Abstract
Adolescence is a particularly vulnerable neurodevelopmental period marked by high rates of engagement with risky alcohol use. This review summarizes the cognitive and neural consequences following alcohol use during adolescence from longitudinal design studies in humans and animals. Findings from human adolescent studies suggest that binge drinking and heavy alcohol use is associated with poorer cognitive functioning on a broad range of neuropsychological assessments, including learning, psychomotor speed, attention, executive functioning, and impulsivity. adolescence is memory, visuospatial functioning, Alcohol use during associated with accelerated decreases in gray matter and attenuated increases in white matter volume, and aberrant neural activity during executive functioning, attentional control, and reward sensitivity tasks, when compared to non-drinking adolescents. Animal studies in rodents and non-human primates have replicated human findings, and suggest cognitive and neural consequences of adolescent alcohol use may persist into adulthood. Novel rodent studies demonstrate that adolescent alcohol use may increase reward responsiveness of the dopamine system to alcohol later in life, as well as disrupt adolescent neurogenesis, potentially through neuroinflammation, with long-lasting neural and behavioral effects into adulthood. Larger longitudinal human cognitive and neuroimaging studies with more diverse samples are currently underway which will improve understanding of the impact of polysubstance use, as well as the interactive effects of substance use, physical and mental health, and demographic factors on cognition and neurodevelopment.
1. Introduction
Adolescence is a critical developmental phase involving significant physical, cognitive, emotional, social, and behavioral changes. Cognitive features of adolescence include heightened reward sensitivity, sensation seeking and impulsive action, and diminished self-control to inhibit emotions and behaviors (1, 2). This contributes to the high rates of engagement in risky behaviors, including the initiation and escalation of alcohol use. Adolescent-specific brain developments may predispose young people to be particularly vulnerable to the potentially serious and long-lasting alcohol-related consequences (3).
Cross-sectional design studies have established a relationship between adolescent alcohol use, brain development, and cognitive function (4). Over the past decade, researchers have attempted to understand the direction of this relationship. Considering that it would be highly unethical to randomize youth to different alcohol-using groups, human research is limited to natural observational studies. This makes it difficult to discern correlational from causal findings. Prospective, longitudinal designs have been used to help delineate between pre-existing alterations and post-alcohol effects on brain development by assessing youth before they have ever used alcohol or other drugs and continuing to assess them over time as a portion of the participant population naturally transitions into substance use. This design allows for examination of normal developmental neural trajectories in youth who have never used alcohol or drugs during adolescence, and compares their brain maturation to youth who transition into substance use.
A recent review summarized potentially pre-existing neurobiological markers of alcohol use in humans (5). While previous reviews have explored the neurobiological consequences of alcohol use, limitations exist. Some previous reviews have summarized studies examining the impact of one adolescent drinking pattern (4), or one study type (i.e., neuropsychological studies (6), neuroimaging studies (7)). Broader, more inclusive, reviews on the effects of alcohol use exist, although they require updating due to the rapidly expanding evidence base (8, 9). The aim of this review is to therefore provide an update on the growing literature by summarizing the neural and cognitive consequences of varying patterns of alcohol use during adolescence, from prospective longitudinal studies in humans, rodents and non-human primates. In order to provide a broader context of the neural and cognitive consequences of alcohol use, this review begins with an overview of adolescent brain development, and the global prevalence rates of adolescent alcohol use before summarizing the effects of adolescent alcohol use on the brain and behavior from both human and animal studies. A focus has been placed on neuroimaging, neuropsychological, and neurophysiological studies as a means to provide a better understanding of the underlying neurobiological consequences of early alcohol use. Findings from cross-sectional studies are not included.
2. Overview of the adolescent brain
The brain undergoes significant neurodevelopment during adolescence, with maturation continuing until around age 25 (10, 11). Brain gray matter, which includes mostly nerve cell bodies and dendrites, tends to decrease during normal adolescent brain development via removal of weak synaptic connections and changes in the extracellular matrix (11–16). Concurrently, white matter volume and white matter integrity increase over this period with continued myelination of axons, allowing for more efficient communication between brain regions (17–20). Some research suggests that through this process, distributed connectivity and circuitry between distant brain regions is increased relative to more local connectivity (21–23); however, this finding has been debated (24).
Various regions of the brain have time-varying developmental trajectories, with lower order sensorimotor regions maturing first, followed by limbic regions important for processing rewards, and frontal regions associated with higher order cognitive functioning developing later in adolescence and young adulthood (15, 16, 25, 26). Adolescent brain developmental trajectories tend to differ by sex, with female brains developing one to two years earlier than males. For instance, cortical gray matter reaches peak thickness in the parietal lobes at ages 10 (female) and 12 (male), and in the frontal lobes at ages 11 (female) and 12 (male). Although this pattern is reversed for the temporal lobes, which reaches maximal thickness at ages 16 (male) and 17 (female; 17).
Neurotransmitter systems, which transmit chemical signals across synapses, also undergo significant change in adolescence. Dopamine projections to the limbic and frontal regions often peak during adolescence (27, 28). This is associated with amplified neural sensitivity following rewards, compared to adulthood (29, 30). Inhibitory control is generally lower in adolescence than adulthood, reflecting greater excitatory synapses and less GABAergic inhibitory neurotransmitters in higher-order frontal regions, with the ratio reversing in later adolescence and into adulthood (31). Reward hypersensitivity in combination with low inhibition is thought to increase adolescents’ drive for risky and novel experiences, such as alcohol use (30, 32). Neurotoxin exposure, particularly alcohol use, during adolescence can affect healthy brain development, with even minor changes in neurodevelopmental trajectories affecting a range of cognitive, emotional, and social functioning (4). Alcohol use during adolescence could therefore set the stage for cognitive problems into adulthood, conferring functional consequences throughout life.
3. Global prevalence of adolescent alcohol use
Alcohol use among adolescents is heterogeneous, ranging from low, normative use to heavy, pathological use. Alcohol is the most frequently used substance, as it is generally the easiest for adolescents to access (33). The average age of initiation for alcohol use among US and Australian adolescents is 15 years (34, 35). Across Europe, most adolescents begin drinking alcohol between ages 12 and 16, with 25% of adolescents in this region first consuming alcohol by age 13 (36). The worldwide estimate of adolescents (age 15–19) who drank alcohol in the past month is 27%, ranging from 1 to 44% across countries (Figure 1; 33). Higher rates of past month adolescent drinking occur in higher income countries; the highest rates are observed in the European region (44%), and the lowest rates are observed in the Eastern Mediterranean region (1.2%; 33, 37). Past month alcohol use among adolescents in other countries ranges from 38% in the Americans and Western Pacific regions, to 21% in Africa and Southeast Asia, and 14% in Japan (33, 38).
