Abstract
Aims:
This study was designed to compare the risk of long-term health outcomes, including microvascular, macrovascular complications and mortality, across 4 cohorts: triple-goal, dual-goal, single-goal, and no-goal achievers.
Methods:
A retrospective cohort of 53,120 patients with T2DM were identified (97.51% male, 61.49% whites) from the Veterans Affairs (VA) electronic medical records VISN 16 data warehouse (2004–2010). Propensity score weight (PSW) was used to balance demographic characteristics and complication history at baseline. The PSW adjusted hazard ratios (aHR) from Cox proportional hazard models were used to compare complications and all-cause mortality over an average of 4 years of follow-up.
Results:
At baseline, 25.43% (13,507) patients achieved triple-goal, while 41.36% (21,972) and 26.37% (14,010) patients achieved dual-goal and single-goal, respectively. During the follow-up period, triple-goal achievement was associated with risk reductions of complications and all-cause mortality when compared to all other groups of achieving dual or single-goal. Across different combinations of dual-goal achievement, the cohort with LDL-C goal achievement had lower risk of complication events and mortality, compared to those that achieved other goals but failed to reach LDL-C goal.
Conclusions:
Achievement of triple-goal was associated with better health outcomes among veterans with T2DM compared to those that did not, while LDL-C has more weight of influence. Multi-faceted treatment strategies targeting hypertension, hyperglycemia and hyperlipidemia may improve health outcome in veterans with T2DM.
Keywords: Type 2 diabetes mellitus, Blood glucose, Blood pressure, Blood lipid, Diabetes related complications
1. Backround
If the current trends continue, one out of three adults in the United States is projected to have diabetes mellitus (DM) by 2050 [1]. It was estimated that diabetes had direct medical costs of US $176 billion in 2012 in the United States and average medical cost of patients with DM was 2.3 times higher than those without diabetes [2]. The economic burden associated with diabetes is largely driven by its complications for patients. At least 30% of people with type 2 diabetes mellitus (T2DM) experience macrovascular and microvascular complications [3–5]. Due to the high incidence and severity of complications, high health utilization and expenditure are associated with diabetes complications. The average inpatient care durations were 15.2, 25.5, and 21.2 days for T2DM related coronary artery disease (CAD), cardiovascular disease (CVD) and any other complication episodes, respectively [2,4].
Evidence-based treatment guidelines for management of patients with T2DM have been developed and updated frequently. However, there has been considerable confusion created by changes in guidelines based on conflicting data from clinical trials or lack of such data. OPTIMISE study has demonstrated that benchmarking with clear goals of HbA1c, LDL-C and blood pressure helps with achieving treatment goals [6]. Clarification of the association between the number of goal achievement and the benefit of long-term health outcome is significant and meaningful for diabetes management. Few studies have considered whether long-term clinical outcomes associated with triple-goal achievement in the population with type 2 diabetes. One study found BP and LDL-C control both related to lower risk of CVD hospitalization, however, no such risk reduction was found by well HbA1c control [7]. Among Chinese population with T2DM, achieving two or more treatment targets out of triple targets was associated with lowering risk of CHD incidence [8].
In addition, a report from the Center for Disease Control and Prevention (CDC) suggests that only a small proportion of people with diabetes are meeting all 3 goals [9]. Meeting dual-goal has been demonstrated to be associated with better clinical outcomes compared to single- or no-goal achievement among patients with T2DM [10]. Only a single-center clinical trial in T2DM (Steno) has attempted to control all the risk factors for diabetes-related complications, yet with a small sample size. This trial has demonstrated significant reductions in long-term cardiovascular events, microvascular complications and mortality [11,12]. However, due to the nature of the trial and the population studied, its applicability to the U.S. population is very limited. Therefore, this study aimed to examine the impacts of achieving three treatment goals which are commonly defined (i.e., glycated hemoglobin (HbA1c) (<7%) and low-density lipoprotein cholesterol (LDL-C) (<100 mg/dl) and blood pressure (BP) <140/90 mm Hg) on health outcomes among veterans in the United States.
2. Research design and methods
2.1. Study design
A retrospective cohort study was conducted to evaluate the patients’ characteristics and compare the risk of long-term complications/mortality among four groups of patients: achieve three goals, achieve two goals, achieve at least one goal or achieve no goals.
2.2. Data source
Our study utilized Veteran Affairs electronic medical records (VA EMRs) data warehouse from the Veterans Integrated Services Network 16 (VISN 16). This database covers more than 445,000 veterans from VISN 16’s 10 medical centers and 40 community based outpatient clinics, which represents about 7.8% of U.S. veterans.
The VISN 16 data warehouse contains demographic, medical records (inpatient and outpatient), lab (HbA1c test and lipid profile included LDL-C) and vital data (height, body weight, and blood pressure) for veterans served in the network of Arkansas, Louisiana, Mississippi, Oklahoma, and parts of Alabama, Florida, Missouri, and Texas. The EMRs are updated monthly and maintained by the VISN 16 Information Technology Development Group. The records for veterans between January 2004, and June 2010 were used for our analyses.
2.3. Sample selection
Adult patients (aged ≥18 years old) had at least two T2DM records (ICD-9-CM: 250.x0 and 250.x2) and no more than one type 1 diabetes mellitus diagnosis (ICD-9-CM: 250.x1 or 250.x3) between January 1, 2004 and June 30, 2010 were identified from dataset. Eligible patients had at least one measurement after 6 months of the first T2DM diagnosis for BP, HbA1c and LDL such that the gap between the three measurements was less than 30 days apart. The earliest date for BP or HbA1c or LDL tests was considered as the index date. Patients who had at least one laboratory measurement for HbA1c and LDL within 1-year after the index date and were enrolled in the VA for at least 12 months following the index date were selected into our final sample.
