Abstract
TRAIL-activating therapy is promising in treating various cancers, including pancreatic cancer, a highly malignant neoplasm with poor prognosis. However, many pancreatic cancer cells are resistant to TRAIL-induced apoptosis despite their expression of intact death receptors (DRs). Protein O-GlcNAcylation is a versatile posttranslational modification that regulates various biological processes. Elevated protein O-GlcNAcylation has been recently linked to cancer cell growth and survival. In this study, we evaluated the role of protein O-GlcNAcylation in pancreatic cancer TRAIL resistance, and identified higher levels of O-GlcNAcylation in TRAIL-resistant pancreatic cancer cells. With gain- and loss- of function of the O-GlcNAc-adding enzyme, O-GlcNActransferase (OGT), we determined that increasing O-GlcNAcylation rendered TRAIL-sensitive cells more resistant to TRA-8-induced apoptosis, while inhibiting O-GlcNAcylation promoted TRA-8-induced apoptosis in TRAIL-resistance cells. Furthermore, we demonstrated that OGT knockdown sensitized TRAIL-resistant cells to TRA-8 therapy in a mouse model in vivo. Mechanistic studies revealed direct O-GlcNAc modifications of DR5, which regulated TRA-8-induced DR5 oligomerization. We further defined that DR5 O-GlcNAcylation was independent of FADD, the adaptor protein for the downstream death-inducing signaling. These studies have demonstrated an important role of protein O-GlcNAcylation in regulating TRAIL resistance of pancreatic cancer cells; and uncovered the contribution of O-GlcNAcylation to DR5 oligomerization and thus mediating death receptor-inducing signaling.
Keywords: O-GlcNAcylation, Pancreatic Cancer, DR5, TRAIL, Apoptosis
Introduction
Pancreatic cancer is one of the most lethal malignancies with a 5-year survival rate below 8%1, 2. Due to its late detection, most pancreatic cancers in advanced stages are not resectable. Chemotherapy, radiotherapy and immunotherapy are very critical treatments for improving the quality of the life of patients. However, the major problem is the development of resistance to conventional therapeutics over time. Therefore, designing novel therapeutic strategies that will reverse resistance or sensitize resistant cancer cells to chemotherapeutics or immunotherapeutics remains an important clinical challenge2, 3.
Dysregulation of cell apoptosis is not only involved in pathogenesis and progression of various cancers but also related to resistance of cancer cells to chemotherapy, radiotherapy and immunotherapy induced-cytotoxicity4. The tumor necrosis factor (TNF)-related apoptosis inducing ligand (TRAIL) induces apoptosis of cells through binding death receptor 4 or 5 (DR4 and DR5). Upon ligand binding, death receptors trigger the assembly of the death-inducing signaling complex (DISC), leading to activation of caspases 8/10 and downstream caspase-3 that induces cellular apoptosis5. Unlike the other extrinsic apoptosis-inducing ligands such as TNF-α and Fas ligand that are toxic to normal cells, TRAIL selectively induces apoptosis in tumor cells while leaving normal cell intact, which makes it a preferred agent for cancer therapy5. Many TRAIL-activating therapies have been tested in clinical trials, such as recombinant human TRAIL and antibodies directed against TRAIL receptors. The antibodies for DR5, including conatumumab (AMG655, antibody for DR5) and tigatuzumab (CS-1008/TRA-8, antibody for DR5), have been used in clinical trials to treat pancreatic cancers. However, such therapeutics exhibit limited efficacy in few selected populations, most pancreatic cancer patients, particularly those in advanced stages, are resistant to therapies with TRAIL and DR4/DR5 agonist antibodies6-8. Experimental studies have demonstrated the resistance of pancreatic cancer cells to TRAIL-induced apoptosis, despite their expression of the death receptors and other components of the apoptosis machinery9-11. Therefore, it is postulated that resistance to TRAIL-induced apoptosis contributes to failure of pancreatic cancer cells to TRAIL therapies in the clinical trials, thus further understanding of the molecular and cellular mechanisms of TRAIL resistance are necessary for the successful application of TRAIL and DR4 or DR5 antibodies in cancer therapy.