Figure 1:
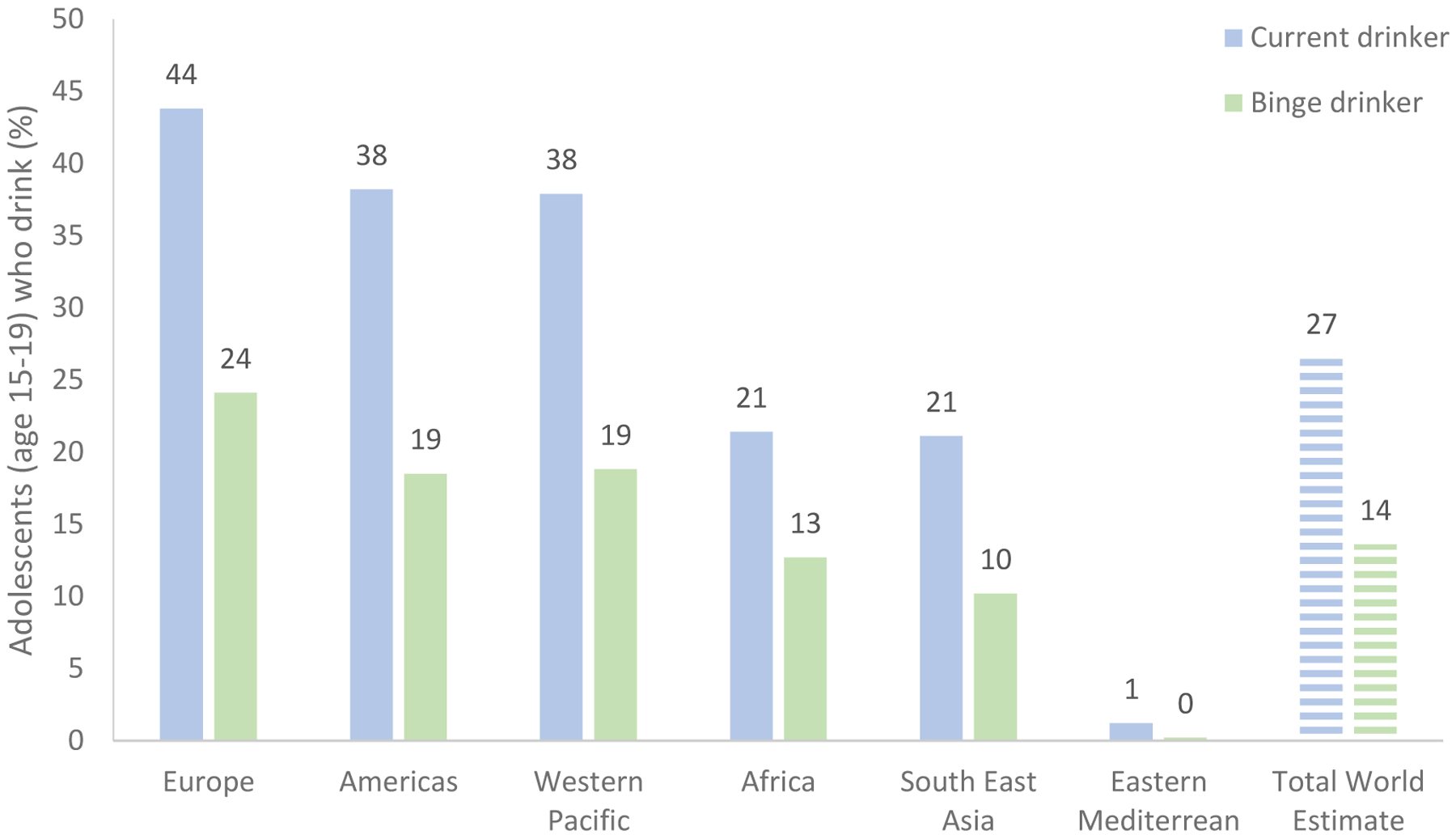
Prevalence of current alcohol use and binge drinking in adolescents aged 15 to 19. In this data, binge drinking was defined as 60+ grams of pure alcohol (~4 standard US drinks) on at least one occasion per month (33).
It is also important to consider common drinking patterns among adolescents, therefore many studies use the alcohol use classification summarized in Figure 2 (39, 40). While rates of heavy drinking are highest among young people aged 20 to 24, heavy alcohol use among adolescents remains a concern. Binge drinking is a pattern of alcohol use that raises blood alcohol concentration (BAC) levels consumption of four or more standard drinks for females and five or more drinks for to 0.08 g/dL, which typically occurs after the males within a two hour period (39). Binge drinking in young people aged 15 to 19 is particularly prevalent (Figure 1), with global estimates of 14% reporting this drinking pattern over the previous month (33). The highest rates of binge drinking are in the European region (24%; 33), particularly in Austria, Cyprus, and Denmark where more than 50% of students report this binge drinking pattern (41). In the US, 4% and 14% of US adolescents aged 14 and 18, respectively, report binge drinking in the previous two weeks (42). Similarly in Australia, 2% and 17% of 14 and 17 year olds report binge drinking in the previous week (43). Approximately 13% of adolescents in Africa and 10% of adolescents in South East Asia report past month binge drinking (33).
Figure 2:
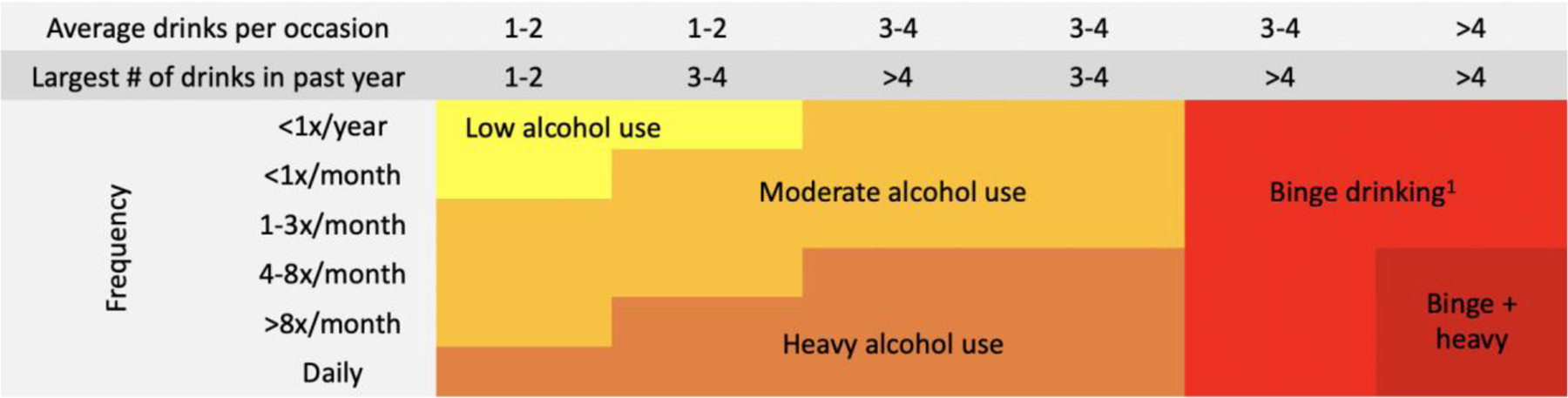
Alcohol use classification chart.
1 Binge drinking is typically ≥4 drinks within 2 hours (females) and ≥5 drinks within 2 hours (males), where the blood alcohol concentration (BAC) level rises to 0.08 g/dL (39). The chart is based on the National Institute on Alcohol Abuse and Alcoholism, and Substance Abuse and Mental Health Services Administration levels of alcohol use definitions (39, 40).
As noted previously with neurodevelopment trajectories, gender differences are also reported in alcohol use estimates. Worldwide estimates of alcohol use also show higher rates of drinking occur among young males than females (33). Globally, 22% of males and 5% of females binge drink during adolescence. When focusing on country-specific adolescent binge drinking, rates are reported as 36% of males and 12% of females in Europe; 30% of males and 6% of females in the Americas and Western Pacific Regions; and approximately 17–21% of adolescent males and 3–4% of adolescent females binge drink in Africa and South East Asia.
Overall, these general and gender-specific in general alcohol use prevalence rates represent a recent decline and binge drinking that parallels an increase in the number of adolescents who abstain from alcohol use altogether (44–47). Despite these declines, adolescent alcohol consumption remains a major public health concern. There is clear evidence that adolescent alcohol use is associated with a wide range of adverse outcomes in both the short and long term. Negative consequences of adolescent alcohol use include gradual attrition of cognitive functions and aberrant neural development trajectories (4).
4. Adolescent alcohol effects on the human brain
Prospective longitudinal neuropsychological, neuroimaging, and neurophysiological studies have identified cognitive and neural consequences directly related to initiation and escalation of adolescent alcohol use. Overall, adolescent alcohol use has been found to negatively affect cognition, brain structure, and function (Table 1); however, the level to which alcohol use and different patterns of drinking affects male and female brain functioning has been debated. Research in this field is also limited to natural observational studies, and it is common for a portion of adolescents to use multiple substances (e.g., alcohol and cannabis use). While studies may try to statistically control for other drug use to parse the relative contribution of alcohol use on brain functioning, this method is imperfect given the high collinearity between alcohol and other drug use variables as well as potential interactive effects. Longitudinal studies with very large sample sizes are currently underway and may help to answer these important issues (48–50).