2.4. Data preparation
Our longitudinal data was prepared with each cycle length of 6 months starting from the index date. Six months before index date was defined as baseline period. Complication events and BP, HbA1c and LDL-C levels were specified for each cycle. From the longitudinal data, we have repeated laboratory measurements over time and treated the key influential factor, goal-achievement, as time-varying variable. The average of BP, HbA1c and LDL-C estimates for each cycle were estimated using the area under the curve (AUC) method [13]: for each patient, any two adjacent BP or HbA1c or LDL-C readings were connected by straight lines over time, irrespective of whether they were in the same cycle or different cycles; then trapezoidal areas under each curve were determined, added together, and divided by the time of cycle.
After calculating the BP, HbA1c and LDL-C levels for each cycle, we determined the goal achievement status based on the standard of HbA1c < 7.0%, LDL-C < 100 mg/dl and BP < 14 0/90 mmHg. Patients with T2DM who reached one and only one standard of the above three were all recorded as single-goal achievers. Similarly, reaching any two of the three goals were defined as dual-goal achievers, all three goals were identified as triple-goal achievers, and no-goal achievers were those who did not meet any goal. We further specified the detailed goal-achievement combination as major covariate with 8 categories (triple-goal, HbA1c + BP, HbA1c + LDL-C, BP + LDL-C, HbA1c only, BP only, LDL-C only, and none).
2.5. Baseline patient characteristics
Patient characteristics in the baseline period were exhibited in Table 1. The baseline information included age, gender, race, body mass index (BMI), residential region, the number of follow-up cycles; and the presence of microvascular, including retinopathy (ICD-9: 249.6, 250.6, 353.5, 356.9, 536.3, 713.5, 337.1, 357.2, 354, and 355), nephropathy (ICD-9: 249.4, 250.4, and 791.0) and neuropathy (ICD-9: 249.5, 250.5, 362.0, 362.1, and 379.23); and macrovascular complications, including atherosclerosis (ICD-9: 440), aneurysm (ICD-9: 441 and 442), embolism (ICD-9: 444 and 445), peripheral vascular disease (PVD) (ICD-9: 249.7, 250.7, 443, 447, and 785.4), cerebrovascular disease (ICD-9: 430–438), and coronary artery disease (CAD) (ICD-9: 410–414) at baseline period.
Table 1 –
Patient characteristics adjusted by propensity score weighting at baseline period.
| Characteristics | All patients | Triple-goal achievers | Dual-goal achievers | Single-goal achievers | No-goal achievers | p-values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 52209 | 13279 | 25.43% | 21594 | 41.36% | 13763 | 26.36% | 3573 | 6.84% | <.0001 | |
| Demographics | |||||||||||
| Age (years; mean ± SD) | 64.99 | ±10.44 | 65.01 | ±10.25 | 64.97 | ±10.49 | 64.99 | ±10.58 | 65.01 | ±10.22 | 0.9792 |
| Male, N (%) | 50883 | 97.46% | 12940 | 97.44% | 21051 | 97.48% | 13413 | 97.45% | 3480 | 97.41% | 0.9911 |
| Race, N (%) | |||||||||||
| White | 32164 | 61.61% | 8160 | 61.45% | 13304 | 61.61% | 8471 | 61.55% | 2229 | 62.39% | 0.9294 |
| Black | 9382 | 17.97% | 2412 | 18.16% | 3859 | 17.87% | 2470 | 17.95% | 640 | 17.92% | |
| Others | 10663 | 20.42% | 2707 | 20.39% | 4431 | 20.52% | 2821 | 20.50% | 704 | 19.69% | |
| BMI (mean ± SD) | 31.76 | ±6.21 | 31.42 | ±6.03 | 31.74 | ±6.20 | 31.99 | ±6.30 | 32.22 | ±6.57 | <.0001 |
| State, N (%) | |||||||||||
| Arkansas | 10805 | 20.70% | 2738 | 20.62% | 4471 | 20.70% | 2843 | 20.65% | 754 | 21.10% | 1.0000 |
| Louisiana | 11373 | 21.78% | 2908 | 21.90% | 4690 | 21.72% | 3004 | 21.83% | 770 | 21.57% | |
| Mississippi | 10605 | 20.31% | 2693 | 20.28% | 4397 | 20.36% | 2804 | 20.38% | 711 | 19.91% | |
| Oklahoma | 10211 | 19.56% | 2602 | 19.60% | 4224 | 19.56% | 2688 | 19.53% | 696 | 19.47% | |
| Texas | 9216 | 17.65% | 2338 | 17.61% | 3812 | 17.65% | 2424 | 17.61% | 641 | 17.95% | |
| Diabetes-related complications (N, %) | |||||||||||
| Microvascular complications | |||||||||||
| Retinopathy | 3013 | 5.77% | 769 | 5.79% | 1246 | 5.77% | 794 | 5.77% | 204 | 5.72% | 0.9990 |
| Nephropathy | 1594 | 3.05% | 398 | 3.00% | 667 | 3.09% | 421 | 3.06% | 108 | 3.04% | 0.9697 |
| Neuropathy | 6274 | 12.02% | 1590 | 11.98% | 2602 | 12.05% | 1654 | 12.02% | 427 | 11.95% | 0.9961 |
| Macrovascular complications | |||||||||||
| Atherosclerosis, aneurysm, or embolism | 892 | 1.71% | 226 | 1.70% | 371 | 1.72% | 234 | 1.70% | 61 | 1.71% | 0.9996 |
| Peripheral vascular disease | 2567 | 4.92% | 652 | 4.91% | 1064 | 4.93% | 676 | 4.91% | 175 | 4.89% | 0.9996 |
| Cerebrovascular disease | 3076 | 5.89% | 788 | 5.94% | 1261 | 5.84% | 809 | 5.87% | 218 | 6.11% | 0.9227 |
| Coronary artery disease | 12436 | 23.82% | 3146 | 23.69% | 5171 | 23.95% | 3276 | 23.80% | 843 | 23.59% | 0.9355 |
A descriptive summary of baseline characteristics was presented for all patients. Comparisons were made among goal-achievement cohorts using chi-square tests for categorical variables and ANOVA tests for continuous variables.