Increase in cancer risk has been demonstrated in patients with diabetes mellitus and in individuals with abnormal glucose tolerance, mainly within digestive sites including pancreas12-14. Nutritional conditions, excess body weight, and insulin resistance can modulate tumor development by modifying circulation factors that affect signaling pathways involved in cell growth, proliferation, and apoptosis15. In addition to being the major source for intracellular energy-producing and biosynthesis, glucose metabolism via the hexosamine biosynthesis pathway generates uridine-diphosphate-β-D-N-acetylglucosamine (UDP-GlcNAc), an active sugar donor for glycosylation, including the O-linked β-N-acetylglucosamine modification (O-GlcNAcylation), a protein posttranslational modification that regulates many aspects of molecular and cellular functions16-20. O-GlcNAcylation is a widespread posttranslational modification that is achieved by addition of a GlcNAc moiety to the hydroxyl group (O-linked) of the serine or threonine residues on the target proteins. O-GlcNAcylation is dynamically controlled by only two specific enzymes, which maintains O-GlcNAc homeostasis in normal physiological conditions. The addition of O-GlcNAc to proteins is catalyzed by the O-GlcNActransferase (OGT), whereas the removal of O-GlcNAc is mediated by O-GlcNAcase (OGA). Excess of nutrients intake, hyperglycemia, and other metabolic perturbations associated with diabetes mellitus and obesity contribute to abnormally elevated O-GlcNAcylation of key signaling molecules and transcription factors. Consequently, disruption of O-GlcNAc homeostasis and chronically increased O-GlcNAcylation are evident in pathological conditions, such as diabetes mellitus, cardiovascular diseases, Alzheimer disease and cancers21-23.
Emerging studies have reported the elevated O-GlcNAcylation and aberrant expression of OGT and OGA in many cancers, such as breast cancer, prostate cancer, lung cancer, liver cancer and pancreatic cancer24-27, which may contributed to the dysregulation of cancer cell growth and invasion28, 29. However, it is entirely unknown whether protein O-GlcNAcylation may contribute to TRAIL resistance of pancreatic cancer. The current studies determined protein O-GlcNAcylation levels in pancreatic cancer cells that exhibit different sensitivity to TRAIL-induced apoptosis. TRAIL resistant pancreatic cancer cells have higher levels of O-GlcNAcylation compared with those of TRAIL sensitive pancreatic cancer cells. Using gain- or loss-of-function of OGT, a key role of protein O-GlcNAcylation in regulating sensitivity of pancreatic cancer cells to TRA-8-induced apoptosis was demonstrated. More importantly, inhibition of O-GlcNAcylation sensitized TRAIL-resistant pancreatic cancer cells to TRA-8 therapy in animal model in vivo. These studies has uncovered a novel function of protein O-GlcNAcylation in regulating the resistance of pancreatic cancer cells to TRAIL-induced apoptosis, which supports the strategy to enhance efficacy of TRAIL therapy via inhibiting O-GlcNAcylation in resistant pancreatic cancers.
Materials and Methods
Cell lines and in vitro studies
The human pancreatic cancer cell lines MiaPaPc-2, BxPC-3 and PANC-1 were purchased from the American Type Culture Collection. The S2VP10 cells, derived from the Suit-2 cells as reported30, were originally provided by Dr. M. Hollingsworth (University of Nebraska). Cell line authentication was determined by short tandem repeat analysis (STR) performed by the American Type Culture Collection. OGT overexpression MiaPaPc-2 and BxPC-3 cells were generated by transfection of pCMV-flag-OGT (OGT, Addgene, #29760); and OGT knockdown S2VP10 and PANC-1 cells were generated by infected with lentiviruses containing a short hairpin RNA (shRNA) specific for OGT (shOGT, Addgene, #10878). PANC-1 cells with FADD knockdown were established as we previously described by shRNA targeting FADD (Open Biosystems)31. All stable clones were selected by 2 μg/ml puromycin as we previously reported31. Sequencing analysis was performed to confirm the correct sequence, and protein expression was validated by Western blot analysis. Apoptosis was induced by TRA-8, a DR5 agonist antibody that was generated as previously described32; and determined by Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining kit (BD Biosciences, San Jose, CA) and analyzed by flow cytometry as we previously reported11.
Western blot analysis
Western blotting was performed as previously described10. Proteins were extracted and quantified using a BCA protein assay kit (Thermo Scientific). Proteins were separated by SDS-PAGE and transferred to Immobilon P or nitrocellulose membranes, which were blocked in 5% non-fat milk and then incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies sequentially. The antibodies used include anti-DR5 (Cat# 2019, Prosci, 1:1000), anti-OGT (Sigma Aldrich, # 06264, 1:1000), O-GlcNAc (RL2, Sigma Aldrich, 1:1000), FADD (Sigma Aldrich, F8063, 1:2000) and anti-β-actin (Sigma Aldrich, #A5541-2MI, 1:10000). Signals were detected using Amershom™ ECL™ Western Blotting Detection reagents (GE Healthcare).
Immunoprecipitation
Cell lysates were incubated with rabbit anti-DR5 (Prosci, #2019, 1:100) or mouse anti-O-GlcNAc (RL2, Sigma Aldrich, 1:100) overnight at 4°C. Immunoprecipitated complexes were recovered by incubation with protein G-agarose beads (Thermo Fisher, #20398), which was washed and boiled in 2xLaemmli sample buffer, proteins were then subjected to Western blot analysis using appropriate antibodies.
