenic body and tail region (Figs. 1A and B), and echogenic thrombus in the splenic vein lumen (Fig. 1C), which is indicative of SI. Ultrasound was repeated daily over 4 days showed gradual enlargement of the lesion; the risk of severe complications such as splenic rupture prompted us to recommend splenectomy. The owner consented, and the dog underwent a surgical exploration of the abdominal cavity while in the dorsal recumbent position. The spleen was generally congestive and enlarged, with well-demarcated multifocal nodules throughout the splenic parenchyma; the rest of the abdomen was unremarkable (Fig. 2A). Total splenectomy was performed, and the abdomen lavaged with warmed sterile saline and closed routinely. The dog recovered uneventfully with routine postoperative antibiotics.
Fig. 1.
Abdominal ultrasound image of an occlusive splenic vein thrombus in a dog with immune-mediated hemolytic anemia (IMHA). (A) Hypoechoic, heterogenous, poorly marginated and irregular shaped structures on the splenic body and tail region, (B) No blood flow on E-flow on the splenic lesion, and (C) Abdominal ultrasound image demonstrating echogenic thrombus in the lumen of the splenic vein (arrow)
Fig. 2.
Gross morphology of the splenic lesion in a dog with splenic infarction (SI). (A) Spleen is generally congestive, and an enlarged and well-demarcated multifocal lesion (circles) is identified, (B) Cross section of the spleen shows clear distinction of the infarct from the normal parenchyma, and (C) White-colored, elastic, and linear materials are visible in the splenic vessel (arrow)
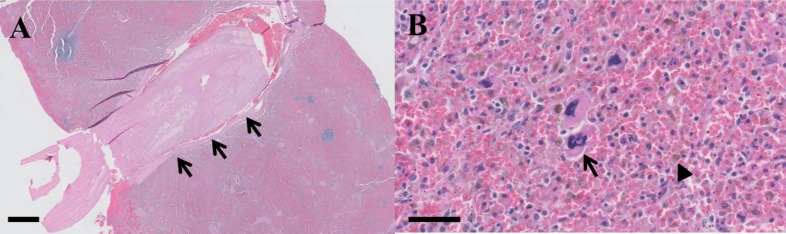
Cross-section of the splenic nodule showed it to be distinct from the surrounding parenchyma, tan to yellow in color (Fig. 2B). The splenic vein contained white linear elastic material (Fig. 2C). Histopathology of the splenic tissue was consistent with SI, revealing multiple areas of necrosis and hemorrhage, and a large thrombus in the splenic blood vessel (Fig. 3A). Additionally, the red pulp contained large numbers of hemosiderin-laden macrophages, lymphocytes, small numbers of erythroid precursors and megakaryocytes (Fig. 3B).
Fig. 3.
Histological features of the spleen in a dog with splenic infarction (SI). (A) Large thrombus in the splenic blood vessel (arrows) which may result in multiple areas of necrosis and hemorrhage, (H&E, ×10; scale bar= 1 mm), and (B) The red pulp contains many hemosiderin-laden macrophages (arrowhead), lymphocytes, and a few erythroid precursors and megakaryocytes (arrow), (H&E, ×400; scale bar= 50 µm)
Results
After splenectomy, the dog’s condition improved, and packed cell volume and platelet count promptly returned to normal. The patient was discharged 2 days after surgery with instructions for oral immuno-suppressive medication. Prednisone therapy was weaned and stopped after 1 month. The patient did not suffer recurrence and remained healthy after 2 years, at the time of this submission.
Discussion
Splenic infarction is a rare pathology, most commonly associated with hypercoagulable conditions in dogs (Friedenberg et al., 2015 ▶; Schattner et al., 2015 ▶). In humans, SI is usually clinically silent, discovered only during splenectomy for hematologic or other indications, or on autopsy (Nores et al., 1998 ▶). Early diagnosis of SI in dogs may also be difficult because clinical signs are usually nonspecific and as yet incompletely described (Hardie et al., 1995 ▶; Friedenberg et al., 2015 ▶). Abdominal ultrasound is an essential diagnostic tool for SI (Hardie et al., 1995 ▶). Ultrasonographic evidence of splenomegaly is considered non-specific, but focal or diffuse splenic hypoechogenicity combined with lack of splenic blood flow visualized by color Doppler is highly suggestive of SI (Hardie et al., 1995 ▶; Bokman et al., 2014 ▶; Friedenberg et al., 2015 ▶). The infarct may be segmental or involve the entire spleen, and typically results from arterial or venous compromise. In the case we report here, abdominal ultrasound findings were consistent with SI with splenic venous thrombosis. We excluded differential diagnoses of splenic pathologies such as hematoma, abscess, torsion, and neoplasia.
