Abstract
Objective:
We aimed to identify risk factors for seizures after intracerebral hemorrhage, and to validate the prognostic value of the previously reported CAVE score (0–4 points: cortical involvement, age <65, volume >10 mL, and early seizures within 7 days of hemorrhage).
Patients and Methods:
Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) was a prospective study of spontaneous intracerebral hemorrhage. We included patients who did not have a prior history of seizure and survived to discharge. Univariate analysis and multiple logistic regression modeling were used to identify risk factors for seizure.
Results:
From 2010 to 2015, 3,000 cases were recruited, and 2,507 patients were included in this study. Seizures after hospital discharge developed in 77 patients (3.1%). Patients with lobar (cortical) hemorrhage (OR 3.0, 95% CI 1.8–5.0), larger hematoma volume (OR 1.5 per cm3, 95% CI 1.2–2.0), and surgical evacuation of hematoma (OR 2.6, 95% CI 1.4–4.8) had a higher risk of late seizure, and older patients had a lower risk (OR 0.88 per 5-year interval increase, 95% CI 0.81–0.95). The CAVE score was highly associated with seizure development (OR 2.5 per unit score increase, 95% CI 2.0–3.2, p<0.0001). The CAVS score, substituting surgical evacuation for early seizure, increased the OR per unit score to 2.8 (95% CI 2.2–3.5).
Conclusions:
Lobar hemorrhage, larger hematoma volume, younger age, and surgical evacuation are strongly associated with the development of seizures. We validated the CAVE score in a multi-ethnic population, and found the CAVS score to have similar predictive value while representing the current practice of AED use.
Keywords: Intracerebral hemorrhage, seizure, epilepsy, post-stroke complication, antiepileptic drug, CAVE score
Introduction
Seizures are common following intracerebral hemorrhage (ICH), affecting at least 10% of patients in the acute and long-term period.[1-3] New-onset seizures after ICH are traditionally divided into early seizures (those occurring within 1–2 weeks of hemorrhage) and late seizures (those occurring thereafter).[4-6] Early and late seizures are thought to have different underlying mechanisms, and therefore have distinct clinical implications.[1] Furthermore, previous studies have suggested that late seizures may be associated with a higher likelihood of epilepsy.[7,8] Despite the significant disease burden of late seizures after ICH, previous studies have not reached a consensus on key risk factors for late seizures. Several studies were done before the widespread use of modern antiepileptic therapies.[1,9,10] Large scale retrospective reviews have looked at ischemic strokes or all types of strokes, but thus far only one study has proposed a risk stratification system specifically for late seizure after spontaneous ICH.[11-13]
In a retrospective analysis of the Helsinki ICH Study, Haapaniemi et al. formulated the CAVE score in an effort to quantify the risk of late seizure after ICH.[13] The 0–4 point score system assigned one point for each of the following: cortical hemorrhage, age younger than 65 years, volume greater than 10 mL, and early seizures within 7 days of hemorrhage. A higher CAVE score was associated with a greater risk of subsequent late seizure development. The investigators validated the CAVE score in a cohort of 325 ICH patients admitted to a single center in Lille, France (Prognosis of InTra-Cerebral Hemorrhage).[14] However, there has been a lack of external validation in a large, multi-ethnic population.
The purpose of our study was to identify independent risk factors for late seizure development after ICH and to validate the prognostic value of the CAVE score, using a large multi-ethnic cohort.
Materials and Methods
Study design
The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study was a multicenter prospective study of ICH with 19 recruitment centers within the United States, which recruited 1000 non-Hispanic white, 1000 non-Hispanic black, and 1000 Hispanic cases of ICH. The methodology of the ERICH study has been published previously.[15] ICH was defined as spontaneous, nontraumatic, sudden onset of headache, change in level of consciousness, or focal neurological deficit accompanied by a focal collection of blood within the brain parenchyma seen on neuroimaging or at autopsy, and not attributable to hemorrhagic conversion of cerebral infarction.[16,17] To qualify for the ERICH study, patients had to be at least 18 years of age, a resident within 75 miles of the recruiting center (100 miles for areas with <1 million population) for at least 6 months, of self-reported white, black, or Hispanic race/ethnicity, diagnosed with spontaneous ICH, and able (by self or legal representative) to provide informed consent. The study used hot-pursuit to enroll subjects, and recruitment centers reviewed emergency room, admission, and neuro-ICU admission logs to identify potential ICH cases. Although patients with a history of seizure and those who died prior to discharge were included in the ERICH study, they were excluded from the present analysis.
Standard protocol approval and patient consents
All participants or their representatives gave written informed consent. The ERICH study was approved by all local institutional review boards prior to enrollment, and conformed to ethical guidelines for human studies.