Animal studies
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham. Eight-week old male athymic mice (Jackson Laboratory) were used for tumor inoculation as we described 11, 33, 34. Stable OGT knockdown (shOGT) or control cells (shScr) were inoculated subcutaneously into the flanks of mice (1.0 ×106 cells/site, 5 mice/each group). Tumor sizes were measured every week and volumes were determined using the formula volume=length×width2/2. After tumor volumes reached to 100 mm3 mice began to be intraperitoneal injected with either TRA-8 (TRA-8, 200 μg per mouse, once per week) or 0.9% sodium chloride (Control) for 3 weeks. At the end of the experiment, tumors were removed from mice, half of each tumor was homogenized for Western blot analysis and the other half was fixed in 4% paraformaldehyde and embedded in paraffin for histology analysis by hematoxylin and eosin stain (H&E).
TUNEL staining 11, 34
TUNEL staining was conducted on tumor sections (DeadEnd Fluorometric TUNEL System; Promega) to determine cell death, while DAPI staining was used to localize nuclei. Stained specimens were examined microscopically (Leica M165 FC). For quantitative analysis, cell numbers were counted under a microscope (×200). Four fields in each slide were counted and the percentage of apoptotic cells was calculated.
Statistical Analysis
Differences between two groups were identified with unpaired Student’s t-test or One-Way ANOVA. For multiple groups, one-way analysis of variance and Student-Newman-Keuls tests were performed to identify differences. Significance was defined as p < 0.05.
Results
O-GlcNAcylation levels in TRAIL sensitive and resistant pancreatic cancer cells.
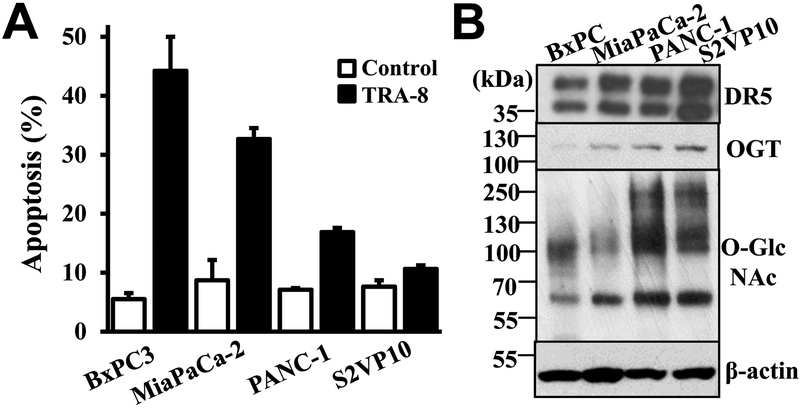
To determine whether protein O-GlcNAcylation is associated with the resistance of pancreatic cancer cells to TRA-8-induced apoptosis, we analyzed protein O-GlcNAcylation and the expression of the O-GlcNAc adding enzyme, OGT, in pancreatic cancer cells that exhibit different sensitivity to TRA-8-induced apoptosis (Figure 1A). Western blot analysis demonstrated that the expression of the death receptor, DR5, was not associated with TRA-8 sensitivity; while higher levels of OGT and protein O-GlcNAcylation in TRA-8 resistant PANC-1 and S2VP10 cells, compared with those in TRA-8 sensitive MiaPaPc-2 and BxPC-3 pancreatic cancer cells (Figure 1B). These results imply that higher levels of OGT and protein O-GlcNAcylation are associated with the resistance of pancreatic cancer cells to TRA-8-induced apoptosis.
Figure 1. O-GlcNAcylation in pancreatic cancer cells with different sensitivity to TRAIL induced apoptosis.
A) TRA-8 induced apoptosis. Pancreatic cancer cells, BxPC-3, MiaPaCa-2, PANC-1 and S2VP10, were exposed to TRA-8 (1 μg/ml) for 24 hours; and apoptotic cells were detected by flow cytometry. Results shown are means ± SD of three independent experiments performed in duplicate. B) O-GlcNAcylation in pancreatic cell lines, as determined by Western blot analysis using specific antibodies for, DR5, OGT and O-GlcNAcylation. The expression of ß-actin was used as a loading control. Representative blots from three independent experiments are shown.
Increased O-GlcNAcylation inhibits TRA-8-induced apoptosis in sensitive pancreatic cancer cells.
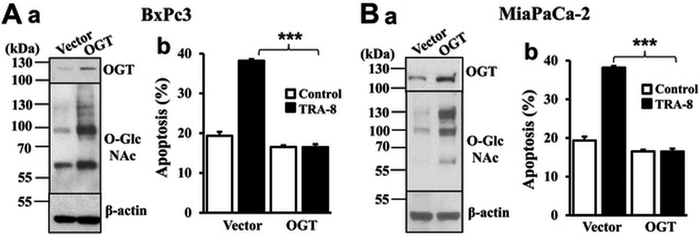
The direct effects of OGT and O-GlcNAcylation on pancreatic cancer TRAIL resistance were tested by increasing OGT expression in the TRAIL sensitive MiaPaPc-2 and BxPC-3 pancreatic cancer cells. Increased expression of OGT and protein O-GlcNAcylation was evident in the OGT stably expressed cells (Figure 2, Aa and Ba, OGT) compared with the control cells (Figure 2, Aa and Ba, Vector). Importantly, increased O-GlcNAcylation decreased sensitivity to TRA-8-induced apoptosis in both TRAIL-sensitive pancreatic cancer cell lines (Figure 2, Ab and Bb, black bars). These results support a causative effect of increased O-GlcNAcylation on TRA-8-induced apoptosis in pancreatic cancer cells.