Table 1:
Summary of consequences of adolescent alcohol use in humans and rodents
| Humans | Rodents | |
|---|---|---|
| Cognitive | Binge/heavy drinking vs control: | Sustained effects into adulthood: |
| ↓ Immediate recall (short-term memory) (53, 63) | ↓ Executive functioning (92–94) | |
| ↓ Delayed retention, recall (long-term memory) (53, 57, 58, 63) | ↑ Risk-taking (94, 95, 97–100) | |
| ↓ Learning (57, 58) | ↑ Impulsivity (94, 95, 97–100) | |
| ↓ Visuospatial function (58) | ||
| ↓ Working memory (52, 53) | Adolescent-specific effects: | |
| ↓ Learning (101–103) | ||
| Dose-dependent relationships: | ↓ Memory (101–103) | |
| ↑ Alcohol use = | ||
| ↓ General cognitive functioning (57) | ||
| ↓ Verbal memory (54, 60, 61) | ||
| ↓ Executive functioning (60) | ||
| ↓ Semantic clustering (60) | ||
| ↓ Reading skills (60) | ||
| ↓ Visuospatial function (54) (females only (62)) | ||
| ↑ Impulsivity (59) | ||
| ↑ Withdrawal / hangovers symptoms = | ||
| ↓ General cognitive functioning (60) | ||
| ↓ Psychomotor speed (54) | ||
| ↓ Attention (males) (62) | ||
| Neural | Binge/heavy drinking vs control: | Sustained effects into adulthood: |
| ↓ Gray matter volume, particularly frontal, temporal (65–69) | ↓ Gray matter volume (106) | |
| ↓ White matter growth (66–70) | ↓ Cortical thickness (107) | |
| ↓ White matter integrity (71–73) | ↓ White matter integrity (106, 109–112) | |
| ↑ Cerebrospinal fluid volume cerebellum (66) | ↓ Synaptic plasticity (101) | |
| ↑ Brain activation during executive functioning (79, 80) | ↓ Connectivity between brain regions (108) | |
| ↓ Brain activation during reward | ↓ Neurogenesis (129–135) | |
| sensitivity tasks (81, 82) | ↑ Neuroinflammation (138) | |
| ↑ P3 amplitdue, particular fronto-parietal during executive functioning and attentional control (85–87) | ||
| Dose-dependent relationships: | ||
| Dose-dependent relationships: | ↑ GABA inhibitory tone on dopamine system = | |
| ↑ Alcohol use = | ↑ Risky decision-making (124) | |
| ↑ Gray matter frontal volume (65) | ↑ Cholinergic tone = | |
| ↑ Brain activation during reward sensitivity tasks (81) | ↑ Disinhibition (99) | |
| ↑ Risk-taking (125) | ||
| ↓ Executive functioning (127) |
Neuropsychological consequences of alcohol use
Neuropsychological test batteries enable tracking of cognitive skills over time to detect potential effects of alcohol use on cognition and intellectual development. Alcohol-induced deficits are arguably even more impactful for adolescents than adults, given that educational attainment, learning, and ongoing neural development are the most critical developmental tasks of adolescence. Notably, alcohol use behaviors at ages 12 to 14 predict lower educational achievement in later years, even after accounting for confounding factors such as sex and externalizing behavior (51). A recent meta-analysis of cross-sectional studies reported adolescent binge drinking was associated with an overall cognitive deficit and specific impairments in decision-making and inhibition (4). Herein, we report on longitudinal studies that have identified potential negative effects of adolescent binge drinking and heavy alcohol use on memory, learning, visuospatial function, executive function, reading ability and impulsivity.
The Avon Longitudinal Study of Parents and Children is an ongoing population-based study in the UK. Utilizing data from 3,141 adolescents, frequent binge drinkers exhibited poorer working memory compared to the low alcohol group. However, this association was attenuated when adjusting for sociodemographic variables, tobacco, and cannabis use (52). In a sample of 89 young people who did not have a history of psychiatric disorders and did not regularly consume other drugs, consistent binge drinking over two years in late adolescence was associated with poorer immediate and delayed recall, retention, and working memory, compared to non-binge drinkers (53). Conversely, a four-year study of 234 adolescents unexpectedly found that more alcohol use predicted better working memory, driven largely by a relationship between recent blackout history and auditory attention scores, when controlling for age, socioeconomic status, abstinence, gender, and baseline performance (54). Although, this was in contrast to other findings in this study which demonstrated that more alcohol use days predicted worse verbal memory and visuospatial ability. Approximately 40% of the cohort had tried cannabis, and 18% had tried other illicit drugs. No follow-up tests supported the unexpected working memory finding, such as removing gender and other covariates from the regression models. The authors conclude that unreliability of self-report alcohol use data may have also contributed to the unexpected result. A study using eight years of data from 2,226 youth in the Tracking Adolescents’ Individual Lives Survey (TRAILS) found that light and heavy adolescent alcohol use was not associated with deterioration in executive functioning, compared to no alcohol use, when controlling for baseline performance, age, and tobacco use (55). A four-year study of 92 adolescents found low alcohol consumption was associated with subtle improvements in inhibitory control (56). No negative effect of low-level alcohol use on the development of school grades, spatial working memory or rapid visual processing was found. Therefore, binge drinking may have specific detrimental effects on executive functioning, in comparison to lighter doses. Inconsistent findings may also partly reflect psychiatric and other substance use comorbidities.
A 10-year longitudinal study followed heavy alcohol using and control youth from age 16 until early adulthood (~age 25). Youth diagnosed with a psychiatric disorder, besides conduct disorder, were excluded from the study at intake. Heavy alcohol use and withdrawal symptoms were associated with worsening verbal memory and learning over time (57, 58), as well as relative declines in visuospatial function (58). Heavier use patterns, and greater hangover and withdrawal symptoms over time were related to worse cognitive functioning, suggesting a dose-dependent relationship between alcohol use and cognitive functioning (57). Dose-dependent relationships between alcohol use and cognitive impairment have been replicated in other studies. Higher total life-time drinks predicts escalated impulsive choice (59), and poorer cognitive flexibility, verbal recall, semantic clustering, and reading skills (60). Higher drinking days over a four-year period predicted worse verbal memory and visuospatial ability (54). Higher estimated peak BAC over six years predicted worse verbal learning, and immediate, short and long-term delayed and cued recall (61). Greater post-drinking effects predict worse psychomotor speed (54), and more withdrawal symptoms over the past month are associated with greater decrements in cognitive functioning (60). Overall, heavy alcohol use during adolescence has been associated with a range of cognitive deficits, with some cognitive domains showing dose-dependent relationships where greater alcohol use is associated with poorer cognitive functioning (see Table 1).
Sex-related neuropsychological consequences of alcohol use
Adolescent alcohol use may differentially impact male and female cognitive function, furthering the implications of noted gender differences within brain development and alcohol use estimates. A five-year longitudinal study followed 89 young adolescents from ages 14 to 19, where a portion transitioned into moderate (14%) or heavy (33%) alcohol use (62). Conduct disorder was present in 15% (female) and 39% (male) of drinkers, and 0% of controls. Drinkers had consumed alcohol at moderate or heavy levels for an average of 2.8 years since initiation (SD=1.3). For females, more drinking days in the past year predicted a greater reduction in visuospatial performance from baseline to follow-up. For males, a tendency was seen for more hangover symptoms in the previous year to predict relative worsening of sustained attention. While drinkers had used cannabis and other drugs, these substances did not predict any change in cognitive functioning. A six-year study followed 155 older adolescents from age 18 every 22-months. Consistent binge drinkers, who continued to engage in binge drinking behavior throughout the entirety of the study, represented 35%, 23% and 10% of the sample at first follow up one, two and three, respectively. Consistent binge drinkers presented difficulties in immediate and delayed recall, with similar deficits for males and females compared to controls (63), while no disadvantage for either sex was observed for decision-making ability (64). This suggests that some cognitive domains may be differentially impacted in adolescent males and females who drink, while other domains may be similarly affected. Further longitudinal research on sex differences in other cognitive domains known to be affected by alcohol use (i.e., learning, executive functions, impulsivity) should be conducted.
Structural brain consequences
Adolescent alcohol-induced alterations in neurodevelopmental trajectories (including accelerated decreases in gray matter volume, attenuated increases in white matter volume and density, and poorer white matter integrity) may underlie some long-term cognitive deficits. Here, longitudinal studies reporting on structural brain changes following alcohol use in adolescence are discussed. The National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA) is a nationally representative prospective longitudinal study being conducted in the US, designed to disentangle the complex relationships between onset, escalation, and desistance of alcohol use in adolescence and neuromaturation (50). At baseline all adolescents were no/low alcohol, tobacco, cannabis and other drug use consumers. Approximately 50% of the cohort endorsed ≥1 externalizing and ≥2 internalizing symptoms. By the two-year follow-up assessment, 356 participants were no/low alcohol consumers, 65 had initiated moderate drinking, and 62 had initiated heavy drinking (65). Adolescents who remained no/low alcohol consumers served as a control group for estimating typical developmental trajectories over the same age range as the drinkers. Youth who initiated heavy drinking showed abnormal neurodevelopmental trajectories compared to continuously non-/low-drinking controls with accelerated decreases in frontal gray matter volume. Marginal differences in frontal gray matter were also observed in moderate drinkers, and although not significant, their intermediate position between no/low and heavy drinkers suggests a dose-dependent effect (65). By the three- to four-year follow up assessment, 328 youth were no/low drinkers, 120 were moderate drinkers and 100 were heavy drinkers (66). Moderate and heavy drinkers continued to exhibit altered neurodevelopmental trajectories, including accelerated cerebellar gray matter declines, white matter expansion, and cerebrospinal fluid volume expansion relative to controls. Cannabis co-use did not contribute to these effects (65, 66).