Propensity score weighting (PSW), the inverse probability weighting estimator, was used for improving the comparability across goal achievement cohorts. Propensity score (PS) was estimated by multinomial logistic regression while adjusting for age, race, residential state, tobacco, and history of microvascular/macrovascular complications at baseline. The inversed and normalized PS for each group was considered as PSW, which was used to adjust in the analysis for long-term clinical outcome [14]. By the PSW, the characteristics and history of complication have been adjusted by the goal-achievement status at baseline.
2.6. Long-term clinical outcome
In our study, longitudinal data were used to estimate the risk of complication events and mortality. Long-term clinical outcomes included microvascular events, macrovascular events and all-cause death. The composite outcome of microvascular complication was defined by any events of neuropathy, nephropathy, or retinopathy. The composite outcome of macrovascular complication was defined by any records of atherosclerosis, aneurysm, embolism, peripheral vascular diseases (PVD), cerebrovascular disease, or coronary artery disease (CAD). Additionally, myocardial infarction (MI) (ICD-9: 410), stroke, acute coronary syndromes (ACS) (ICD-9: 410 and 411.1) and congestive heart failure (CHF) (ICD-9: 398.91 and 428) were used as independent clinical outcomes. Specifically, the time to the first observation of each clinical outcome event from the index date was used as dependent variable for the Cox proportional hazard regression models. The primary explanatory variable was goal-achievement status as the time-varying variable for the PSW weighted multivariate-adjusted Cox model. Other potentially confounding variables were controlled for in the analysis including age, gender, race, residential state, presence of the microvascular/macrovascular complications at baseline, and pre-exiting comorbidity (hypoglycemia (ICD-9: 249.8, 250.8, 250.80, 250.82, 251.0, 251.1, 251.2), hypertension (ICD-9: 401–405), hyperlipidemia (ICD-9: 272.0–272.4), renal disease (ICD-9: 250.4, 590, 593, 791.0)) at baseline, tobacco use, Charlson Comorbidity Index (CCI) at baseline [15]; and time varying body mass index (BMI). The triple-goal achievement served as the reference category in the models. The risk of complication in relation to triple-goal status was quantified in terms of adjusted hazard ratios (aHRs). Data analyses were performed using SAS 9.4 (Cary, NC).
3. Results
Our study identified 149,613 Patients with at least two T2DM diagnoses (ICD-9-CM code: 250.x0 or 250.x2) records between January 1, 2004 and June 30, 2010. Among them, 138,709 patients with T2DM were allowed to have at most one type 1 diabetes mellitus (T1DM): 131,474 patients without T1DM; 8409 patients with only one recorded T1DM diagnosis, that was likely to be advertently coded. Further, out of 64,608 patients with at least one BP, HbA1c and LDL test during 6 months after their first T2DM diagnosis, 56,917 patients had at least one more BP, HbA1c and LDL test within 1 year after the first recorded test (index date). The final analytic sample included 53,120 adult patients who met all the selection criteria and had at least 12 months follow up after the index date (Appendix: Fig. A1). On average, selected patients were followed 7.98 (±2.44) cycles (6 months per cycle), which was about 4 years follow-up.
There were 25.43% (13,507) of triple-goal achievers, while 41.36% (21,972), 26.37% (14,010) and 6.84% (3631) patients achieved dual-goal, single-goal, and none of the goals at baseline, respectively. Among the whole selected population, there were 25.43% (13,507) of triple-goal achievers and 6.84% (3631) patients without any goal achievement at baseline. Among 26.37% (14,010) single-goal achievers, 13.10% (6958), 6.47% (3438) and 6.80% (3614) patients only achieved the BP, LDL-C and HbA1c goal, respectively. Among 41.36% (21,972) patients with T2DM who achieved the dual-goal achievement at baseline, 17.34% (9213) patients achieved the dual-goal of BP and HbA1c; 15.90% (8444) patients achieved the dual-goal of BP and LDL-C; and 8.12% (4315) patients achieved the dual-goal of LDL-C and HbA1c (Appendix: Fig. A1).
PSW was used to balance the differences between comparison groups at baseline and the Table 1 showed the PS weighted results of the demographic characteristics. All characteristics, except BMI (P < .0001), were comparable across the four achievement groups after PSW weighting (P > .05). At baseline, the average age at baseline was about 65 years old. Most of patients were male (97%); 62% patients were white and the average BMI was 31.76 kg/m2. For the diabetes-related complications at baseline, neuropathy was the most prevalent (12.02%) microvascular complications followed by 5.77% patients had retinopathy and 3.05% patients had nephropathy at baseline. Among the macrovascular complications, CAD had the highest prevalence (23.82%), while 1.71% had diagnosis of atherosclerosis, aneurysm, or embolism, 4.89% had PVD, and 5.80% had cerebrovascular disease.