Figure 2. Overexpression of OGT attenuates TRA-8 induced apoptosis in sensitive pancreatic cancer cells.
A) BxPC-3, and B) MiaPaCa-2 cells were transfected with OGT expressing vector or control vector. Stable clones were selected by neomycin. a. Protein O-GlcNAcylation and OGT expression, as determined by Western blot analysis using specific antibodies. The expression of ß-actin was used as a loading control. Representative blots from three independent experiments are shown. b. TRA-8 induced apoptosis. Cells were exposed to TRA-8 (1 μg/ml) for 24 hours, and apoptosis were determined by flow cytometry (n=3, ***p<0.001).
Inhibition of O-GlcNAcylation promotes TRA-8-induced apoptosis in resistant pancreatic cancer cells.
On the other hand, the effects of reduced O-GlcNAcylation on TRA-8-induced apoptosis in TRAIL resistance PANC-1 and S2VP10 pancreatic cancer cells were tested by knocking down OGT. Knockdown of OGT concurrently inhibited O-GlcNAcylation as validated by Western blot analysis (Figure 3Aa and Ba). Flow cytometry analysis demonstrated markedly increased TRA-8-induced apoptosis in the OGT knockdown (shOGT) PANC-1 and S2VP10 cells compared with the parent cells with control shRNA (shScr) (Figure 3Ab and Bb). These studies suggest that increased O-GlcNAcylation is important for TRAIL resistance. Taken together, these data support a novel function of protein O-GlcNAcylation in regulating sensitivity of pancreatic cancer cells to TRA-8-induced apoptosis in vitro.
Figure 3. Downregulation of OGT promotes TRA-8-induced apoptosis in resistant pancreatic cancer cells.
A) PANC-1, and B) S2VP10 cells were infected with lentiviruses carrying scrambled shRNA (shScr) or shRNA for OGT (shOGT), and selected by puromycin. a. Protein O-GlcNAcylation and OGT expression, as determined by Western blot analysis using specific antibodies. The expression of ß-actin was used as a loading control. Representative blots from three experiments are shown. b. TRA-8 induced apoptosis. Cells were exposed to TRA-8 (1 μg/ml) for 24 hours, and apoptosis were determined by flow cytometry (n=3, ***p<0.001).
Inhibition of O-GlcNAcylation sensitizes resistant pancreatic cancer toTRA-8 therapy in vivo.
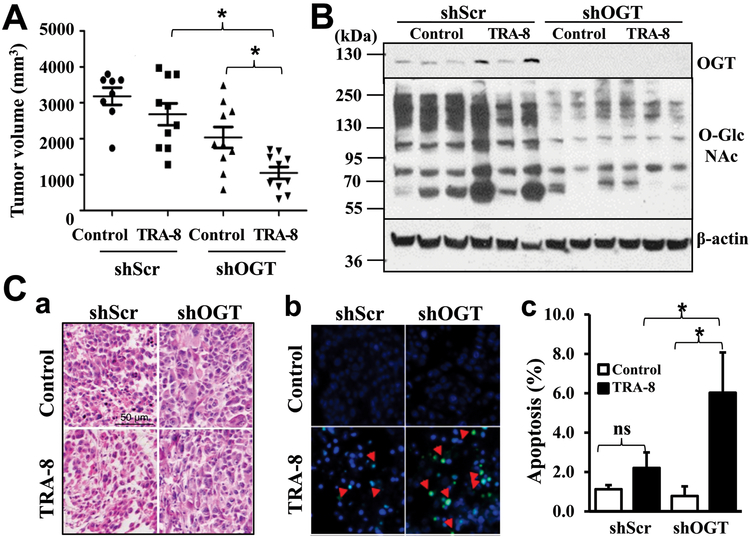
To determine how inhibition of OGT and O-GlcNAcylation affects TRAIL resistance of pancreatic cancer in vivo, we evaluated pancreatic cancer tumorigenesis and their sensitivity to TRAIL therapy in a mouse xenograft model. TRAIL-resistant S2VP10 cells with OGT knockdown (shOGT) or control shRNA (shScr) were grafted in vivo and characterized. As expected, TRA-8 treatment did not affect tumorigenesis of the control cells (Figure 4A, shScr), whereas TRA-8 therapy markedly reduced tumor sizes in the OGT knockdown S2VP10 cells (Figure 4A, shOGT). Western blot analysis validated the OGT knockdown and decreased O-GlcNAcylation in the isolated tumors, which were not affected by the TRA-8 treatment (Figure 4B). Consistent with reduced tumor sizes, TUNEL staining of tumor sections revealed significantly increased cell death in pancreatic tumors with the OGT knockdown (Figure 4C). Similar to the in vitro observation, these studies further demonstrated the function of O-GlcNAcylation in modulating the sensitivity of resistant pancreatic cancer cells to TRA-8 therapy in vivo.