These findings replicate earlier longitudinal studies with smaller sample sizes showing adolescent heavy drinkers had altered neurodevelopmental trajectories, including accelerated decreases in gray matter in frontal and temporal lobes (67–69), and attenuated increases in white matter growth over time of the frontal, temporal and occipital lobes, cingulate, corpus callosum, and pons, compared to non-using controls (67, 69). A prospective four-year study measured within-subject changes in brain volume for males and females. Heavy-drinking males and females showed similar deviations in neural developmental trajectories compared to continuously non-drinking controls, including accelerated decreases in gray matter volume (particularly in frontal and temporal regions), and attenuated increases in white matter volume over the follow-up, even after controlling for cannabis and other substance use (67).
In a sample of 113 alcohol-naïve adolescents aged 11 to 16 at baseline, 45 went on to binge drink before turning 21. Binge drinking throughout adolescence predicted altered frontostriatal white matter microstructural development when compared to developmental trajectories of non-using healthy adolescents (70). Three studies examining adolescents who used alcohol and cannabis showed these youth had consistently poorer white matter integrity across 7 to 20 clusters compared to controls, as well as poorer cognitive functioning over an 18-month (71) to three-year period (72, 73). Mixed findings were reported for the specific effects of alcohol, with two studies reporting that heavy drinking predicts worsening white matter integrity (71, 72) with either no effect (71) or added effect (72) of co-occurring cannabis use. A third study reported that white matter integrity effects were driven by heavy cannabis initiation (73). The right superior longitudinal fasciculus, connecting the frontoparietal-temporal networks, was the only consistent white matter tract across studies to show poorer white matter integrity among alcohol users compared to control.
Overall, binge and heavy drinking appears to affect the normal developmental trajectories of gray and white matter maturation during adolescence, particularly in the frontal and temporal lobes, and interconnecting networks. Some studies have reported accompanying cognitive deficits alongside aberrant neurodevelopmental trajectories. Patterns observed among alcohol-using youth may represent accelerated but non-beneficial pruning of gray matter, attenuated connective efficiency of white matter tracts, or alternatively, premature cortical gray matter decline similar to volume declines related to accelerated aging in adult alcoholics (74) or even “normal” aging (75, 76). Adults who engage in sustained problematic drinking exhibit similar structural alterations and have speeded gray and white matter decline, which suggests alcohol use is associated with accelerated brain aging (74, 75, 77). Existing studies tend to group youth by “drinkers” versus “controls”. To address this methodological limitation, the Adolescent Brain Cognitive Development (ABCD) Study is underway with a larger sample size (~12,000) which will allow more nuanced investigation of the dose-dependent effect of alcohol on neural development (48, 78).
Functional brain consequences
Task-based functional neuroimaging studies measure brain activation by detecting changes in blood direction while participants complete tasks. These studies can help link structural brain changes with behavioral and cognitive deficits following alcohol initiation in adolescence. Functional neuroimaging studies have identified potential effects of alcohol use on adolescent brain activation during tasks of working memory, inhibitory control, and reward sensitivity. In a longitudinal study, 40 12- to 16-year-old adolescents were scanned before they ever used alcohol or drugs and then were rescanned approximately three years later (79, 80). In total, 15% of adolescents who transitioned into heavy drinking by late adolescence presented with conduct disorder. These heavy drinking adolescents showed less baseline brain activation in frontal and parietal regions during a visual working memory (79) and inhibition task (80) when compared to controls. Neural activation during these tasks increased from baseline to follow-up in youth who initiated drinking compared to decreased activation in those who remained abstinent over the follow-up. This suggests that youth who initiate heavy drinking may require more executive cognitive control to perform at the same level as non-users.
Heavy alcohol use may also affect reactivity and sensitivity to reward. Adolescent binge drinking was associated with less cerebellar (81) and dorsal striatum (82) activation during a monetary reward and decision making task, respectively. More drinks per drinking day predicted less activation within these regions among binge drinkers (81). This suggests binge drinking may affect the emotional component of reward processing and decision making, as damage to the posterior cerebellum has been associated with cognitive and emotional deficits (83), while the dorsal striatum is integral to incorporating emotional information into reward and decision-making (84).
Neurophysiological studies conducted in Spain over a two-year period have measured event-related potential (ERP) components among consistent binge drinking and non-binge drinking youth during inhibitory and complex attention tasks (85–87). In separate studies of 38 to 57 participants, consistent binge drinkers exhibited increased P3 amplitude (related to working memory and inhibitory control) in the central, parietal and frontal regions, as well as increased activation in the prefrontal cortex and insula during inhibitory responses, compared to non- or low-drinkers (86, 87). Consistent binge drinkers also reported increased P3b amplitude in the central and parietal regions during an attentional control task compared to controls, with more pronounced differences observed after two years of consistent binge drinking (85).
Taken together these studies suggest that neural differences are observable as a consequence of alcohol use, mirroring the behavioral findings from neuropsychological and neurostructural studies. Functional changes were not examined in relation to neuropsychological deficits; thus, it is not possible to infer whether changes in neural response were related to poorer cognitive outcomes. Sex differences in neural activation following the uptake of alcohol use in adolescence remains unknown. Of note, these functional findings come from small samples (< 30 drinkers in each study) and include mostly Caucasian participants from high socioeconomic status groups. More longitudinal fMRI and ERP studies in larger, more diverse samples are needed to better understand the specific effect of alcohol on neural functioning in adolescence.
Neurobiological consequences: Integrating findings from human studies
Determining how adolescent alcohol use may lead to overt cognitive and behavioral deficits is critical, and early structural and functional brain changes may help us understand this relationship. Following adolescent alcohol initiation, structural brain changes appear to occur. Studies have consistently reported accelerated decreases in gray matter volume and attenuated white matter growth of the frontal and temporal lobes, with poorer white matter integrity throughout related networks (65–73). The frontal lobe is thought to be critical for higher-order cognitive control, and the temporal lobe plays an important role in learning and memory (88, 89). Damage to these regions may result in overt cognitive impairments. Likewise, neuropsychological studies demonstrate a possible dose-dependent response of alcohol use on executive functioning ability (53, 55) and learning and memory (54, 60, 61). Preliminary functional neuroimaging and neurophysiological research complements findings from neuropsychological and structural neuroimaging studies; transitions into heavy alcohol use and binge drinking result in increased neural activation in fronto-parietal regions during executive functioning and attentional control tasks (79, 80, 85–87). This suggests that heavy alcohol use initiation and continuation may have a cumulative effect on brain activity, and anomalous activity may reflect degradation of underlying attentional and executive functioninging mechanisms. Heavy drinkers may therefore require more executive cognitive control to perform at the same level as non-users. Overall, integration of human neuroimaging, neuropsychological, and neurophysiological studies suggest that moderate to heavy alcohol use may initially result in structural brain changes, and with heavier binge doses, the resulting neural impairments may lead to more overt functional consequences (i.e., cognitive functioning deficits).
It is important to note that previous reviews illustrate that pre-morbid cognitive and neural vulnerabilities predispose some adolescents to initiate, and misuse, alcohol (4, 5). Presently, it is not clear whether neurobiological deficits are the direct results of adolescent alcohol use, irrespective of predispositions, or whether those youth exhibiting vulnerability markers prior to alcohol initiation then experience worse neurobiological outcomes following uptake. Larger prospective longitudinal studies that are currently underway will help disentangle these complex relationships (48, 78).