Table 2 presents the multivariate regression analysis results for the long-term clinical outcomes. All clinical outcomes, including vascular events outcomes, specific complications, and all-cause death, had decreased risk among patients with more goal-achievement. Compared to the dual-goal achievement, the triple-goal group had significantly lower risks of the composite macrovascular complications (aHR 0.989, 95% CI [0.981, 0.996]), MI (aHR 0.991, 95% CI [0.984, 0.998]), cerebrovascular disease (aHR 0.990, 95% CI [0.983, 0.998]), acute coronary syndromes (ACS) (aHR 0.990, 95% CI [0.983, 0.997]), congestive heart failure (CHF) (aHR 0.991, 95% CI [0.984, 0.998]) and all-cause death (aHR 0.989, 95% CI [0.982, 0.996]) after controlling for the covariates and PSW. However, no significant differences were found for the composite microvascular complication (aHR 0.993, 95% CI [0.986, 1.000]).
Table 2 –
Multivariate Analysis with Propensity Score Weighting of Long-Term Clinical Outcomes by Goal Achievement Status.
| Complications/death | Triple-goal vs. No-goal | Triple-goal vs. Single-goal | Triple-goal vs. Dual-goal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aHR* | 95% CI | aHR* | 95% Cl | aHR* | 95% CI | ||||
| Microvascular complications | 0.977 | 0.963 | 0.990 | 0.987 | 0.978 | 0.995 | 0.993 | 0.986 | 1.000 |
| Macrovascular complications | 0.965 | 0.952 | 0.979 | 0.977 | 0.969 | 0.985 | 0.989 | 0.981 | 0.996 |
| Myocardial infarction | 0.967 | 0.954 | 0.980 | 0.979 | 0.972 | 0.987 | 0.991 | 0.984 | 0.998 |
| Cerebrovascular disease | 0.972 | 0.958 | 0.985 | 0.980 | 0.972 | 0.988 | 0.990 | 0.983 | 0.998 |
| Acute coronary syndromes | 0.967 | 0.954 | 0.980 | 0.978 | 0.971 | 0.987 | 0.990 | 0.983 | 0.997 |
| Congestive heart failure | 0.966 | 0.953 | 0.979 | 0.978 | 0.970 | 0.986 | 0.991 | 0.984 | 0.998 |
| All-cause death | 0.964 | 0.951 | 0.978 | 0.977 | 0.969 | 0.984 | 0.989 | 0.982 | 0.996 |
aHR: Adjusted Hazard Ratio; compared with Triple-goal achievers to other goal achievement which were associated with higher risk for complications/all-cause mortality.
Compared to the single-goal achievers, the triple-goal achievers had significantly lower risks of microvascular complications (aHR 0.987, 95% CI [0.978, 0.995]), macrovascular complications (aHR 0.977, 95% CI [0.969, 0.985]), MI (aHR 0.979, 95% CI [0.972, 0.987]), cerebrovascular disease (aHR 0.980, 95% CI [0.972, 0.988]), ACS (aHR 0.978, 95% CI [0.971, 0.987]), CHF (aHR 0.978, 95% CI [0.970, 0.986]) and all-cause death (aHR 0.977, 95% CI [0.969, 0.984]). Furthermore, the magnitude of relative risks further reduced when patients with triple-goal achievement compared to the none-goal achievers.
For further ascertaining the influence of specific goal, additional multivariate analysis of triple-goal compared to detailed combinations of dual-goal achievement were shown in Table 3. Compared with the dual-goal of HbA1c and BP achievement, the triple-goal achievers (i.e., achieving additional LDL-C goal) had significantly lower risk of composite microvascular complications (aHR 0.978, 95% CI [0.967, 0.988]), composite macrovascular complications (aHR 0.979, 95% CI [0.969, 0.990]), MI (aHR 0.978, 95% CI [0.968, 0.988]), cerebrovascular disease (aHR 0.978, 95% CI [0.968, 0.988]), ACS (aHR 0.978, 95% CI [0.967, 0.987]), CHF (aHR 0.977, 95% CI [0.966, 0.986]) and all-cause death (aHR 0.976, 95% CI [0.966, 0.986]). However, compared with the dual-goal combinations of HbA1c+LDL-C and BP+LDL-C, the triple-goal achievement did not show significant effect on the risk of complications or all-cause death.
Table 3 –
Propensity score weighted multivariate analysis of long-term clinical outcomes compared with triple-goal and dual-goal achievers.
| Complications/death | Triple-goal vs. A1c+BP | Triple-goal vs. A1c+LDL-C | Triple-goal vs. LDL-C+BP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aHR* | 95% CI | aHR* | 95% Cl | aHR* | 95% CI | ||||
| Microvascular complications | 0.978 | 0.967 | 0.988 | 0.996 | 0.984 | 1.008 | 1.001 | 0.992 | 1.009 |
| Macrovascular complications | 0.979 | 0.969 | 0.990 | 1.002 | 0.990 | 1.014 | 0.992 | 0.983 | 1.000 |
| Myocardial infarction | 0.978 | 0.968 | 0.988 | 0.994 | 0.983 | 1.006 | 0.997 | 0.989 | 1.005 |
| Cerebrovascular disease | 0.978 | 0.968 | 0.988 | 0.999 | 0.988 | 1.011 | 0.995 | 0.987 | 1.004 |
| Acute coronary syndromes | 0.978 | 0.967 | 0.987 | 0.994 | 0.983 | 1.006 | 0.997 | 0.989 | 1.005 |
| Congestive heart failure | 0.977 | 0.966 | 0.986 | 0.995 | 0.983 | 1.007 | 0.998 | 0.990 | 1.007 |
| All-cause death | 0.976 | 0.966 | 0.986 | 0.993 | 0.981 | 1.005 | 0.995 | 0.987 | 1.004 |
aHR: Adjusted Hazard Ratio; compared with Triple-goal achievers to other goal achievement which were associated with higher risk for complications/all-cause mortality.