Figure 4. OGT knockdown increases sensitivity of resistant pancreatic cancer to TRA-8 therapy in mice.
S2VP10 cells stably infected with scrambled shRNA (shScr) or shRNA for OGT (shOGT) were injected into nude mice, which were then subjected to control vehicle (Control) or TRA-8 treatment for 3 weeks. A) Tumor volumes, in each group are shown (n=8-10/each group, *p<0.05); B) Protein O-GlcNAcylation and OGT expression, in representative tumors in each group was determined by Western blot analysis. The expression of β-actin was used as a loading control. C) Cell death in tumors was analyzed by TUNEL staining. a. Representative H&E staining images from each group (Scale bar=50μm). b. Representative TUNEL staining images (red arrows indicating positive staining). b. Quantitative analysis of TUNEL positive cells as percentage of total cells in the tumor sections (ns=not significant, *p<0.05).
O-GlcNAcylation regulates oligomerization of DR5.
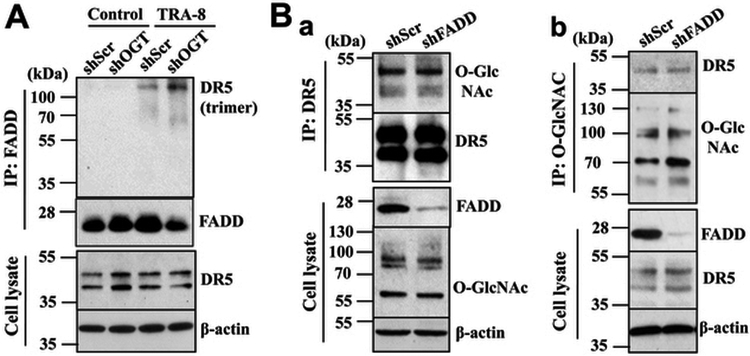
We have previously demonstrated that strategies to increase expression of the key TRAIL-signaling receptor, the death receptor 5 (DR5), promotes TRA-8-induced apoptosis in resistant pancreatic cancer cells33; although the expression of DR5 is intact in the TRAIL-resistant pancreatic cancer cells, including PANC-1 and Suit-2, the parental cells for S2VP1011. Accordingly, we first analyzed whether inhibition of O-GlcNAcylation may induce augmentation of DR5 expression, thus promoting TRA-8-induced apoptosis. Western blot analysis of DR5 expression in the OGT knockdown PANC-1 suggest that inhibition O-GlcNAcylation did not affect DR5 expression (Figure 5A, DR5, cell lysate). Therefore, increased TRA-8-induced apoptosis by reduced O-GlcNAcylation in the TRAIL-resistant pancreatic cancer cells may not be attributed to differential expression of DR5.
Figure 5. OGT knockdown enhances TRA-8-induced DR5 oligomerization in pancreatic cancer cells.
A) DR5 oligomerization, in PANC-1 pancreatic cancer cells stably infected with scrambled shRNA (shScr) or shRNA for OGT (shOGT) with or without exposure to TRA-8 (1 μg/ml) for 1 hour, followed by immunoprecipitation using anti-FADD antibody. Representative blots from three experiments are shown. B) FADD downregulation does not affect DR5 O-GlcNAcylation. Pancreatic cancer cells PANC-1 cells were stably infected with scrambled shRNA (shScr) or shRNA for FADD (shFADD). O-GlcNAcylation of DR5 was determined by immunoprecipitation with a. an anti-DR5 antibody; and b. an anti-O-GlcNAcylation antibody. Western blot analysis was performed using specific antibodies. Representative blots from at least two independent experiments are shown.
Ligand-induced DR5 homo-trimerization is the essential first step and prerequisite for the formation of death-inducing signaling complex (DISC), which recruits the Fas-associated death domain (FADD) and procaspase-8 that leads to caspase-8 activation and apoptosis35, 36. Therefore, inhibition of TRAIL-induced DR5 trimerization attenuates TRAIL-induced apoptosis, thus rendering resistance of cancer cells to TRAIL therapy. To determine whether O-GlcNAcylation might regulate TRA-8-induced apoptosis via modulating TRA-8-induced DR5 trimerization, we performed immunoprecipitation with the anti-FADD and analyzed FADD-associated DR5 using non-reducing SDS-PAGE. As shown in Figure 5A (IP: FADD), FADD was not associated with high molecular mass DR5 (oligomerization) without stimulation with the DR5 agonist antibody TRA-8. Increased FADD binding to trimerized DR5 was demonstrated in the OGT knockdown cells, supporting the notion that the inhibition of O-GlcNAcylation might promote DR5 oligomerization and DISC recruitment of FADD.