5. Cognitive and neural functioning following alcohol remittance
Studies have examined the effects of alcohol remittance (i.e., discontinuation of alcohol use) in adolescence on cognitive and neural functioning. A 10-year study found remitted youth, who had previously met criteria for an alcohol use disorder, performed similarly to youth with persistent disorders on tasks measuring visuospatial functioning and language abilities (58). The majority of youth in this study also met criteria for at least one other substance use disorder. Similarly, no improvements were reported for immediate or delayed recall in a sample of 20 young people who had stopped binge drinking for two years (63). However, another two-year study which included 16 ex-binge drinkers found some improvement in delayed recall which reflected an intermediate position between binge and non-drinkers at age 21 (53). Longer-term abandonment of binge drinking (two to four years) in healthy older adolescents who occasionally report cannabis and/or tobacco use, was associated with improvements in immediate recall which matched non-drinking control performance (63), and improvements in long-term memory (63) and working memory (90) which again reflected an intermediate position between binge and non-drinkers.
One functional neuroimaging study reported that, after one month of abstinence, adolescents who previously drank heavily no longer exhibited alterations in reward activation to alcohol cues, highlighting the potential for adolescents to benefit from early intervention and recover from the short-term effects of alcohol (91). Overall, these results provide mixed evidence as to whether cognitive functioning in adolescents who drink heavily can be modified or improved after abstinence, reductions in drinking, or treatment. While there is preliminary support that abstinence may be related to recovery in brain functioning, more evidence is required. Future research is needed to clarify when cognitive and neural recovery is most likely, and if certain cognitive and neural domains are more malleable than others following changes in substance use. This knowledge will benefit practitioners working with adolescents and can ultimately inform alcohol use treatment practices.
6. Adolescent alcohol effects in animals
Human research is limited to natural observational studies which have typically assessed youth into early adulthood at the latest. Conversely, researchers have much higher levels of control over experimental conditions in animal studies, including frequency, amount and duration of alcohol exposure, and have often assessed rodents or non-human primates into late adulthood after the termination of alcohol use. Therefore, animal studies can provide helpful insight into knowledge gaps from human literature on consequences of adolescent alcohol use. Notably, much of the work using rodent models has been conducted only in males; where possible, rodent research testing both sexes is reported.
Comparable cross-species findings
Animal studies can never completely reproduce all human features, and there have been notable differences in analyses used to examine the consequences of alcohol use on the adolescent human (e.g., cognitive, neuroimaging) and rodent brain (e.g., molecular, cellular). However, rodent studies have started to use measures that are similar to those used in human studies, and have provided evidence for cross-species similarities in findings. Partly consistent with human research, cognitive studies in male rodents have shown that adolescent alcohol use predicts poorer executive functioning in adulthood, including cognitive flexibility (92, 93), set shifting (94), and extinction of responses following termination of reinforcer cues (94–96). Adolescent alcohol use in male rodents has also been associated with poorer inhibition, reflecting heightened impulsivity and risk taking in adulthood (94, 95, 97–100). Similar to human studies, moderate alcohol use and binge drinking in male and female rodents predicts alterations in learning and memory during adolescence (101–103), however this may have minimal effects on later learning and memory in adulthood (104, 105). In terms of neural consequences of adolescent alcohol use, adult male and female rodents show attenuated neurodevelopment, including reduced volume in the corpus callosum (106), attenuated thickness in frontal regions (107), decreases in connectivity between frontal regions, the nucleus accumbens and dorsal striatum (108), poorer white matter integrity (106, 109–112) and impaired synaptic plasticity (101), similar to human adolescent studies. Interestingly, greater volume reductions were predictive of later relapse drinking in adult rats (106). Experimental rodent studies also support cross-sectional findings in human studies (113, 114) that females may be more vulnerable than males to the neurotoxic effects of alcohol (106).
Non-human primate findings parallel rodent and human findings. In a recent study, rhesus macaques were imaged before and after one year of alcohol exposure. Findings showed that brain volume increased in controls throughout adolescence into early adulthood; however, heavy drinking macaques showed reduced rates of brain growth over the follow-up period, particularly in white matter regions and the thalamus, in a dose-dependent fashion (115). These structural changes may be associated with cognitive aberrations continuing into adulthood.
Adolescent versus adult alcohol use in rodents
Studies that have compared equivalent exposures to alcohol in adolescent and adult animals have found that the effects of alcohol exposure during adulthood are generally less pronounced than after comparable alcohol exposure in adolescence (116). Adolescents are less sensitive than adults to many of the intoxicating alcohol effects that serve as cues to stop drinking, such as alcohol’s motor-impairing, sedative, social-inhibiting, and hangover-inducing effects (117). Comparatively, adolescents are more sensitive than adults to desirable consequences of low levels of alcohol use, including social facilitation and rewarding effects (117). Rodent studies show that as adults, former adolescent alcohol-exposed animals still exhibit ‘adolescent-like’ insensitivities to alcohol’s motor-impairing, sedative, and taste aversive effects (118–120), while retaining adolescent-typical increased sensitivities to alcohol’s rewarding effects (119, 121). This may contribute to consistent drinking patterns from adolescence into adulthood.
Novel rodent findings
Rodent studies provide novel insight into areas which have not yet been studied in great detail in humans, such as effects of adolescent alcohol use on neurotransmitters, neurogenesis, and neuroinflammation. There are marked developments that occur in the dopamine neurotransmitter system during adolescence, important for reward-motivated behavior. Limited human research shows dopamine system development is disrupted following alcohol use, although most studies have focused on older, alcohol-dependent adults (122). Findings from rodent studies suggest the dopamine system is particularly sensitive to the effects of alcohol use during adolescence (for review, see 123). Following alcohol use, adolescent male rodents show increased GABA inhibitory tone on the dopamine system neurons in the nucleus accumbens (124). This decreases tonic dopamine tone and increases phasic dopamine responses to rewarding and risky activities, and in turn, appears to increase risky decision-making following alcohol use. Preliminary evidence also suggests these dopamine system changes enhance later reactivity to the rewarding, but not harmful, effects of alcohol (123), although this requires further investigation in both animal and human studies.
Adolescent alcohol use also appears to disrupt other neurotransmitter systems, including the cholinergic system of the basal forebrain (116). These neurons play critical roles in cognitive functions, including learning and memory. Multiple studies show that repeated alcohol use during adolescence reduces the number of neurons showing immunoreactivity to choline O-acetyltransferase (ChAT) in the basal forebrain (92, 99, 107, 125–127). This decline in ChAT immunoreactivity is associated with greater disinhibitory behavior (99), increased risky behavior (125), and decreased performance on set-shifting tasks (127) in adulthood following alcohol remittance. This suggests adolescent alcohol use leads to loss of cholinergic tone which has lasting functional consequences.
Neurogenesis involves formation of new neurons and integration into functional neural networks, which is a critical component of nervous system development (128). Rates of neurogenesis are influenced by environmental factors. Repeated alcohol use in adolescence, but not adulthood, decreases neurogenesis (129), and such changes may be evident long after alcohol use has stopped (129–131). The mechanisms underlying neurogenesis disruptions following adolescent alcohol use remains unclear. One suggestion is the suppression of neurotrophins, such as brain-derived neurotrophic factor (BDNF), which is a regulator of the survival and differentiation of newly generated neurons. Adolescent alcohol use appears to decrease BDNF expression in the hippocampus and interrupts neurogenesis (132–135). Further evidence of the role of BDNF in neurogenesis disruption comes from a study where a BDNF agonist was administered to male rodents previously exposed to alcohol (133). Administration resulted in neurogenesis, and reversed depression-like symptoms observed during alcohol withdrawal and abstinence following repeated alcohol use in adolescence.
Repeated exposure to alcohol during adolescence also induces long-lasting neural and behavioural changes via the induction of neuroinflammation. Alcohol stimulates the release of innate pro-inflammatory cytokines that can disrupt synaptic plasticity and lead to neuropathology and cell death (136, 137). Studies including male and female mice demonstrate that females are more vulnerable than males to the neuroinflammatory effects of alcohol (138). Rodent studies have examined ways to reduce neuroinflammation caused from adolescent alcohol use. For instance, administration of a neuroimmune drug, ibudilast, reduced alcohol drinking in dependent male rodents by 50% (139), and administration of an anti-inflammatory drug, indomethacin, prevented cell death, and reduced cognitive and motor deficits that were evident after adolescent alcohol exposure (140). Furthermore, female rodents with altered gene expression of TLR4, which reduced inflammatory activation following alcohol use, did not show behaviors consistent with adolescent alcohol use, such as anxiety and heightened reward sensitivity to alcohol (141). Overall, animal studies provide evidence of lasting impacts of adolescent alcohol use into adulthood, with growing evidence of retention of adolescent-like phenotypes.