Data collection and classification
A standardized data collection protocol, including personal interview and chart abstraction, was used. Data items included demographics, patient medical history, medication and substance use prior to ICH onset, and details of the acute ICH hospitalization. CT scans of each patient were read by a central imaging core to determine ICH location and volume. Surviving patients were contacted for follow-up at 3, 6, and 12 months post discharge, and information on complications, functional status, office visits, hospitalizations, and medication use were collected. Seizures occurring during the hospitalization were recorded in the “complications” section of the chart abstraction form. For the present analysis we defined any seizure that occurred during hospitalization as “early seizure” and defined seizures that occurred after discharge, including first-ever and recurrent seizure, as “late seizure.” We identified seizures after discharge by reviewing individual follow-up interview records for any emergency room visit, hospitalization, office visit, or consultation that described a seizure or seizure-like episode. We did not include nonspecific episodes that did not have a clinician’s diagnosis of seizure or possible seizure. Treatment with an anti-epileptic drug (AED) during hospitalization was classified as use for prophylaxis (in cases where there was no diagnosis of seizure), for clinical seizure, or for both prophylaxis and subsequent treatment of seizure. For the present analysis, ICH location was classified as lobar (cortical) or non-lobar; lobar hemorrhages included ICH involving the frontal, parietal, temporal, and occipital lobes, or any combination of these areas, and non-lobar hemorrhages included those involving the deep, cerebellar, and brainstem regions. Procedures included intraventricular catheterization, ventriculostomy, and shunt placement. Surgical hematoma evacuation was considered separately.
Statistical Analysis
Descriptive statistics are presented as mean (SD), median (IQR) or frequency (percentage) depending on the type of variable. Initial analyses revealed a non-normal distribution for hematoma volume; therefore, hematoma volume was analyzed as the natural logarithm of volume plus one, and geometric mean with confidence intervals are presented as descriptive statistics. A multiple logistic regression model included variables significant (p<0.10) in univariate analysis to identify significant independent risk factors associated with late seizure after ICH by backward elimination (p<0.05). Variables that were significant in the univariate analysis but found to have dependencies on multicollinearity testing were not included in the multivariate analysis. Receiver operating characteristic (ROC) curves were generated and c-statistics calculated for late seizure models based on the CAVE score and the alternative CAVS score. Analyses were performed using SAS version 9.3 (Cary, N.C.).
Results
Of the 3000 patients with verified cases of spontaneous ICH enrolled in the ERICH study from September 13, 2010 through September 7, 2015, 178 had a history of seizure, and 315 additional patients died prior to discharge, and were therefore excluded. The remaining 2507 cases were included in the analysis. Among these cases, 205 died between discharge and three months post onset, 62 died between three and six months, and 69 died between six and twelve months; 1906 completed follow-up at three months, 1806 at six months, and 1667 at twelve months. The remaining cases were lost to follow-up. After the initial analysis of the 2507 cases of ICH, we performed a secondary analysis on 2156 cases, excluding 214 patients who died without a single follow-up and 137 patients who were lost to follow-up, to minimize selection bias.
Table 1 shows the baseline characteristics of the 2507 patients included in the analysis. Per study design, an essentially even representation of white (32.5%), black (33.6%) and Hispanic (33.9%) cases was observed. Seizures were documented in 186 (7.4%) patients during the initial hospitalization for ICH, of which 83 (3.4%) had seizure as one of the presenting symptoms. The calculated CAVE score was distributed from 0 to 4, with the majority of patients having a score of 1 or 2. One or more AED was prescribed during hospitalization in 1028 (41.0%) cases, 936 (37.3%) for prophylaxis of seizure, 161 (6.4%) for clinical seizure, and 69 (2.7%) for both prophylaxis and subsequent treatment of seizure. Levetiracetam was the most commonly used AED (915 cases), followed by phenytoin/fosphenytoin (158 cases). Upon discharge, at least one AED was prescribed in 591 (23.6%) cases. Of the 186 patients who had early seizures, 128 (68.8%) were discharged on one or more AED. At the time of the third follow-up at 12 months, 1223 of 1584 participants provided medication lists, of whom 241 (19.7%) were on at least one AED.
Table 1.