Detailed combinations of the dual-goal achievement were further compared with each specific single-goal achievement in PSW adjusted multivariate Cox regression model (Table 4). Among the six comparison groups of dual-goal, achieving the LDL-C goal in addition to achieving the HbA1c goal only or the BP goal only showed significant benefits on lowering the risk of complications and all-cause of death. Furthermore, achieving the HbA1c goal in addition to the BP goal compared to the BP-goal achievement only was associated with lower risk of the composite macrovascular complication.
Table 4 –
Propensity score weighted multivariate analysis of long-term clinical outcomes compared with dual-goal and single-goal achievers.
| Complications/death | Ale + BP vs A1e | Ale + BP vs BP | A1e + LDL-C vs A1e | A1e + LDL-C vs LDL-C | LDL-C + BP vs LDL-C | LDL-C + BP vs BP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aHR* | 95% CI | aHR* | 95% CI | aHR* | 95% CI | aHR* | 95% CI | aHR* | 95% CI | aHR* | 95% CI | |
| Microvascular complications | 1.005 | (0.988, 1.021) | 0.996 | (0.983, 1.009) | 0.986 | (0.969, 1.003) | 1.008 | (0.993, 1.023) | 1.003 | (0.991, 1.016) | 0.973 | (0.962, 0.984) |
| Macrovascular complications | 0.996 | (0.980, 1.013) | 0.982 | (0.969, 0.995) | 0.974 | (0.958, 0.992) | 0.990 | (0.975, 1.005) | 1.000 | (0.988, 1.012) | 0.970 | (0.959, 0.981) |
| Myocardial infarction | 0.995 | (0.979, 1.011) | 0.988 | (0.976, 1.001) | 0.978 | (0.962, 0.995) | 1.000 | (0.985, 1.014) | 0.997 | (0.985, 1.009) | 0.969 | (0.959, 0.980) |
| Cerebrovascular disease | 0.999 | (0.983, 1.016) | 0.987 | (0.975, 1.000) | 0.978 | (0.961, 0.995) | 0.997 | (0.983, 1.011) | 1.001 | (0.989, 1.013) | 0.970 | (0.959, 0.981) |
| Acute coronary syndromes | 0.995 | (0.979, 1.011) | 0.989 | (0.977, 1.001) | 0.978 | (0.961, 0.994) | 0.999 | (0.985, 1.014) | 0.997 | (0.985, 1.008) | 0.970 | (0.959, 0.980) |
| Congestive heart failure | 0.994 | (0.978, 1.010) | 0.989 | (0.976, 1.001) | 0.975 | (0.959, 0.992) | 1.001 | (0.986, 1.015) | 0.998 | (0.986, 1.010) | 0.967 | (0.956, 0.978) |
| All-cause death | 0.996 | (0.980, 1.012) | 0.988 | (0.976, 1.001) | 0.978 | (0.962, 0.995) | 0.998 | (0.983, 1.012) | 0.995 | (0.984, 1.007) | 0.969 | (0.958, 0.980) |
aHR: Adjusted Hazard Ratio; compared with Triple-goal achievers to other goal achievement which were associated with higher risk for complications/all-cause mortality.
4. Discussion
To our knowledge, this is the first large scale retrospective study that was designed to compare the differences in long-term health outcomes associated with triple-goal achievement in patients with T2DM. Our study with the positive association between higher level of goal-achievement and better long-term clinical outcomes suggested that better management of HbA1c, BP and LDL-C has a significant effect in lowering the risks of all-cause death and composite micro-or macrovascular complications.
Achieving the triple-goal, including BP, HbA1c and LDL-C goal in our study, was associated with additional benefits on reducing death and diabetes related complications than dual-goal achievers. The risks of all other studied complications and all-cause death were found lower in the triple-goal achievement group than the group achieved any dual-goal, except the microvascular complication. One more goal achieved is associated with about 1% reduction every 6 months in risk of complications and mortality. The results of comparison of dual-goal and single-goal achievers in our study were consistent with the dual-goal achievement study in 2013 (10). And compared to triple-goal achievement group, the effect increased along with number of goals reached. The risk reduction was around 2% compared triple-goal and single-goal achievers, while it became about 3% when triple-goal achievers compared to no-goal achievers. Notably, goal-achievement status was measured by a cycle of 6 months, which means the comparative risk reduction was associated with the goal-achievement status difference (e.g., triple-goal compared to dual-goal achievers) over a course of 6 months. Longer time of maintaining diabetes treatment goals over 4 years on average may carry much greater reduction in the risks of complications or mortality. Furthermore, LDL-C was identified as the most influential contributor from the results of analyses comparing specific goal-achievement combinations in this study. This result confirmed the finding in UKPDS study which showed lowering LDL-C had largest effect size in reducing risk of CAD, compared to controlling HbA1c, SBP [16].
Our results of low risk of various complications associated with patients who meet more treatment goals were consistent with the Steno-2 study, which has been the only long-term randomized clinical trial (RCT) of managing blood glucose, BP, lipid by intensive therapy among patients with diabetes. However, the Steno study limited to a small number of patients at a single site in Denmark. Our study replicated the findings of diabetes management aimed at achieving triple-goal is beneficial in the US population. Furthermore, intensive treatment aimed at achieving specific targets of blood glucose, BP, blood lipid in a clinical trial is different from achieving those goals in community settings. In the Steno-2 study, only DBP and LDL-C level reached the recommended targets among the patients that received intensive therapy. Systolic BP (SBP) was dropped to around 130 mmHg at the end of intervention but raised to 140 mm Hg at the end of follow-up, which was close to the goal we used in this study. The Steno-2 failed to reach the HbA1c goal of 7% at the end of intervention (mean = 7.9%) and at end of follow-up (mean = 7.7%) [11,12]. Even though the goals were not fully reached in Steno-2 study, the intensive treatment targeted at hyperglycemia, hypertension and dyslipidemia was effective in reducing mortality and the risk of cardiovascular complications. Therefore, our study has confirmed the findings from Steno-2 study, and demonstrated that, beyond clinical trials, multi-faceted diabetes management in community practice may lead to lower risk of complications and mortality.