7. Future directions and conclusions
Recent prospective, longitudinal designs have greatly increased our knowledge of the complex relationship between adolescent brain development and alcohol use by parsing out the pre-existing vulnerabilities from the consequential effects of use (142). However, with high heterogeneity in patterns of alcohol and other substance use during this critical neurodevelopmental period, more research is needed to determine what developmental processes and cognitive domains may be most responsive to prevention and treatment initiatives. The larger multi-site studies currently underway (e.g., ABCD, NCANDA) will hopefully help disentangle the complicated picture of substance co-use, the interactive effects of adolescent substance use and psychopathology, sex and other demographic factors, health habits, and genetic vulnerabilities, among other important factors related to substance use. It is necessary to understand substance-specific effects, especially given growing US legalization and rise in rates of cannabis use, the dramatic rise in adolescent e-cigarette use, and global concerns regarding opioid dependency and associated deaths. These larger studies are positioned to differentiate the specific neural developmental effects of alcohol as well as cannabis, tobacco, e-cigarettes, opioids, cocaine, hallucinogens, and amphetamines. Future studies also need to make concerted efforts to enroll more adolescents with diverse backgrounds, as substance use effects may not generalize across ethnicities and cultures (most research to date has been in Caucasian youth from upper middle class families), various family structures, or psychopathology profiles. This knowledge will benefit practitioners working with adolescents, and hopefully, inform future substance use prevention and intervention initiatives
Better understanding the dose-dependent effects of substances will enable improved public health information to inform policies regarding limiting amounts of adolescent use and controlling potency of substance-containing products. Specifically, it will be useful to know how adolescent binge drinking compared to lower levels of drinking differentially affects cognition and behavior. Additionally, a greater understanding of short compared to longer-term neural and cognitive effects of alcohol use and remittance in adolescence through to adulthood is needed to better inform treatment. Researchers are starting to track these changes in short and longer-term effects using neural markers of substance use to better understand how an individual is responding to treatment (143). Targeting cognitive makers of substance use through cognitive retraining treatment strategies has demonstrated some success in reducing alcohol use (144), as well as in a range of clinical populations including various substance use disorders (145). Researchers are also beginning to investigate the effectiveness of cognitive training as a prevention initiative for adolescent substance use (146–148), although early findings suggest this method may need to be supplemented with a substance use prevention program (149).
Of note, all of the human longitudinal studies in this review relied on youth self-report of substance use. Some of the existing studies also used ranges for self-report questionnaires, which weakens the ability to understand dose-dependent relationships. Substance use researchers are beginning to incorporate real time measures via smart phone technology, more sophisticated biological markers (i.e., blood, urine, saliva, and hair samples), as well as daily reporting or real-time tracking of drug use through youths’ smart phones and wearable devices (150). These nuanced tools will help improve the accuracy and reliability of reports to better quantify the frequency and amount of alcohol consumed. Better neuroimaging standards, such as scanning under neutral conditions to control for factors like time since last alcohol use, and more consistency in measures used to assess cognitive functioning are also suggested as an area of future research.
Cross-species findings show comparability in effects of alcohol use on the adolescent brain and behavior, and novel experimental rodent studies on the consequences of alcohol use can guide future work in human adolescents. For instance, researchers are now focused on quantification of various neurochemicals and transmitters in the brain measured through Magnetic Resonance Spectroscopy (MRS; (151). Understanding such neurochemical changes could help us better understand the neurobiological effects of substance use, the mechanisms of change, and alterations incurred through psychotherapy or pharmacological treatment.
Overall, it is clear that adolescent alcohol use is associated with neural and cognitive consequences (see Table 1 for summary). Drawing on the most recent longitudinal studies, this review has integrated findings from human neuropsychological and neuroimaging studies, and the animal literature. Neurobiological research suggests a dose-dependent relationship may occur between alcohol use with brain differences and cognitive deficits. Structural and functional brain changes may initially occur following moderate to heavy alcohol doses, while more overt cognitive deficits may be the result of neural insults from heavy and binge doses. Future longitudinal studies should examine the mediating role of brain structure and function on associations between adolescent alcohol use and cognitive and behavioral consequences. Emerging work has begun to characterize the time-limited and potentially recoverable, versus persisting neural and cognitive effects of alcohol use. Current findings and future research has the potential to significantly improve global health by informing the development of prevention and intervention strategies to address alcohol mechanisms associated with neural and cognitive consequences in adolescence.
Highlights.
Adolescence is a critical neurodevelopmental period marked by rising alcohol use.
This review summarizes the neural and cognitive effects of alcohol use.
Adolescent alcohol use is related to changes in brain structure and function.
Heavy alcohol use is associated with poorer cognitive functioning.
Funding:
This work was supported by the National Health and Medical Research Council (GNT1169377; Lees), the National Institute on Alcohol and Alcoholism (K23 AA025399; Squeglia, U01 DA041093; Squeglia), and the National Institute on Drug Abuse (5T32DA024635; Meredith).
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. [DOI] [PubMed] [Google Scholar]
- 2.Romer D, Reyna VF, Satterthwaite TD. Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev Cogn Neurosci. 2017;27:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spear LP. Alcohol consumption in adolescence: a translational perspective. Curr Addict Rep. 2016;3:50–61. [Google Scholar]
- 4.Lees B, Mewton L, Stapinski LA, Squeglia LM, Rae CD, Teesson M. Neurobiological and cognitive profile of young binge drinkers: A systematic review and meta-analysis. Neuropsychology Review. 2019;29(3):357–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squeglia LM, Cservenka A. Adolescence and Drug Use Vulnerability: Findings from Neuroimaging. Current opinion in behavioral sciences. 2017;13:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbia C, López-Caneda E, Corral M, Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neuroscience & Biobehavioral Reviews. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Ewing SW, Sakhardande A, Blakemore S-J. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage: Clinical. 2014;5:420–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol. 2013;9:703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermens DF, Lagopoulos J, Tobias-Webb J, De Regt T, Dore G, Juckes L, et al. Pathways to alcohol-induced brain impairment in young people: A review. Cortex. 2013;49(1):3–17. [DOI] [PubMed] [Google Scholar]
- 10.Giedd JN. The teen brain: insights from neuroimaging. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2008;42(4):335–43. [DOI] [PubMed] [Google Scholar]
- 11.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paus T Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9(2):60–8. [DOI] [PubMed] [Google Scholar]
- 13.Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature neuroscience. 1999;2(10):859–61. [DOI] [PubMed] [Google Scholar]
- 16.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. [DOI] [PubMed] [Google Scholar]
- 18.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(30):10937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–52. [DOI] [PubMed] [Google Scholar]
- 20.Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm (Vienna). 2013;120(9):1369–95. [DOI] [PubMed] [Google Scholar]
- 21.Baker STE, Lubman DI, Yücel M, Allen NB, Whittle S, Fulcher BD, et al. Developmental Changes in Brain Network Hub Connectivity in Late Adolescence. The Journal of Neuroscience. 2015;35(24):9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 2009;5(5):e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, et al. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013;64:671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(14):3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst M The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoogendam JM, Kahn RS, Hillegers MH, van Buuren M, Vink M. Different developmental trajectories for anticipation and receipt of reward during adolescence. Dev Cogn Neurosci. 2013;6:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon NW, Moghaddam B. Neural processing of reward in adolescent rodents. Dev Cogn Neurosci. 2015;11:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doremus-Fitzwater TL, Spear LP. Reward-centricity and attenuated aversions: an adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev. 2016;70:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Global status report on alcohol and health 2018. Geneva; 2018. [Google Scholar]
- 34.Aiken A, Clare PJ, Wadolowski M, Hutchinson D, Najman JM, Slade T, et al. Age of Alcohol Initiation and Progression to Binge Drinking in Adolescence: A Prospective Cohort Study. Alcoholism, clinical and experimental research. 2018;42(1):100–10. [DOI] [PubMed] [Google Scholar]
- 35.Richmond-Rakerd LS, Slutske WS, Wood PK. Age of initiation and substance use progression: A multivariate latent growth analysis. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2017;31(6):664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Adolescent alcohol-related behaviours: trends and inequalities in the WHO European Region, 2002–2014. 2018. [Google Scholar]
- 37.Inchley J, Currie D, Vieno A, Torsheim T, Ferreira-Borges C, Weber MM, et al. Adolescent alcohol-related behaviours: Trends and inequalities in the WHO European Region, 2002–2014. Copenhagen, Denmark: WHO Regional Office for Eurpose; 2018. 94 p. [Google Scholar]
- 38.Morioka H, Itani O, Kaneita Y, Ikeda M, Kondo S, Yamamoto R, et al. Associations between sleep disturbance and alcohol drinking: A large-scale epidemiological study of adolescents in Japan. Alcohol. 2013;47(8):619–28. [DOI] [PubMed] [Google Scholar]
- 39.National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. 2018.