Baseline characteristics of the ERICH cohort (n=2507)
| n (%) | |
|---|---|
| Age, mean (SD) | 61.3 (14.4) |
| Female | 1030 (41.1) |
| Race/Ethnicity | |
| White | 815 (32.5) |
| Black | 842 (33.6) |
| Hispanic | 850 (33.9) |
| Heavy alcohol use (> 5 drinks per day) | 230 (9.2) |
| Diabetes | 706 (28.2) |
| Prior history of ischemic stroke | 287 (11.4) |
| Prior history of Alzheimer’s disease/ dementia | 157 (6.3) |
| GCS at presentation, median (IQR) | 15 (13, 15) |
| ICH location | |
| Lobar | 736 (29.4) |
| Non-lobar | 1714 (68.4) |
| Volume of hematoma (cc), geometric mean (95% CI) | 9.2 (8.8, 9.7) |
| Treatment with AED during hospitalization | 1028 (41.0) |
| Seizure during hospitalization | 186 (7.4) |
| Length of stay, mean (SD) | 13.4 (16.0) |
| Length of stay, median (IQR) | 8 (5, 17) |
| CAVE* score | |
| 0 | 392 (15.6) |
| 1 | 935 (37.3) |
| 2 | 852 (34.0) |
| 3 | 287 (11.4) |
| 4 | 41 (1.6) |
Early seizure defined as seizure during hospitalization
Following hospital discharge, 77 (3.1%) patients had one or more seizures within 12 months. Table 2 compares these 77 patients with the remaining group of 2430 patients with regard to risk factors associated with late seizure. On average, patients who had late seizures were about 4.4 years younger, had lower GCS at presentation, and had a larger volume of hematoma. A significantly larger proportion of patients who had late seizures were treated with an AED during hospitalization, had lobar hemorrhage, underwent catheter or shunt placement, underwent surgical evacuation, and had early seizures during the initial hospitalization. Among those who had late seizures, 13 (16.9%) had one or more early seizures during their hospital stay. In the multivariable analysis, larger hematoma volume, lobar location of ICH, surgical hematoma evacuation, AED treatment during hospitalization and younger age (OR of 0.88 per 5-year increase in age indicates that older patients had a lower risk of seizure) were associated with late seizures. AED treatment during hospitalization was excluded due to multicollinearity. Regarding race/ethnicity, the proportions of late seizures in black and Hispanic patients were not significantly different from those in white patients. Heavy alcohol use (greater than 5 drinks per day) and prior history of ischemic stroke or dementia were also not associated with the likelihood of developing late seizures.
Table 2.
Univariate and multivariate analysis of factors associated with late seizure
| No late seizure (n=2,430) |
Late seizure (n=77) |
Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value | |
|---|---|---|---|---|---|---|
| Age, mean ± SD | 61.4 ± 14.4 | 57.0 ± 12.7 | 0.98 (0.96, 0.99) | 0.0086 | 0.98 (0.96, 0.99) | 0.0044 |
| Age in 5-year intervals | 0.90 (0.83, 0.97) | 0.0086 | 0.88 (0.81, 0.96) | 0.0044 | ||
| Race/Ethnicity | ||||||
| White non-Hispanic | 792 (32.6%) | 23 (29.9%) | Reference | |||
| Black non-Hispanic | 821 (33.8%) | 21 (27.3%) | 0.9 (0.5, 1.6) | 0.6782 | ||
| Hispanic | 817 (33.6%) | 33 (42.9%) | 1.4 (0.8, 2.4) | 0.2322 | ||
| Heavy alcohol use (> 5 drinks per day) | 220 (9.2%) | 10 (13.5%) | 1.5 (0.8, 3.0) | 0.2174 | ||
| Diabetes | 691 (28.4%) | 15 (19.5%) | 0.6 (0.3, 1.1) | 0.0881 | ||
| Treatment with AED during hospitalization | 975 (40.1%) | 53 (68.8%) | 3.3 (2.0, 5.4) | <0.0001 | 1.72 (1.00, 2.96) | 0.0488 |
| GCS at presentation, median (IQR) | 15 (13, 15) | 14 (9.5, 15) | 0.91 (0.86, 0.97) | 0.0016 | ||