Although thromboembolic causes include cardiovascular disorders, hyperadrenocorticism, and neoplasia, we know of no published reports of SI in canine IMHA patients. Immune-mediated hemolytic anemia is caused by immune-mediated destruction of red blood cells and confers a high risk of thromboembolic complications (Carr et al., 2002 ▶; Swann et al., 2016 ▶). Corticosteroid, a first-line treatment of IMHA, may also cause hypercoagulable states by reducing plasminogen activator concentrations (Laurenson et al., 2010 ▶). A retrospective study of 72 dogs with IMHA found thrombi in the major vessels of approximately 80% of dogs on postmortem examination, implicating thromboembolism as a major cause of mortality (Carr et al., 2002 ▶). Our patient presented with normal D-dimer levels but was diagnosed fortuitously with splenic vein thrombosis and SI during abdominal ultrasound to investigate her progressive anemia and concurrent thrombocytopenia. In cases of cardiovascular thromboembolism, related infarcts are affected by multiple emboli; but infarcts induced by hematological diseases such as IMHA are usually limited to the spleen (Park et al., 2011 ▶). In human medicine, immune-mediated mechanisms related to cryoglobulin synthesis may cause splenic infarct (Usküdar et al., 2012 ▶). Our study revealed neither laboratory evidence of hypercoagulability, nor any cardiac cause, with localization of the infarct in the spleen. Enlarged spleens appear prone to infarction (Hardie et al., 1995 ▶), and splenomegaly in our patient was likely due to either extramedullary hematopoiesis or IMHA; splenic infarct may have arisen due to splenomegaly or immune-mediated mechanisms.
Concurrent thrombocytopenia occurs in 50-70% of dogs with IMHA, possibly because of increased platelet loss caused by disseminated intravascular coagulation, hemorrhage, or immune-mediated destruction (Swann et al., 2016 ▶). We identified thrombocytopenia concurrent with IMHA in our patient; both conditions improved after splenectomy and immunosuppressive treatment. The patient’s anemia likely resulted from sequestration of erythrocytes within the enlarged spleen (Horgan et al., 2009 ▶); her transient leukopenia was probably due to consumption of WBCs by acute inflammation associated with the poorly perfused splenic parenchyma.
We report pathologic changes of the spleen that are consistent with hypersplenism: splenic myeloid metaplasia, thrombosis, infarction, erythrophagocytosis, and splenomegaly (Zamokas et al., 2016 ▶). Similar findings were reported in a retrospective study of 65 dogs that highlighted overlap with human conditions such as hypersplenism, myeloid metaplasia, and hemophagocytic syndrome (Spangler and Kass, 1999 ▶; Weiss, 2007 ▶). Hypersplenism is a group of syndromes that involve splenomegaly and peripheral cytopenia with various etiologies (George, 2014 ▶; Lv et al., 2016 ▶). In a previous report, a case of splenic torsion resulted in clinical signs and laboratory changes similar to those seen in dogs with hypersplenism (Schnier, 2010 ▶). However, an association of hypersplenism with SI as observed in the present case has not been reported previously. Our patient’s rapid recovery from progressive anemia, hemolysis, and thrombocytopenia after splenectomy suggests that these were all attributable to hypersplenism.
For both humans, and dogs, the first-line treatment of IMHA is glucocorticoids (Horgan et al., 2009 ▶). Splenectomy is the second line treatment in humans who are unresponsive to glucocorticoid therapy, yielding a response rate of 60-75% (Horgan et al., 2009 ▶). In veterinary medicine, two retrospective case series have reported that splenectomy may be useful to treat cases of immune-mediated thrombocytopenia, anemia, and Evan’s syndrome that are seemingly refractory to medication in dogs (Feldman et al., 1985 ▶; Horgan et al., 2009 ▶). They state that splenectomy reduces the requirements for transfusion or medication postoperatively, and potentially allows the rapid normalization of hematologic abnormalities (Feldman et al., 1985 ▶; Horgan et al., 2009 ▶). However, neither study enrolled a control group, and moreover, the underlying pathophysiology in association with splenic lesions, such as infarction, was not well addressed (Swann et al., 2019 ▶). Therefore, in veterinary patients, splenectomy is not commonly performed because of the possible risks of inducing immunodeficiency, which may further precipitate secondary infection (Horgan et al., 2009 ▶). However, as in the present case, splenectomy may in fact be favorable for veterinary patients, given the alternative risks of SI with possible severe complications, such as hypersplenism secondary to IMHA.
As in the present case, cases of SI possibly caused by hypercoagulable state in IMHA have not been reported previously, especially that in which splenectomy was indicated because of the risk of spleen rupture. Similarly, in humans, SI secondary to occlusion of the major splenic vessel may progress to splenic abscess or rupture, complications that usually require surgical intervention (Nores et al., 1998 ▶; Ozakin et al., 2016 ▶). In our patient, we chose splenectomy to resolve refractory IMHA, leading to a dramatic recovery. These clinical courses suggest that concurrent spleen infarction may be responsible for refractory IMHA.