It is interesting to note that LDL-C has been highlighted in current study and our previous dual-goal achievement study. It was demonstrated that LDL-C is the only goal which has consistently demonstrated benefits on risk reduction, regardless of combinations treatment goals. In the 2015 published guideline, the goal of LDL-C was removed from lipid control [17]. And statin was widely recommended for patients with T2DM to routinely control blood lipid. Combination therapy with statin in ACCORD study did not reduce the LDL-C significantly [18]. The effect of statin and the newly recommended lipid control therapy is still controversial. A recent study demonstrated that further LDL-C lowering with ezetimibe led to improved outcomes [19] and clinical trials are ongoing with newer and more powerful therapies that could lead to much lower levels of LDL-C [20]. Our findings may fill the gap of understanding the role of controlling LDL-C and may contribute to clinical practice when physicians feel confused about the controversy and the changes in guidelines.
The BP goal our study chose was consistent with the current American Diabetes Association (ADA) [21] and 8th Joint National Committee (JNC 8) recommendations for patients with T2DM [22]. The guidelines tend to be conservative on BP treatment goal (≤140/90 mmHg) and suggest the stringent goal (≤130/80 mmHg) is more appropriate for younger patients with T2DM. The mean age of the patients selected in our study was about 65 years old (SD = 10.44), so the stringent BP goal may unsuitable. From the ACCORD results, the group with the treatment targeting SBP < 120 mm Hg had no significantly lower risk of death or primary composite outcome (i.e., nonfatal MI, nonfatal stroke and cardiovascular-related death) compared to the group with targeting SBP < 1 40 mm Hg [23]. It established the stringent SBP standard had no additional benefits than the target of <140 mmHg [24,25].
In our study, we also estimated the incremental benefit of achieving HbA1c goal for those patients who accomplished BP goal, LDL-C goal or both already. And we found that, after achieving the LDL-C goal or dual-goal of LDL-C and BP, additional achieving HbA1c did not show any further benefits on lowering complication or death risk. But compared to the patients achieved BP goal only, adding one more HbA1c achievement can reduce the risk of macrovascular complications in the long-term. Isolated treatment goal of HbA1c (<7%) has drawn a lot of attention and has been well investigated. However, with setting different goal of HbA1c in clinical trials, the benefits were not confirmed. In the ADVANCE study, the intensive glucose control group with HbA1c level < 6.5%, which achieved the goal of HbA1c (<7%), had a significant effect on reducing the risk of major macrovascular and microvascular events compared to the group with standard control (mean HbA1c at 7.3%) [26], but the risk reduction on death was not found at the end of the post-trial follow-up [25]. However, the intensive glucose lowering therapy in ACCORD study showed significant increase of mortality from reducing the HbA1c to 6.4% compared to the standard therapy group (mean HbA1c at 7.5%) [27]. A single goal of HbA1c may be not appropriate for all patients with T2DM in different health condition and demographics. The flexibility to health professionals and patients need to be considered while addressing the risk of inappropriate management (e.g., under- or over-treatment). To this end, an optimal individualized HbA1c goal should be studied in the future research.
5. Limitations and strengths
This is a retrospective observational study and causality cannot be assured. Based on the nature of design, the estimation may still be affected by unobserved confounders. A cross-sectional study found that triple goal achievers, compared to less or none of goal achievers, had shorter diabetes duration, lower waist fat, better b-cell function [28]. Those VA EMR unavailable factors, including the diabetes severity and duration, may lead to bias in the analysis. Further, patients with at least two lab measurement results were selected for comparing the patients with different goal achievement status. Unfortunately, patients with T2DM who had no test results in record cannot be reached in our study. And categories of anti-diabetic medication, blood pressure and lipid lowering medications were measured by receiving it or not. Dosage and medication adherence were not considered in this study. Secondly, data on the health care services provided by the providers outside of the VA health system cannot be identified in the database, even though the patients enrolled in VA rarely used the service out of the system. Finally, most of the selected participants are males in our study, which limits the capacity of generalizability of the female group. Similar studies in general population should be performed in the future.
The findings of associations between long-term outcomes and goal-achievement status are strengthened by using the PSW approach for balancing baseline characteristics and medical history. The four comparison groups based on patient’s triple-goal, dual-goal, single-goal and non-goal achievement were not well-balanced at baseline period (Appendix Table A1). To overcome the deficiency of retrospective observational study design and do not limit the generalizability of patients with T2DM at the same time, we chose to use PSW, instead of stringent selection criteria, to find comparable groups. By applying PSW, all characteristics at baseline, except BMI, were balanced across four groups, and comparability was significantly improved. Furthermore, we controlled the influential covariates in the regression model. And we identified a relatively large sample size, which is more representative of the VA population, and limiting the influence of patients with extreme observation values. Comparisons across multiple groups with multi-status may offer more meaningful and specific evidences for routine chronic care.
6. Conclusion
Achieving three goals of HbA1c, BP and LDL-C among U.S. veterans with T2DM is associated with lower risk of complications or death compared to none, any one, or two goal achievements with average 4 years follow-up. Achievement of LDL-C goal may be more beneficial for risk reduction on complications and mortality than the other two goals. To inform changes in clinical practice in the future, a pragmatic prospective randomized trial targeting all three goals is needed to confirm the finding, and may drive future guidelines in the US.
