Abstract
Objective:
Subjective excessive daytime sleepiness, commonly measured with the Epworth Sleepiness Scale (ESS), is associated with cognitive impairment in Parkinson disease (PD). Significant correlation between subject and informant responses has been reported in neurologically healthy individuals. We sought to assess this correlation in patients with PD.
Patients and Methods:
854 individuals in the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) had subject as well as informant-completed ESS completed within one year of a movement disorder exam and cognitive assessment. Correlations were evaluated using Spearman’s rank correlation coefficients.
Results:
Overall, 397/854(46.5%) were female with mean age of 77.5 (SD 8.3). 572 (67%) were cognitively normal (CogNL), 135 (15.8%) had mild cognitive impairment (MCI) and 147 (17.2%) dementia. Spearman R correlations (all with p ≤ 0.001) between subject and informant ESS responses were 0.73 overall, 0.67 for the CogNL group, 0.79 for the MCI group, 0.79 for those with dementia. Of 175 with clinically probable PD, 115 (65.7%) were CogNL, 38 had MCI, and 22 (12.6%) dementia. For subjects with PD correlations (all with p<0.001) were 0.65 for PD-CogNL, 0.83 for PD-MCI, and 0.70 for those with PD-dementia.
Conclusion:
These significant correlations between subject and informant-completed ESS can be useful in guiding clinical trials designed to assess efficacy of potential treatments for excessive daytime sleepiness for the general population and for patients with PD, even those having cognitive impairment.
Keywords: Parkinson disease, cognitive impairment, mild cognitive impairment, dementia, excessive daytime sleepiness, Epworth Sleepiness Scale
1. Introduction
Subjective excessive daytime sleepiness (EDS), commonly measured with the Epworth Sleepiness Scale (ESS), is more common in Parkinson disease (PD) than in healthy controls. [1] While EDS in newly diagnosed PD subjects is no more common than age-matched healthy controls, [2] it becomes increasingly common with PD progression.[3] EDS is also correlated with dementia,[4] which may eventually develop in up to 80% of PD patients.[5] Though cognitive impairment might be expected to affect insight about EDS, correlation between responses of the PD patient and a caregiver, or other close relative informant, to the ESS has, to our knowledge, never been studied.
2. Patients and Methods
2.1. Study design
Data were derived from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), a longitudinal cohort study of adults in Maricopa County, Arizona in the United States with a focus on recruitment of individuals with Parkinson disease, dementia and neurologically healthy controls.[6] The study was approved by the Western IRB and all subjects signed written informed consent. We identified 854 individuals with subject as well as informant-completed ESS and completed within one year of a movement disorder exam as well as neuropsychological assessment. Clinical diagnosis had been reviewed and assigned annually according to standard diagnostic criteria and based upon all available test results by a consensus committee consisting of expert neurologists, psychiatrists and neuropsychologists. [6]
2.2. Statistical methods
Data were analyzed using SAS 9.3 software. Paired t-test was used to compare mean differences in subject-informant responses to the ESS. Subject and informant ESS correlations were estimated using the Spearman rank correlation coefficient.
3. Results
3.1. Subject characteristics
Of the 854 subjects identified, 397(46.5%) were female with mean age of 77.5 (SD 8.3). Cognitive status was 572 (67%) cognitively normal (CogNL), 135 (15.8%) mild cognitive impairment (MCI) and 147 (17.2%) dementia. There were 175 subjects with clinically probable PD, 115 (65.7%) were CogNL, 38 (21.7%) had MCI, and 22 (12.6%) dementia.
3.2. Subject-CR ESS Scores
Mean subject-informant scores (table 1) were significantly different for overall sample, MCI, dementia, and non-PD subjects overall (p<0.01). These differences did not meet statistical significance for any of the PD groups.
Table 1.