To further determine how O-GlcNAcylation affects oligomerization of DR5, we evaluated whether DR5 is directly O-GlcNAcylated in pancreatic cancer cells (Figure 5Ba, b). Co-immunoprecipitation studies using both anti-DR5 (Figure 5Ba) and anti-O-GlcNAcylation (Figure 5Bb) antibodies suggest that DR5 is modified by O-GlcNAcylation. Furthermore, with the use of PANC-1 cells with FADD knockdown, we demonstrated that FADD knockdown did not affect DR5 O-GlcNAcylation, indicating that DR5 O-GlcNAcylation is independent of the adaptor protein FADD.
Discussion
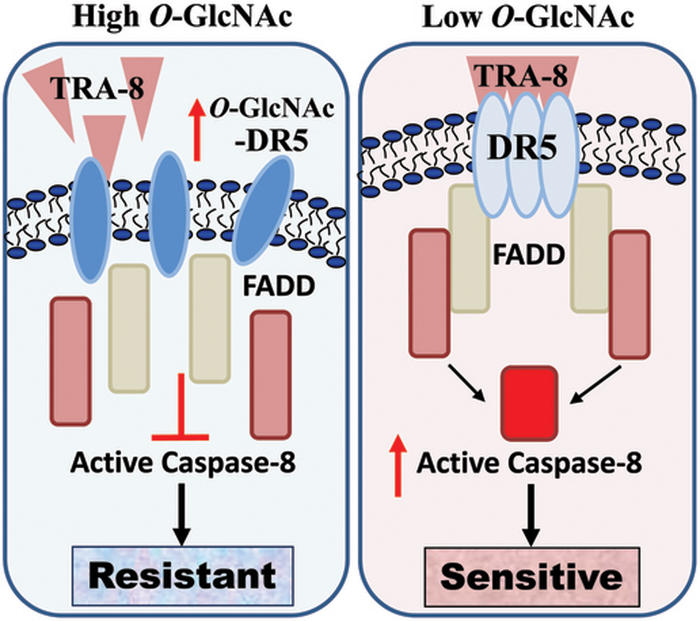
Numerous human cancer cells, including pancreatic cancer cells, are resistant to TRAIL-induced apoptosis and the molecular mechanisms underlying the resistance remain unclear, which makes it become a major huddle for the use of TRAIL-activating agents for cancer therapy despite its highly promising efficacy in targeting cancer cells. Our ongoing efforts in the search of strategies to sensitize TRAIL-induced apoptosis in resistant pancreatic cancer cells have uncovered that targeting the death inducing signaling complex (DISC) by either inducing expression of DR5 or converting survival signals to apoptotic singling is effective in promoting apoptosis of the TRAIL-resistant cancer cells10, 11, 33, 34. The present studies offer novel molecular insights into sensitizing TRA-8-induced apoptosis of resistant pancreatic cancer cells at the essential initial step of the DISC formation, oligomerization of the death receptor, via inhibiting O-GlcNAcylation of DR5 (Figure 6).
Figure 6. O-GlcNAcylation regulation of TRAIL sensitivity of pancreatic cancer cells.
Increased O-GlcNAcylation of DR5 decrease TRAIL-induced DR5 oligomerization and the activation of apoptotic signaling, which leads to TRAIL resistance of the pancreatic cancer cells. Inhibition of O-GlcNAcylation of DR5 increases TRAIL-induced DR5 oligomerization and activation of apoptotic signals, and thus rendering the TRAIL-resistant pancreatic cancer cells more sensitive to TRAIL therapy.
We identified a positive correlation between increased OGT and O-GlcNAcylation and elevated TRAIL resistance of pancreatic cancer cells, which has improved our current understanding of the contributions of O-GlcNAcylation and their determinants, OGT and OGA to cancer cell growth and invasion24-29. In addition, we demonstrated a definitive role of OGT-mediated O-GlcNAcylation in regulating the sensitivity of pancreatic cancer cells to TRAIL-induced apoptosis using both gain- and loss-of-function of OGT strategies in vitro. Furthermore, inhibition of O-GlcNAcylation was found to sensitize the TRAIL-resistant pancreatic cancer cells to TRA-8 therapy in vivo, supporting the application of a novel strategy to target O-GlcNAcylation for improving treatment efficacy of TRAIL therapy.