Appendix A
Fig. A1 –
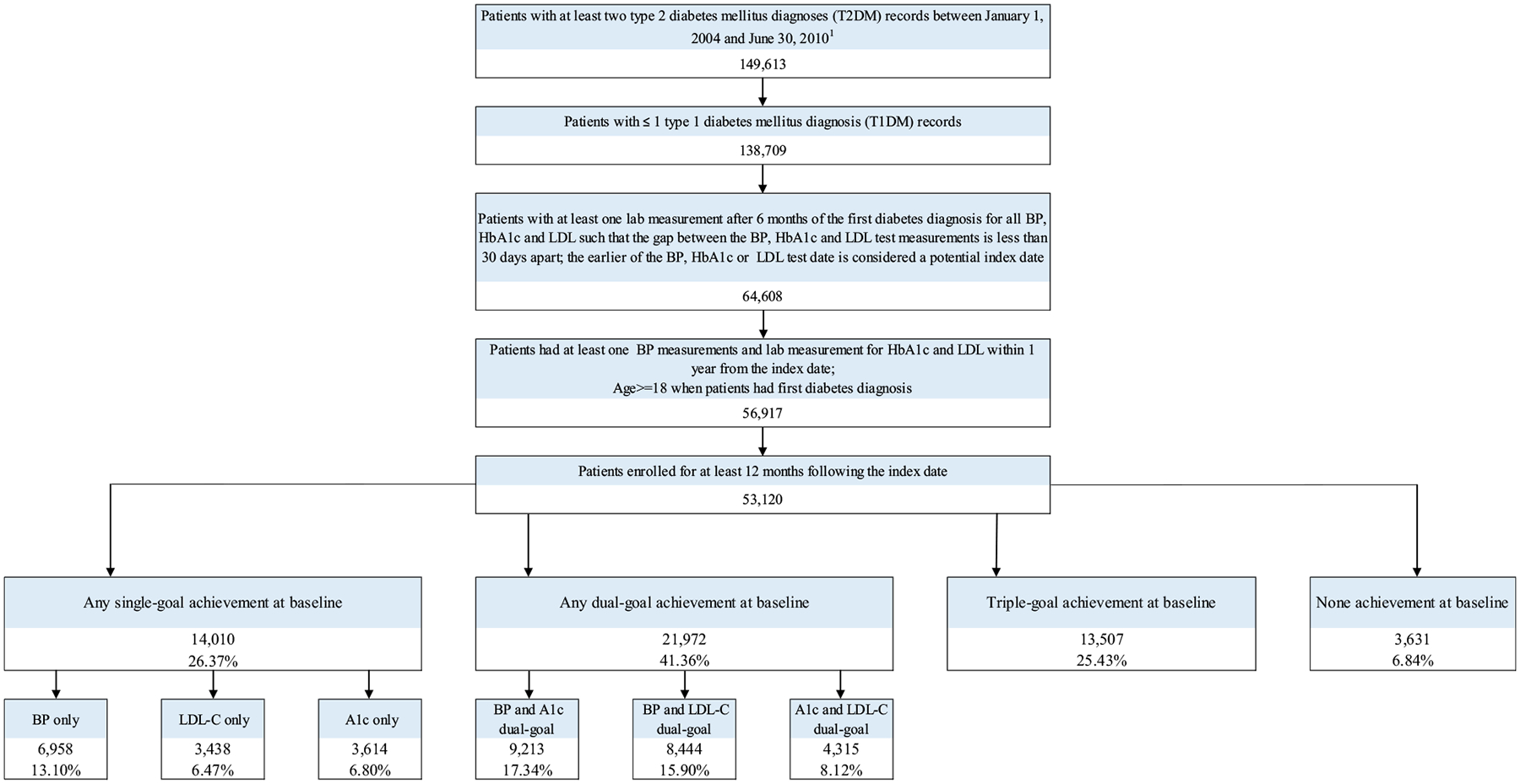
Flow chart for sample selection.
Table A1 –
Patient characteristics at baseline period.