ESS-informant and ESS-participant comparison in the overall cohort and in sub-populations
| Population | ESS-Informant | ESS-Participant | Difference (95% CI) | P value* |
|---|---|---|---|---|
| Overall (N=854); Mean(SD) | 7.7 (5.2) | 7.1 (4.9) | 0.57 (0.34 to 0.81) | <.001 |
| CogNL (N=572); Mean(SD) | 6.6 (4.4) | 6.3 (4.2) | 0.28 (−0.00 to 0.56) | 0.052 |
| MCI (N=135); Mean(SD) | 8.5 (5.7) | 7.7 (5.5) | 0.81 (0.26 to 1.37) | 0.005 |
| DEM (N=147); Mean(SD) | 10.9 (5.9) | 9.4 (5.8) | 1.48 (0.88 to 2.08) | <.001 |
| Non PD (N=679); Mean(SD) | 7.1 (4.9) | 6.5 (4.6) | 0.66 (0.40 to 0.91) | <.001 |
| PD-CogNL (N=115); Mean(SD) | 8.2 (5.0) | 8.1 (4.7) | 0.03 (−0.68 to 0.75) | 0.923 |
| PD-MCI (N=38); Mean(SD) | 12.1 (5.6) | 11.7 (5.7) | 0.37 (−0.64 to 1.37) | 0.463 |
| PDD (N=22); Mean(SD) | 13.8 (5.0) | 12.6 (5.1) | 1.14 (−0.41 to 2.69) | 0.142 |
Paired t-test is used
CogNL: normal; MCI: mild cognitive impairment; PD: Parkinson disease; PDD: Parkinson disease dementia
3.3. Subject-CR ESS Score Correlations
Spearman R correlations between subject and informant ESS responses were 0.73 overall, 0.67 for the CogNL group, 0.79 for the MCI group, 0.79 for those with dementia (figure 1a). For the subjects that did not have PD the r value was 0.70. For subjects with PD the r value was 0.65 for the CogNL cases, 0.83 PD-MCI and 0.70 for those with PD-dementia (figure 1b). P value was <0.001 for all comparisons.
Figure 1a.
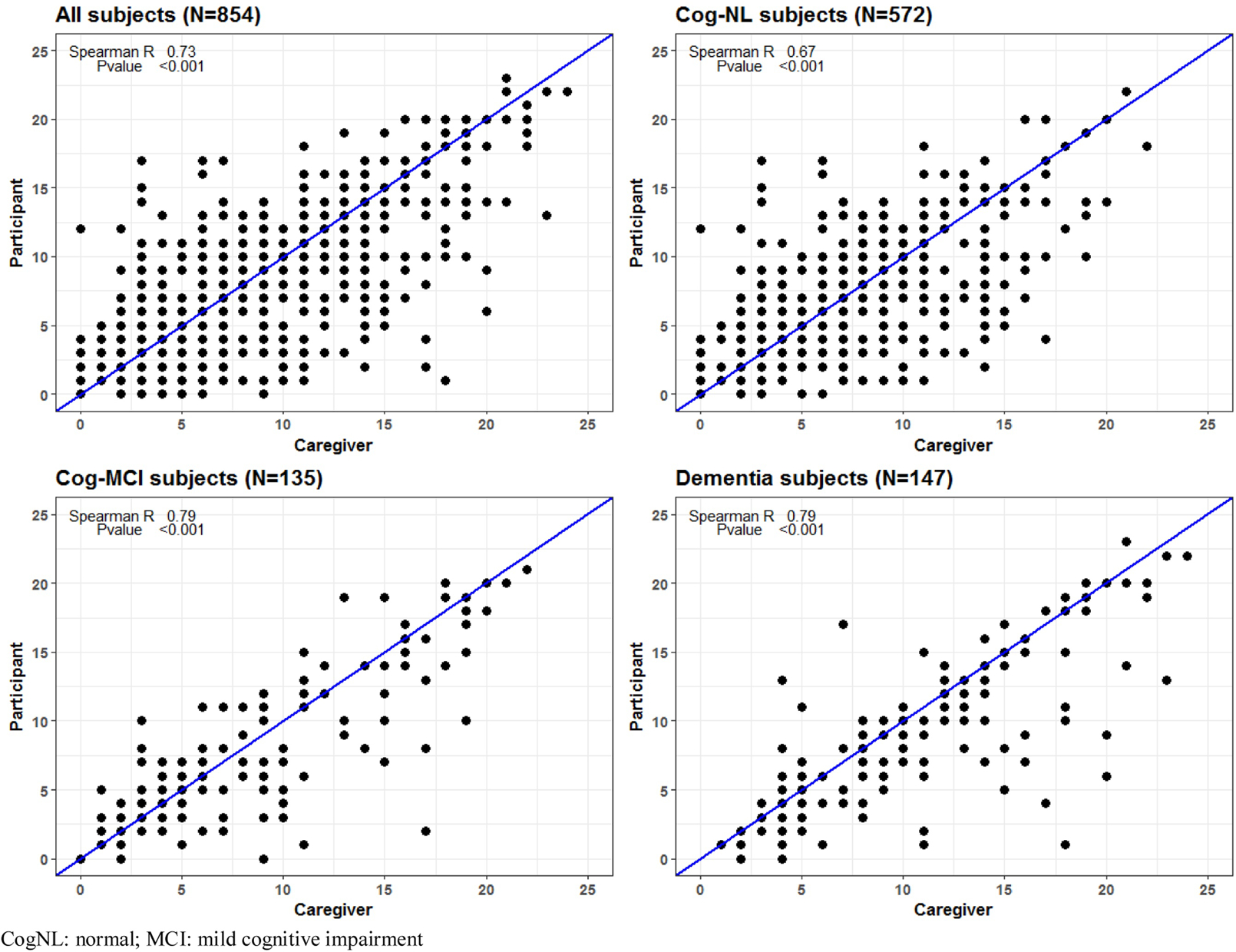
Correlation overall and by cognitive status
Figure 1b.