Mechanistically, our studies revealed a novel modification of DR5 by O-GlcNAcylation, which may regulate TRA-8-induced trimerization of DR5. Consequently, inhibition of O-GlcNAcylation increased TRA-8-induced DR5 trimerization, thus sensitizing TRA-8-induced apoptosis. As O-GlcNAcylation can activate signaling of growth factors that promote cell survival, which in turn contributes to the inhibition of cell death of cancer cells by increased O-GlcNAcylation. For example, O-GlcNAcylation of nuclear factor ҡB enhances its transcriptional activity and thus inhibits apoptosis of pancreatic cancer cells27. Increased O-GlcNAcylation promotes the expression of heat-shock proteins and enhances stability and activity of oncogene c-MYC protein that contribute to the survival of cancer cells in response to stress 37, 38. In addition, we have shown that O-GlcNAcylation promotes PI3K/Akt activity39, an important survival signaling in cancer cells40. In addition, direct effects of O-GlcNAcylation on apoptosis signaling pathways have also been reported. Inhibition of O-GlcNAcylation by the OGT knockout in T-cells results in increased apoptosis in both CD4+ and CD8+ T-cells41. In pancreatic cancer cells, inhibition of OGT induces apoptosis27. A recent study has further shown that O-GlcNAcylation may directly affect the important apoptosis effectors, caspase-3, 8 and 9 proteins, therefore inhibiting activation of the extrinsic apoptotic pathway in lung, cervical and breast cancer cells42. Consistently, our current studies demonstrated that increased O-GlcNAcylation directly modify DR5 that may contribute to TRA-8-induced pancreatic cancer cell apoptosis.
We determined O-GlcNAcylation of DR5 affected TRA-8-induced DR5 trimerization. The requirement for death receptor aggregation and clustering is a characteristic shared by the tumor necrosis factor receptor family members and an essential first step in activating the receptor-mediated signal transduction35, 36. For the TRAIL receptor DR5, ligand-induced DR5 homo-trimerization is prerequisite for the formation of DISC, which recruits the adaptor protein FADD and procaspase-8 that promotes the activation of caspase-8. We demonstrated that inhibition of DR5 O-GlcNAcylation increased TRA-8-induced DR5 trimerization, thus sensitizing TRA-8-induced apoptosis. These findings shed new lights on the longstanding puzzle on TRAIL resistance of cancer cells in the context of the intact expression of DR5. Our findings suggest that levels of O-GlcNAcylation or selective O-GlcNAcylation of DR5 may be a useful indicator to predict the sensitivity of pancreatic cancer patients to TRAIL therapy.
The mechanisms underlying increased protein O-GlcNAcylation in pancreatic cancer are currently not clear. In diabetes mellitus, multifaceted regulatory mechanisms increase O-GlcNAcylation in diabetes, including upregulation of OGT, downregulation of OGA, and increased glucose metabolism via the HBP and UDP-GlcNAc20, 43-45. In addition, insulin stimulation has been shown to promote OGT shuttling from nucleus to cytoplasm, leading to increased O-GlcNAcylation in cytoplasm46, 47. These putative mechanisms may all together contribute to increased O-GlcNAcylation and its modification of key regulatory molecules in the apoptosis machinery. Nonetheless, our studies have demonstrated that increased O-GlcNAcylation of apoptotic proteins, such as DR5 or the death effectors caspases42, may contribute to pancreatic cancer development and their resistance to TRAIL therapy.
Conclusions
In summary, our studies identified a new role of protein O-GlcNAcylation in regulating sensitivity of pancreatic cancer cells to TRA-8-induced apoptosis in vitro and TRA-8 therapy in an animal model of pancreatic cancer in vivo. We reported a novel mechanism underlying direct O-GlcNAc modifications of DR5 that regulates TRA-8-induced DR5 oligomerization and thus apoptosis signaling. These studies open a new avenue for the potential application of protein O-GlcNAcylation and its modification of the death receptors for pancreatic cancer diagnosis, prediction for TRAIL therapy, or even selective targeting to enhance the efficacy for TRAIL therapy.
Acknowledgments
This work was supported by grants from the Veterans Affairs Research Department, BX002296 and BX003617 (YC). The authors have no potential conflicts of interest to disclose.