- 40.Substance Abuse and Mental Health Services Administration. Binge Drinking: Terminology and Patterns of Use 2016. [Available from: https://www.samhsa.gov/capt/tools-learning-resources/binge-drinking-terminology-patterns.
- 41.Kraus L, Guttormsson U, Leifman H, Shsaron Arpa, Sabrina Molinaro, Karin Monshouwer, et al. ESPAD Report 2015: Results from the European school survey project on alcohol and other drugs. Luxembourg: European Monitroing Centre for Drugs and Drug Addiction; 2016. [Google Scholar]
- 42.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2018: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2019. [Google Scholar]
- 43.White V, Williams T. Australian secondary school students’ use of tobacco, alcohol, and over-the-counter and illicit substances in 2014. Cancer Council Victoria; 2016. [Google Scholar]
- 44.Looze M, Raaijmakers Q, Bogt TT, Bendtsen P, Farhat T, Ferreira M, et al. Decreases in adolescent weekly alcohol use in Europe and North America: evidence from 28 countries from 2002 to 2010. Eur J Public Health. 2015;25 Suppl 2:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inchley JCD, Young T, Samdal O, Torsheim T, Augustson L et al. , editors. Growing up unequal: gender and socioeconomic differences in young people’s health and well-being. Health Behaviour in School-aged Children (HBSC) study: international report from the 2013/2014 survey. Copenhagen: WHO Regional Office for Europe; 2016. [Google Scholar]
- 46.Pennay A, Holmes J, Torronen J, Livingston M, Kraus L, Room R. Researching the decline in adolescent drinking: The need for a global and generational approach. Drug and alcohol review. 2018;37 Suppl 1:S115–s9. [DOI] [PubMed] [Google Scholar]
- 47.Pape H, Rossow I, Brunborg GS. Adolescents drink less: How, who and why? A review of the recent research literature. Drug and alcohol review. 2018;37 Suppl 1:S98–s114. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Buchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Molecular psychiatry. 2010;15(12):1128–39. [DOI] [PubMed] [Google Scholar]
- 50.Brown S, Brumback T, Tomlinson K, Cummins K, Thompson W, Nagel B, et al. The national consortium on alcohol and NeuroDevelopment in adolescence (NCANDA): A multisite study of adolescent development and substance use. J Stud Alcohol Drugs. 2015;76(6):895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latvala A, Rose RJ, Pulkkinen L, Dick DM, Korhonen T, Kaprio J. Drinking, smoking, and educational achievement: cross-lagged associations from adolescence to adulthood. Drug Alcohol Depend. 2014;137:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahedy L, Field M, Gage S, Hammerton G, Heron J, Hickman M, et al. Alcohol Use in Adolescence and Later Working Memory: Findings From a Large Population-Based Birth Cohort. Alcohol Alcohol. 2018;53(3):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mota N, Parada M, Crego A, Doallo S, Caamaño-Isorna F, Holguin SR, et al. Binge drinking trajectory and neuropsychological functioning among university students: A longitudinal study. Drug Alcohol Depend. 2013;133:108–14. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen-Louie TT, Castro N, Matt GE, Squeglia LM, Brumback T, Tapert SF. Effects of Emerging Alcohol and Marijuana Use Behaviors on Adolescents’ Neuropsychological Functioning Over Four Years. J Stud Alcohol Drugs. 2015;76(5):738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boelema SR, Harakeh Z, van Zandvoort MJE, Reijneveld SA, Verhulst FC, Ormel J, et al. Adolescent Heavy Drinking Does Not Affect Maturation of Basic Executive Functioning: Longitudinal Findings from the TRAILS Study. PloS one. 2015;10(10):e0139186–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jurk S, Mennigen E, Goschke T, Smolka MN. Low-level alcohol consumption during adolescence and its impact on cognitive control development. Addiction biology. 2018;23(1):313–26. [DOI] [PubMed] [Google Scholar]
- 57.Hanson KL, Cummins K, Tapert SF, Brown SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2011;25(1):127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse. 2011;20(2):135–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones SA, Steele JS, Nagel BJ. Binge drinking and family history of alcoholism are associated with an altered developmental trajectory of impulsive choice across adolescence. Addiction. 2017;112:1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc 2014;20(8):784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen-Louie TT. Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol Clin Exp Res. 2016;40:1895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psycholog Addictive Behav. 2009;23:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carbia C, Cadaveira F, Caamano-Isorna F, Rodriguez-Holguin S, Corral M. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PloS one. 2017;12(2):e0171393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carbia C, Cadaveira F, Caamaño-Isorna F, Rodríguez Holguín S, Corral M. Binge Drinking Trajectory and Decision-Making during Late Adolescence: Gender and Developmental Differences. Frontiers in psychology. 2017;8:783-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, et al. Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. The American journal of psychiatry. 2018;175(4):370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sullivan EV, Brumback T, Tapert SF, Brown SA, Baker FC, Colrain IM, et al. Disturbed Cerebellar Growth Trajectories in Adolescents Who Initiate Alcohol Drinking. Biological psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. Brain development in heavy-drinking adolescents. American Journal of Psychiatry. 2015;172(6):531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, et al. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39(6):345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones SA, Nagel BJ. Altered frontostriatal white matter microstructure is associated with familial alcoholism and future binge drinking in adolescence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2019;44(6):1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism, clinical and experimental research. 2013;37 Suppl 1:E181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry research. 2013;214(3):374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013;3(1):396–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, clinical and experimental research. 1992;16(6):1078–89. [DOI] [PubMed] [Google Scholar]
- 75.Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage. 2013;65:176–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–87. [DOI] [PubMed] [Google Scholar]
- 77.Guggenmos M, Schmack K, Sekutowicz M, Garbusow M, Sebold M, Sommer C, et al. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry. 2017;7(12):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, et al. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience. 2018;32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs. 2012;73(5):749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 2015;16:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones SA, Cservenka A, Nagel BJ. Binge drinking impacts dorsal striatal response during decision making in adolescents. Neuroimage. 2016;129:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balleine BW, Delgado MR, Hikosaka O. The Role of the Dorsal Striatum in Reward and Decision-Making. The Journal of Neuroscience. 2007;27(31):8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez-Caneda E, Cadaveira F, Crego A, Doallo S, Corral M, Gomez-Suarez A, et al. Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol and alcoholism (Oxford, Oxfordshire). 2013;48(4):464–71. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Caneda E, Cadaveira F, Crego A, Gomez-Suarez A, Corral M, Parada M, et al. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction (Abingdon, England). 2012;107(10):1796–808. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Caneda E, Holguin SR, Corral M, Doallo S, Cadaveira F. Evolution of the binge drinking pattern in college students: Neurophysiological correlates. Alcohol. 2014;48(5):407–18. [DOI] [PubMed] [Google Scholar]
- 88.Otero TM, Barker LA. The Frontal Lobes and Executive Functioning In: Goldstein S, Naglieri JA, editors. Handbook of Executive Functioning. New York, NY: Springer New York; 2014. p. 29–44. [Google Scholar]
- 89.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–6. [DOI] [PubMed] [Google Scholar]
- 90.Carbia C, Cadaveira F, Lopez-Caneda E, Caamano-Isorna F, Rodriguez Holguin S, Corral M. Working memory over a six-year period in young binge drinkers. Alcohol. 2017;61:17–23. [DOI] [PubMed] [Google Scholar]
- 91.Brumback T, Squeglia LM, Jacobus J, Pulido C, Tapert SF, Brown SA. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addictive behaviors. 2015;46:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman LG Jr., He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, clinical and experimental research. 2011;35(4):671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2014;116:142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, et al. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(11):2570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Risher ML, Fleming RL, Boutros N, Semenova S, Wilson WA, Levin ED, et al. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PloS one. 2013;8(5):e62940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller KM, Risher ML, Acheson SK, Darlow M, Sexton HG, Schramm-Sapyta N, et al. Behavioral Inefficiency on a Risky Decision-Making Task in Adulthood after Adolescent Intermittent Ethanol Exposure in Rats. Sci Rep. 2017;7(1):4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Torcaso A, Asimes A, Meagher M, Pak TR. Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology. 2017;76:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desikan A, Wills DN, Ehlers CL. Ontogeny and adolescent alcohol exposure in Wistar rats: open field conflict, light/dark box and forced swim test. Pharmacol Biochem Behav. 2014;122:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience. 2011;199:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tapia-Rojas C, Carvajal FJ, Mira RG, Arce C, Lerma-Cabrera JM, Orellana JA, et al. Adolescent Binge Alcohol Exposure Affects the Brain Function Through Mitochondrial Impairment. Mol Neurobiol. 2018;55(5):4473–91. [DOI] [PubMed] [Google Scholar]