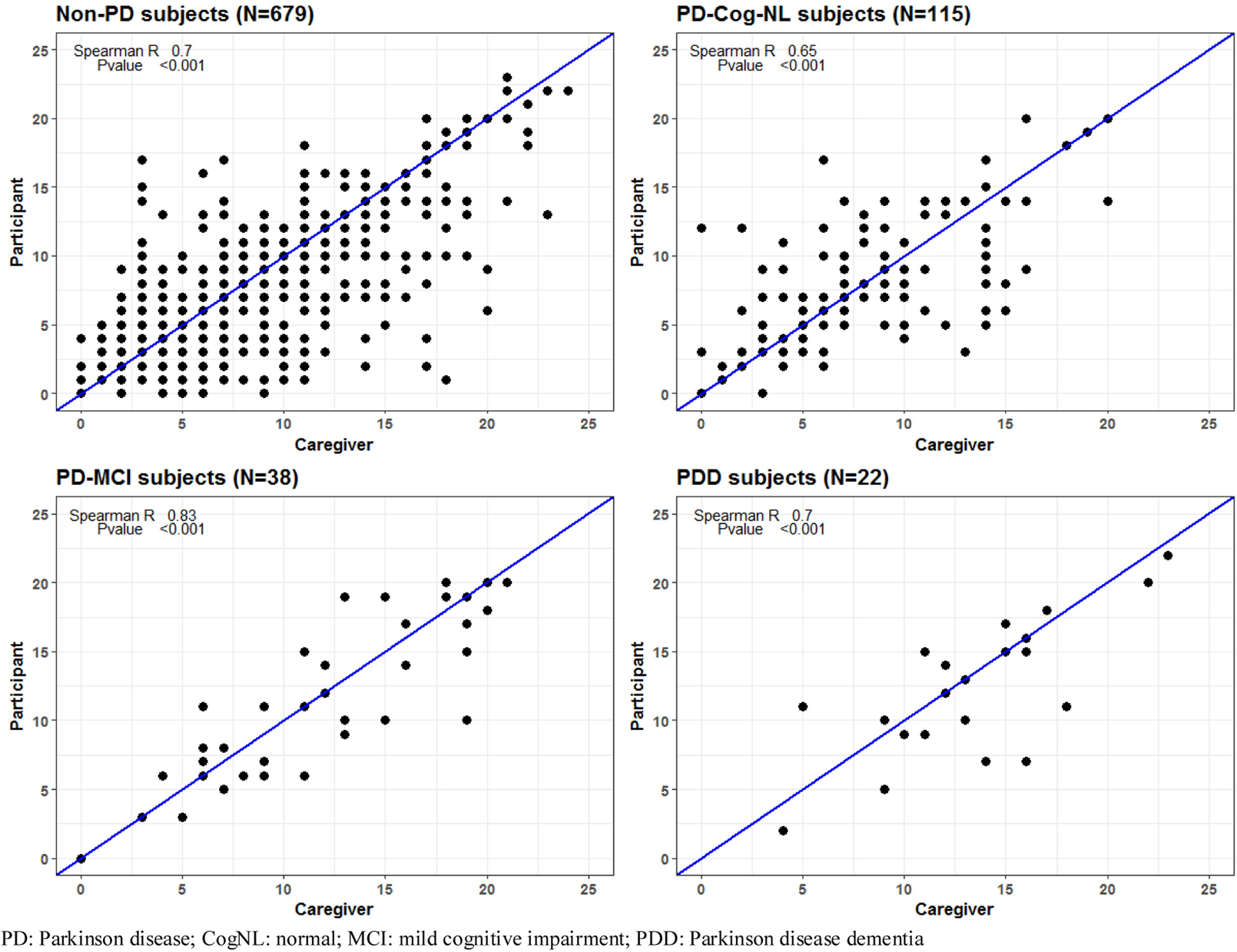
Correlation by PD and cognitive status
4. Discussion
To our knowledge, this is the first study to examine the correlations between subject and informant-competed ESS in subjects with PD. Our data showed excellent correlations between subject and informant-completed ESS scores, regardless of cognitive impairment or whether the subjects had PD with or without MCI or dementia. As data from previous studies in non-PD populations have shown conflicting results,[7] the presented data should be interpreted with caution. One limitation of this study is that, in AZSAND, subject and informant complete the ESS at home so one may influence the other’s responses. However, as the mean differences in ESS score between subject and informant for our overall, as well as non-PD MCI and dementia samples, did meet statistical significance, this may not be a significant factor. Those within the PD-MCI and PD-dementia sample did not, however this may be due to much smaller sample size in these groups. Another limitation is the subjective measure of daytime sleepiness without objective correlation, as ESS has not been shown in PD subjects to correlate with the more objective mean sleep latency test. [8] To address the limitations of this study, additional research is needed using larger sample sizes (with measures taken to prevent informants from influencing subject responses). The significant correlations demonstrated between subject and informant-completed ESS in this study can be useful to guide design of clinical trials to assess efficacy of potential treatments for EDS in the setting of PD, even with associated cognitive impairment.