| Characteristics | All patients | Triple-goal Achievers | Dual-Goal Achievers | Single-goal Achievers | No-goal achievers | p-values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 53120 | 13507 | 25.43% | 21972 | 41.36% | 14010 | 26.37% | 3631 | 6.84% | <.0001 | |
| Demographics | |||||||||||
| Age (years; mean ± SD) | 65.02 | ±10.43 | 67.12 | ±10.08 | 64.94 | ±10.42 | 63.65 | ±10.53 | 62.89 | ±10.03 | <.0001 |
| Male, N (%) | 51795 | 97.51% | 13246 | 98.07% | 21397 | 97.38% | 13638 | 97.34% | 3514 | 96.78% | <.0001 |
| Race, N (%) | |||||||||||
| White | 32665 | 61.49% | 8899 | 65.88% | 13685 | 62.28% | 8156 | 58.22% | 1925 | 53.02% | <.0001 |
| Black | 9411 | 17.72% | 1668 | 12.35% | 3757 | 17.10% | 3009 | 21.48% | 977 | 26.91% | |
| Others | 11044 | 20.79% | 2940 | 21.77% | 4530 | 20.62% | 2845 | 20.31% | 729 | 20.08% | |
| BMI (mean ± SD) | 31.78 | ±6.23 | 31.10 | ±5.92 | 31.76 | ±6.20 | 32.22 | ±6.38 | 32.64 | ±6.70 | <.0001 |
| State, N (%) | |||||||||||
| Arkansas | 11121 | 20.94% | 3019 | 22.35% | 4698 | 21.38% | 2795 | 19.95% | 609 | 16.77% | <.0001 |
| Louisiana | 11520 | 21.69% | 2760 | 20.43% | 4687 | 21.33% | 3169 | 22.62% | 904 | 24.90% | |
| Mississippi | 10643 | 20.04% | 2678 | 19.83% | 4471 | 20.35% | 2827 | 20.18% | 667 | 18.37% | |
| Oklahoma | 10309 | 19.41% | 2780 | 20.58% | 4206 | 19.14% | 2631 | 18.78% | 692 | 19.06% | |
| Texas | 9527 | 17.93% | 2270 | 16.81% | 3910 | 17.80% | 2588 | 18.47% | 759 | 20.90% | |
| Number of cycles in study (mean ± SD) | 7.98 | ±2.44 | 7.73 | ±2.51 | 7.98 | ±2.44 | 8.14 | ±2.39 | 8.21 | ±2.33 | <.0001 |
| Diabetes-related complications (N, %) | |||||||||||
| Microvascular complications | |||||||||||
| Retinopathy | 3051 | 5.74% | 465 | 3.44% | 1203 | 5.48% | 1043 | 7.44% | 340 | 9.36% | <.0001 |
| Nephropathy | 1635 | 3.08% | 351 | 2.60% | 645 | 2.94% | 487 | 3.48% | 152 | 4.19% | <.0001 |
| Neuropathy | 6360 | 11.97% | 1464 | 10.84% | 2638 | 12.01% | 1817 | 12.97% | 441 | 12.15% | <.0001 |
| Macrovascular complications | |||||||||||
| Atherosclerosis, aneurysm, or embolism | 910 | 1.71% | 252 | 1.87% | 383 | 1.74% | 218 | 1.50% | 57 | 1.57% | 0.2140 |
| Peripheral vascular disease | 2597 | 4.89% | 684 | 5.06% | 1019 | 4.64% | 702 | 5.01% | 192 | 5.29% | 0.1350 |
| Cerebrovascular disease | 3083 | 5.80% | 880 | 6.52% | 1245 | 5.67% | 771 | 5.50% | 187 | 5.15% | 0.0004 |
| Coronary artery disease | 12708 | 23.92% | 3821 | 28.29% | 5283 | 24.04% | 2947 | 21.03% | 657 | 18.09% | <.0001 |
Footnotes
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- [1].Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Diabetes A. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36(4):1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27). J Diabetes Complicat 2012;26(2):123–8. [DOI] [PubMed] [Google Scholar]
- [4].Candrilli SD, Meyers JL, Boye K, Bae JP. Health care resource utilization and costs during episodes of care for type 2 diabetes mellitus-related comorbidities. J Diabetes Complicat 2014. [DOI] [PubMed] [Google Scholar]
- [5].Harzallah F, Ncibi N, Alberti H, Ben Brahim A, Smadhi H, Kanoun F, et al. Clinical and metabolic characteristics of newly diagnosed diabetes patients: experience of a university hospital in Tunis. Diabetes Metab 2006;32(6):632–5. [DOI] [PubMed] [Google Scholar]
- [6].Hermans MP, Elisaf M, Michel G, Muls E, Nobels F, Vandenberghe H, et al. Benchmarking is associated with improved quality of care in type 2 diabetes. Diabetes Care 2013;36(11):3388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med 2013;28(5):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kong AP, Yang X, Ko GT, So W-Y, Chan W-B, Ma RC, et al. Effects of treatment targets on subsequent cardiovascular events in Chinese patients with type 2 diabetes. Diabetes Care 2007;30(4):953–9. [DOI] [PubMed] [Google Scholar]
- [9].Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31(1):81–6. [DOI] [PubMed] [Google Scholar]
- [10].Shi L, Ye X, Lu M, Wu EQ, Sharma H, Thomason D, et al. Clinical and economic benefits associated with the achievement of both HbA1c and LDL cholesterol goals in veterans with type 2 diabetes. Diabetes Care 2013;36(10):3297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348(5):383–93. [DOI] [PubMed] [Google Scholar]
- [12].Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358(6):580–91. [DOI] [PubMed] [Google Scholar]
- [13].Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25(2):275–8. [DOI] [PubMed] [Google Scholar]
- [14].McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013;32(19):3388–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- [16].Turner R, Millns H, Neil H, Stratton I, Manley S, Matthews D, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998;316(7134):823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Association AD. Standards of medical care in diabetes–2015: summary of revisions. Diabetes Care 2015;38(Suppl. 1):S4. [DOI] [PubMed] [Google Scholar]
- [18].Group AS, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362(17):1563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372(25):2387–97. [DOI] [PubMed] [Google Scholar]
- [20].Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372(16):1500–9. [DOI] [PubMed] [Google Scholar]
- [21].American Diabetes A Standards of medical care in diabetes–2014. Diabetes Care 2014;37(Suppl. 1). S14–80. [DOI] [PubMed] [Google Scholar]
- [22].James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507–20. [DOI] [PubMed] [Google Scholar]
- [23].Group AS, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362(17):1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patel A, Group AC, MacMahon S, Chalmers J, Neal B, Woodward M, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370(9590):829–40. [DOI] [PubMed] [Google Scholar]
- [25].Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371(15):1392–406. [DOI] [PubMed] [Google Scholar]
- [26].Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358(24):2560–72. [DOI] [PubMed] [Google Scholar]
- [27].Action to Control Cardiovascular Risk in Diabetes Study G, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358(24):2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Camara S, Bouenizabila E, Hermans MP, Ahn SA, Rousseau MF. Novel determinants preventing achievement of major cardiovascular targets in type 2 diabetes. Diabetes Metab Synd: Clin Res Rev 2014;8(3):145–51. [DOI] [PubMed] [Google Scholar]