To our knowledge, this is the first reported veterinary case of SI as a rare complication associated with IMHA, corresponding to the patterns reported in human medicine (Park et al., 2011 ▶; Usküdar et al., 2012 ▶). We conclude that splenic infarcts should be considered in patients with similar cases of refractory IMHA, and diagnostic abdominal Doppler ultrasound must be performed even if the D-dimer value is within normal limits. In dogs with splenic infarct accompanied by severe persistent anemia, with or without thrombocytopenia, immediate splenectomy may save the patients’ life.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- Bokman CL, Sfeir M, Chahwala V, Ginzburg E. Spontaneous massive splenic infarction in the setting of renal transplant and septic shock: a case report and review of the literature. Case. Rep. Med. 2014;2014:510259. doi: 10.1155/2014/510259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune-mediated hemolytic anemia: a retrospective study of 72 dogs. J. Vet. Intern. Med. 2002;16:504–509. doi: 10.1892/0891-6640(2002)016<0504:pffmat>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Feldman BF, Handagama P, Lubberink AA. Splenectomy as adjunctive therapy for immune-mediated thrombocytopenia and hemolytic anemia in the dog. J. Am. Vet. Med. Assoc. 1985;187:617–619. [PubMed] [Google Scholar]
- Friedenberg SG, Balakrishnan N, Guillaumin J, Cooper ES, Lewis K, Russell DS, Breitschwerdt EB. Splenic vasculitis, thrombosis, and infarction in a febrile dog infected with Bartonella henselae. J. Vet. Emerg. Crit. Care. 2015;25:789–794. doi: 10.1111/vec.12367. [DOI] [PubMed] [Google Scholar]
- George MR. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J. Blood. Med. 2014;5:69–86. doi: 10.2147/JBM.S46255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie EM, Vaden SL, Spaulding K, Malarkey DE. Splenic infarction in 16 dogs: a retrospective study. J. Vet. Intern. Med. 1995;9:141–148. doi: 10.1111/j.1939-1676.1995.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Horgan JE, Roberts BK, Schermerhorn T. Splenectomy as an adjunctive treatment for dogs with immune-mediated hemolytic anemia: ten cases (2003-2006) J. Vet. Emerg. Crit. Care. 2009;19:254–261. doi: 10.1111/j.1476-4431.2009.00419.x. [DOI] [PubMed] [Google Scholar]
- Laurenson MP, Hopper K, Herrera MA, Johnson EG. Concurrent diseases and conditions in dogs with splenic vein thrombosis. J. Vet. Intern. Med. 2010;24:1298–1304. doi: 10.1111/j.1939-1676.2010.0593.x. [DOI] [PubMed] [Google Scholar]
- Lv Y, Lau WY, Li Y, Deng J, Han X, Gong X, Liu N, Wu H. Hypersplenism: history and current status. Exp. Ther. Med. 2016;12:2377–2382. doi: 10.3892/etm.2016.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am. Surg. 1998;64:182–188. [PubMed] [Google Scholar]
- Ozakin E, Cetinkaya O, Baloglu Kaya F, Acar N, Cevik AA. A rare cause of acute abdominal pain: splenic infarct (case series) Turk. J. Emerg. Med. 2016;15:96–99. doi: 10.5505/1304.7361.2015.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MY, Kim JA, Yi SY, Chang SH, Um TH, Lee HR. Splenic infarction in a patient with autoimmune hemolytic anemia and protein C deficiency. Korean J. Hematol. 2011;46:274–278. doi: 10.5045/kjh.2011.46.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner A, Adi M, Kitroser E, Klepfish A. Acute splenic infarction at an academic general hospital over 10 years: presentation, etiology, and outcome. Medicine (Baltimore). 2015;94:e1363. doi: 10.1097/MD.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnier LM. A case of splenic torsion with progressive anemia and thrombocytopenia. Can. Vet. J. 2010;51:527–529. [PMC free article] [PubMed] [Google Scholar]
- Spangler WL, Kass PH. Splenic myeloid metaplasia, histiocytosis, and hypersplenism in the dog (65 cases) Vet. Pathol. 1999;36:583–593. doi: 10.1354/vp.36-6-583. [DOI] [PubMed] [Google Scholar]
- Swann JW, Garden OA, Fellman CL, Glanemann B, Goggs R, LeVine DN, Mackin AJ, Whitley NT. ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 2019;33:1141–1172. doi: 10.1111/jvim.15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Woods K, Wu Y, Glanemann B, Garden OA. Characterisation of the immunophenotype ofdogs with primary immune-mediated haemolytic anaemia. PLoS One. 2016;11:e0168296. doi: 10.1371/journal.pone.0168296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usküdar TH, Karahan S, Gümüş U. Splenic infarct in a patient with autoimmune hemolytic anemia. Turk. J. Haematol. 2012;29:432–433. doi: 10.5505/tjh.2012.71324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ. Hemophagocytic syndrome in dogs: 24 cases (1996-2005) J. Am. Vet. Med. Assoc. 2007;230:697–701. doi: 10.2460/javma.230.5.697. [DOI] [PubMed] [Google Scholar]
- Zamokas G, Grigonis A, Babickaitė L, Riškevičienė V, Lasienė K, Juodžiukynienė N. Extra-medullary hematopoiesis (EMH) and other pathological conditions in dog spleens. Med. Weter. 2016;72:768–772. [Google Scholar]