Highlights.
We compared Parkinson disease patient/caregiver Epworth Sleepiness Scale responses
Responses of Parkinson disease participants correlated significantly
Correlations were seen in those with Parkinson’s related cognitive decline
These results may be useful in guiding treatment trials in Parkinson disease
Acknowledgements:
We thank our donors and their families for their contributions of tissue, time and effort to this work.
Funding Sources:
This study was funded by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), the Michael J. Fox Foundation for Parkinson’s Research, the Arizona Alzheimer’s Consortium and Mayo Clinic Foundation.
Disclosures:
DRS received research support from the Arizona Alzheimer’s Consortium, Abbvie, Acadia, Axovant, Biogen, Eli Lilly, Neurocrine, Michael J Fox Foundation, NIH and Teva; consultant fees from Abbvie, Teva, Lundbeck, Merz and Neurocrine; speaker fees from Acadia, Lundbeck, Sunovion, Teva and US World Meds.
CHA has received research funding from the Michael J. Fox Foundation, NIH, US Department of Defense, and the Arizona Biomedical Research Foundation, and has received consulting fees from Acadia, Acorda, Cynapsus, Jazz, Lundbeck, Merz, Minerva, Neurocrine, and Sunovion
NZ receives research support from the Arizona Alzheimer’s Consortium and NIH.
HS: received research support from Cynapsus/Sunovion, Axovant, Impax, US World Meds, Michael J. Fox Foundation and the NIH
CMB: receives grant support from the Arizona Alzheimer’s Consortium, National Institutes of health, MJFF. Research support from Axovant, Biogen, Lilly, Avid, Genentech, AstraZeneca, Merck, Pfizer, Roche, Takeda, Biotie, Neurocrine, Navidea, Novartis, Suven, Abbvie, USC-ALZ Association, and Navidea.
ED-D, KJD, LIS: no disclosures
SHM: receives consulting fees from Abbvie, Medtronic and Adamas. Research support from Jazz Pharmaceuticals, Pharma 2B, Eli Lily and Arizona Biomedical research Consortium (ABRC)
EZ: receives research support from Eli Lilly, Biogen, Pfizer, Merk, and NIH
TGB: receives grant support from the the Arizona Alzheimer’s Consortium, National Institutes of Health and MJFF, performs contracted research for Navidea Biopharamaceuticals and Avid Radiopharmaceuticals, serves as a paid consultant with Genentech, GSK, Roche and Ventana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Adler CH, Hentz JG, Shill HA, Sabbagh MN, Driver-Dunckley E, Evidente VG, et al. Probable RBD is increased in Parkinson’s disease but not in essential tremor or restless legs syndrome. Parkinsonism Relat Disord. 2011;17:456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simuni T, Caspell-Garcia C, Coffey C, Chahine LM, Lasch S, Oertel WH, et al. Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: A case control study. Mov Disord. 2015;30:1371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amara AW, Chahine LM, Caspell-Garcia C, Long JD, Coffey C, Hogl B, et al. Longitudinal assessment of excessive daytime sleepiness in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2017;88:653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson’s disease: a 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry. 2011;82:1033–7. [DOI] [PubMed] [Google Scholar]
- [6].Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35:354–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li Y, Zhang J, Lei F, Liu H, Li Z, Tang X. Self-evaluated and close relative-evaluated Epworth Sleepiness Scale vs. multiple sleep latency test in patients with obstructive sleep apnea. J Clin Sleep Med. 2014;10:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wienecke M, Werth E, Poryazova R, Baumann-Vogel H, Bassetti CL, Weller M, et al. Progressive dopamine and hypocretin deficiencies in Parkinson’s disease: is there an impact on sleep and wakefulness? J Sleep Res. 2012;21:710–7. [DOI] [PubMed] [Google Scholar]