- 102.Marco EM, Peñasco S, Hernández M-D, Gil A, Borcel E, Moya M, et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Frontiers in Behavioral Neuroscience. 2017;11(233). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montesinos J TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun. 2015;45:233–44. [DOI] [PubMed] [Google Scholar]
- 104.Acheson SK, Bearison C, Risher ML, Abdelwahab SH, Wilson WA, Swartzwelder HS. Effects of acute or chronic ethanol exposure during adolescence on behavioral inhibition and efficiency in a modified water maze task. PloS one. 2013;8(10):e77768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Attention Semenova S., impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl). 2012;219(2):433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pascual M, Pla A, Minarro J, Guerri C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol Alcohol. 2014;49(2):187–92. [DOI] [PubMed] [Google Scholar]
- 107.Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PloS one. 2014;9(11):e113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, et al. Adolescent alcohol exposure decreases frontostriatal resting- state functional connectivity in adulthood. Addiction biology. 2018;23(2):810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vetreno RP, Yaxley R, Paniagua B, Crews FT. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addiction biology. 2016;21(4):939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vargas WM, Bengston L, Gilpin NW, Whitcomb BW, Richardson HN. Alcohol binge drinking during adolescence or dependence during adulthood reduces prefrontal myelin in male rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(44):14777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J, et al. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun. 2015;45:233–44. [DOI] [PubMed] [Google Scholar]
- 112.Wolstenholme JT, Mahmood T, Harris GM, Abbas S, Miles MF. Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC. Front Mol Neurosci. 2017;10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Medina KL. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism, clinical and experimental research. 2005;29(9):1590–600. [DOI] [PubMed] [Google Scholar]
- 115.Shnitko TA, Liu Z, Wang X, Grant KA, Kroenke CD. Chronic Alcohol Drinking Slows Brain Development in Adolescent and Young Adult Nonhuman Primates. eneuro. 2019;6(2):ENEURO.0044–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev. 2016;68(4):1074–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spear LP. Adolescent neurodevelopment. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;52(2 Suppl 2):S7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcoholism, clinical and experimental research. 2002;26(7):960–8. [DOI] [PubMed] [Google Scholar]
- 119.Toalston JE, Deehan GA, Hauser SR, Engleman EA, Bell RL, Murphy JM, et al. Reinforcing Properties and Neurochemical Response of Ethanol within the Posterior Ventral Tegmental Area Are Enhanced in Adulthood by Periadolescent Ethanol Consumption. Journal of Pharmacology and Experimental Therapeutics. 2014;351(2):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci. 2015;16:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quoilin C, Didone V, Tirelli E, Quertemont E. Chronic ethanol exposure during adolescence alters the behavioral responsiveness to ethanol in adult mice. Behav Brain Res. 2012;229(1):1–9. [DOI] [PubMed] [Google Scholar]
- 122.Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr Top Behav Neurosci. 2013;13:511–40. [DOI] [PubMed] [Google Scholar]
- 123.Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience. 2018. [DOI] [PubMed] [Google Scholar]
- 124.Schindler AG, Soden ME, Zweifel LS, Clark JJ. Reversal of Alcohol-Induced Dysregulation in Dopamine Network Dynamics May Rescue Maladaptive Decision-making. The Journal of Neuroscience. 2016;36(13):3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boutros N, Semenova S, Liu W, Crews FT, Markou A. Adolescent intermittent ethanol exposure is associated with increased risky choice & decrease of dopaminergic & cholinergic neuron markers in adult rats. Int J Neuropharmacol. 2015;18:pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swartzwelder HS. Adolescent intermittent alcohol exposure: deficits in object recognition memory and forebrain cholinergic markers. P Lo S One. 2015;10:e0140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernandez GM, Savage LM. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience. 2017;361:129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pino A, Fumagalli G, Bifari R, Decimo I. New neurons in adult brain: distribution, molecular mechanisms and therapies. Biochem Pharmacol. 2017;141:4–22. [DOI] [PubMed] [Google Scholar]
- 129.Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu W, Crews FT. Persistent decreases in adult subventricular and hippocampal neurogenesis following adolescent intermittent ethanol exposure. Front Behav Neurosci. 2017;11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sakharkar AJ. A role for histone acetylation mechanisms in adolescent exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 2016;221:4691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scheidt L Ethanol during adolescence decreased the BDNF levels in the hippocampus in adult male Wistar rats, but did not alter aggressive and anxiety-like behaviors. Trends Psychiatry Psychother. 2015;37:143–51. [DOI] [PubMed] [Google Scholar]
- 135.Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: neurotrophin and behavioral adaptation alter long-term, continuous ethanol exposure. P Lo S One. 2016;11:e0149987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crews FT, Vetreno RP. Addiction, adolescence, and innate immune gene induction. Front Psychiatry. 2011;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crews FT. Toll-like receptor signaling and stages of addiction. Psychopharmacology. 2017;234:1483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311(1):27–34. [DOI] [PubMed] [Google Scholar]
- 139.Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Franklin KM, et al. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addiction biology. 2015;20(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pascual M, Blanco M, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioral alterations in adolescent rats. Eur J Neurosci. 2007;25:541–50. [DOI] [PubMed] [Google Scholar]
- 141.Montesinos J, Pascual M, Rodriguez-Arias M, Minarro J, Guerri C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav Immun. 2016;53:159–71. [DOI] [PubMed] [Google Scholar]
- 142.Squeglia LM, Gray KM. Alcohol and Drug Use and the Developing Brain. Current psychiatry reports. 2016;18(5):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cservenka A, Nagel BJ. Neuroscience of alcohol for addiction medicine: Neurobiological targets for prevention and intervention in adolescents. Progress in brain research. 2016;223:215–35. [DOI] [PubMed] [Google Scholar]
- 144.Bowley C, Faricy C, Hegarty B, J Johnstone S, L Smith J, J Kelly P, et al. The effects of inhibitory control training on alcohol consumption, implicit alcohol-related cognitions and brain electrical activity. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;89(3):342–8. [DOI] [PubMed] [Google Scholar]
- 145.Aguinaldo LD, Squeglia LM, Gray KM, Coronado C, Lees B, Tomko RL, et al. Behavioral treatments for adolescent cannabis use disorder: A rationale for cognitive retraining. Curr Addict Rep 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bourque J, Baker TE, Dagher A, Evans AC, Garavan H, Leyton M, et al. Effects of delaying binge drinking on adolescent brain development: A longitudinal neuroimaging study. BMC Psychiatry. 2016;16(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mewton L, Hodge A, Gates N, Visontay R, Teesson M. The Brain Games study: Protocol for a randomised controlled trial of computerised cognitive training for preventing mental illness in adolescents with high-risk personality styles. BMJ Open. 2017;7(9):e017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Leary-Barrett M, Masse B, Pihl RO, Stewart SH, Se’guin JR, Conrod PJ. A cluster-randomised controlled trial evaluating the effects of delaying onset of adolescent substance abuse on cognitive development and addiction following a selective, personality-targeted intervention programme: the Co-Venture trial. Addiction. 2017;112(10):1871–81. [DOI] [PubMed] [Google Scholar]
- 149.Mewton L, Hodge A, Gates N, Visontay R, Lees B, Teesson M. A randomised double-blind trial of cognitive training for the prevention of psychopathology in at-risk youth. Behav Research & Therapy. Under rev [DOI] [PubMed] [Google Scholar]
- 150.Tomko RL, Gray KM, Oppenheimer SR, Wahlquist AE, McClure EA. Using REDCap for ambulatory assessment: Implementation in a clinical trial for smoking cessation to augment in-person data collection. The American Journal of Drug and Alcohol Abuse. 2019;45(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cohen-Gilbert JE, Jensen JE, Silveri MM. Contributions of magnetic resonance spectroscopy to understanding development: potential applications in the study of adolescent alcohol use and abuse. Development and psychopathology. 2014;26(2):405–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