References
- 1.Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018;15(6):333–348. [DOI] [PubMed] [Google Scholar]
- 3.Van Audenaerde JRM, Roeyen G, Darcy PK, et al. Natural killer cells and their therapeutic role in pancreatic cancer: A systematic review. Pharmacol Ther 2018;189:31–44. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 5.Holoch PA, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL): a new path to anti-cancer therapies. Eur J Pharmacol 2009;625(1-3):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Kurzrock R, Hong DS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res 2010;16(23):5883–5891. [DOI] [PubMed] [Google Scholar]
- 7.Forero-Torres A, Shah J, Wood T, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother Radiopharm 2010;25(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Miguel D, Lemke J, Anel A, et al. Onto better TRAILs for cancer treatment. Cell Death Differ 2016;23(5):733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geismann C, Erhart W, Grohmann F, et al. TRAIL/NF-kappaB/CX3CL1 Mediated Onco-Immuno Crosstalk Leading to TRAIL Resistance of Pancreatic Cancer Cell Lines. Int J Mol Sci 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang SZ, Xu F, Zhou T, et al. The long non-coding RNA HOTAIR enhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand. J Biol Chem 2017;292(25):10390–10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan K, Sun Y, Zhou T, et al. PARP-1 regulates resistance of pancreatic cancer to TRAIL therapy. Clin Cancer Res 2013;19(17):4750–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dossus L, Kaaks R. Nutrition, metabolic factors and cancer risk. Best Pract Res Clin Endocrinol Metab 2008;22(4):551–571. [DOI] [PubMed] [Google Scholar]
- 13.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 14.Simon D, Balkau B. Diabetes mellitus, hyperglycaemia and cancer. Diabetes Metab 2010;36(3):182–191. [DOI] [PubMed] [Google Scholar]
- 15.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- 16.Hanover JA. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J 2001;15(11):1865–1876. [DOI] [PubMed] [Google Scholar]
- 17.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007;446(7139):1017–1022. [DOI] [PubMed] [Google Scholar]
- 18.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab 2008;19(10):380–389. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre T, Dehennaut V, Guinez C, et al. Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes and Alzheimer’s disease. Biochim Biophys Acta 2010;1800(2):67–79. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zhao X, Wu H. Metabolic Stress and Cardiovascular Disease in Diabetes MellitusThe Role of Protein O-GlcNAc Modification. Arterioscler Thromb Vasc Biol 2019:ATVBAHA119312192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab 2010;36(6 Pt 1):423–435. [DOI] [PubMed] [Google Scholar]
- 22.Copeland RJ, Han G, Hart GW. O-GlcNAcomics--Revealing roles of O-GlcNAcylation in disease mechanisms and development of potential diagnostics. Proteomics Clin Appl 2013;7(9-10):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan HB, Singh JP, Li MD, et al. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab 2013;24(6):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Y, Gao J, Han C, et al. O-GlcNAcylation is increased in prostate cancer tissues and enhances malignancy of prostate cancer cells. Mol Med Rep 2014;10(2):897–904. [DOI] [PubMed] [Google Scholar]
- 25.Mi W, Gu Y, Han C, et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta 2011;1812(4):514–519. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Mi W, Ge Y, et al. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res 2010;70(15):6344–6351. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem 2013;288(21):15121–15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 2010;35(10):547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrer CM, Sodi VL, Reginato MJ. O-GlcNAcylation in Cancer Biology: Linking Metabolism and Signaling. J Mol Biol 2016;428(16):3282–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitamura N, Iwamura T, Taniguchi S, et al. High collagenolytic activity in spontaneously highly metastatic variants derived from a human pancreatic cancer cell line (SUIT-2) in nude mice. Clin Exp Metastasis 2000;18(7):561–571. [DOI] [PubMed] [Google Scholar]
- 31.Yuan K, Jing G, Chen J, et al. Calmodulin mediates Fas-induced FADD-independent survival signaling in pancreatic cancer cells via activation of Src-extracellular signal-regulated kinase (ERK). J Biol Chem 2011;286(28):24776–24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 2001;7(8):954–960. [DOI] [PubMed] [Google Scholar]
- 33.Yuan K, Yong S, Xu F, et al. Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget 2015;6(28):25308–25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu F, Sun Y, Yang SZ, et al. Cytoplasmic PARP-1 promotes pancreatic cancer tumorigenesis and resistance. Int J Cancer 2019;145(2):474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan FK, Chun HJ, Zheng L, et al. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000;288(5475):2351–2354. [DOI] [PubMed] [Google Scholar]
- 36.Clancy L, Mruk K, Archer K, et al. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci U S A 2005;102(50):18099–18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zachara NE, O’Donnell N, Cheung WD, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 2004;279(29):30133–30142. [DOI] [PubMed] [Google Scholar]
- 38.Itkonen HM, Minner S, Guldvik IJ, et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res 2013;73(16):5277–5287. [DOI] [PubMed] [Google Scholar]
- 39.Heath JM, Sun Y, Yuan K, et al. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res 2014;114(7):1094–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krzeslak A, Forma E, Bernaciak M, et al. Gene expression of O-GlcNAc cycling enzymes in human breast cancers. Clin Exp Med 2012;12(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Donnell N, Zachara NE, Hart GW, et al. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 2004;24(4):1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuh KN, Batt AR, Zaro BW, et al. The New Chemical Reporter 6-Alkynyl-6-deoxy-GlcNAc Reveals O-GlcNAc Modification of the Apoptotic Caspases That Can Block the Cleavage/Activation of Caspase-8. J Am Chem Soc 2017;139(23):7872–7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu K, Paterson AJ, Chin E, et al. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proceedings of the National Academy of Sciences of the United States of America 2000;97(6):2820–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nature reviews Molecular cell biology 2017;18(7):452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. The Journal of biological chemistry 1999;274(45):32015–32022. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Ongusaha PP, Miles PD, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 2008;451(7181):964–969. [DOI] [PubMed] [Google Scholar]
- 47.Whelan SA, Lane MD, Hart GW. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem 2008;283(31):21411–21417. [DOI] [PMC free article] [PubMed] [Google Scholar]