| Volume of hematoma (cc), Gmean (95% CI) | 9.0 (8.5, 9.4) | 22.3 (17.5, 28.4) | 2.1 (1.7, 2.7) | <0.0001 | 1.45 (1.10, 1.91) | 0.0093 |
| Lobar location of ICH | 689 (29.0%) | 47 (61.8%) | 4.0 (2.5, 6.3) | <0.0001 | 2.67 (1.57, 4.54) | 0.0003 |
| Temporal lobe ICH | 240 (10.1%) | 12 (15.8%) | 1.7 (0.9, 3.1) | 0.1123 | ||
| Procedures | ||||||
| Intraventricular catheter/ ventriculostomy/ shunt placement | 425 (17.5%) | 21 (27.3%) | 1.8 (1.1, 3.0) | 0.0292 | ||
| Surgical evacuation of hematoma | 193 (7.9%) | 24 (31.2%) | 5.2 (3.2, 8.7) | <0.0001 | 2.50 (1.38, 4.55) | 0.0027 |
| Early seizure (seizure during hospitalization) | 173 (7.1%) | 13 (16.9%) | 2.7 (1.4, 4.9) | 0.0019 | ||
| Length of stay (days), median (IQR) | 8 (5, 17) | 13 (7, 28) | 1.01 (1.01, 1.02) | 0.0015 |
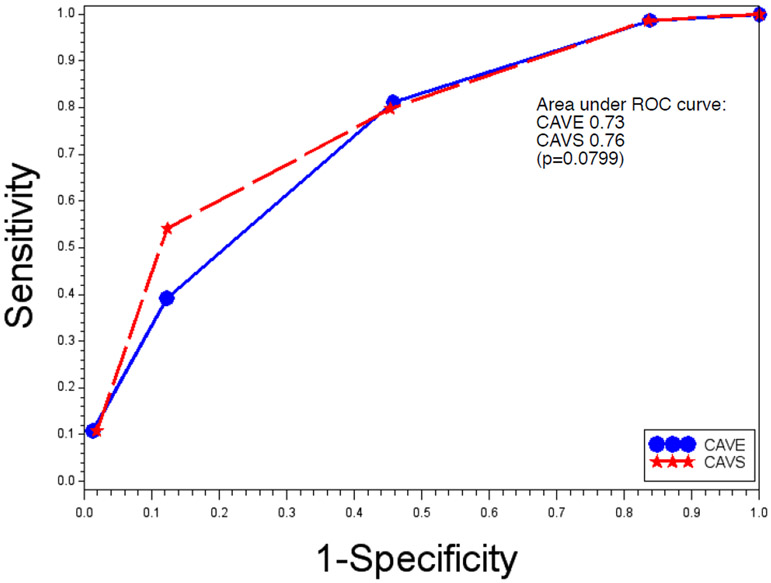
Among the 77 patients who had late seizures, only one patient had a CAVE score of 0, and 62 (80.5%) patients had a score of 2 or above. This was in contrast to the group that did not have late seizures, in which 391 (16.1%) had a score of 0 and 1118 (46.0%) patients had a score of 2 or above (Table 3). In our cohort, the risk of developing late seizures with a CAVE score of 4 was 19.5%. The negative predictive value of a CAVE score of 0 was 99.74%. The odds ratio per unit CAVE score increase was 2.5 (95% CI 2.0–3.2, p<0.0001). As early seizure did not reach independent statistical significance, we substituted the significant factor, surgical evacuation, in the place of early seizure to form a new score: CAVS. The OR per unit CAVS score increase was 2.8 (95% CI 2.2–3.5, p<0.0001). The c-statistic for CAVS was 0.76, compared with 0.73 for CAVE (difference in area under the ROC curve p=0.0799) (Figure 1).
Table 3.
CAVE* score and seizure incidence
| No late seizure (n=2,430) |
Late seizure (n=77) |
Incidence of late seizure per score** |
OR per unit score increase (95% CI) |
p-value | |
|---|---|---|---|---|---|
| CAVE* score |
2.5 (2.0, 3.2) | <0.0001 | |||
| 0 | 391 (16.1) | 1 (1.3) | 0.26% | ||
| 1 | 921 (37.9) | 14 (18.2) | 1.5% | ||
| 2 | 820 (33.7) | 32 (41.6) | 3.8% | ||
| 3 | 265 (10.9) | 22 (28.6) | 7.7% | ||
| 4 | 33 (1.4) | 8 (10.4) | 19.5% |
Early seizure defined as seizure during hospitalization
Calculated based on ERICH cohort
Figure 1.
CAVE and CAVS, receiver operating characteristic (ROC) curve
The secondary analysis on 2156 cases, excluding patients who died without follow-up and those lost to follow-up, revealed overall unchanged results. Risk factors for late seizure in this group remained the same as those in the original analysis (Table 4).
Table 4.
Secondary analysis of factors associated with late seizure, excluding patients who died without follow up or were lost to follow up (n=2156)
| No late seizure (n=2079) |
Late seizure (n=77) |
Univariate OR (95% CI) |
p-value | Multivariate OR (95% CI) |
p-value | |
|---|---|---|---|---|---|---|
| Age, mean ±SD | 60.2 (14.0) | 57.0 (12.7) | 0.98 (0.97, 1.00) | 0.0473 | 0.98 (0.96, 1.00) | 0.0209 |
| Age in 5-year intervals | 0.92 (0.84, 1.00) | 0.0473 | 0.90 (0.83, 0.98) | 0.0209 | ||
| Race/Ethnicity | ||||||
| White non-Hispanic | 654 (31.5%) | 23 (29.9%) | Reference | |||
| Black non-Hispanic | 701 (33.7%) | 21 (27.3%) | 0.9 (0.5, 1.6) | 0.6014 | ||
| Hispanic | 724 (34.8%) | 33 (42.9%) | 1.3 (0.8, 2.2) | 0.3487 | ||
| Heavy alcohol use (> 5 drinks per day) | 198 (9.7%) | 10 (13.5%) | 1.5 (0.7, 2.9) | 0.2820 | ||
| Diabetes | 581 (28.0%) | 15 (19.5%) | 0.6 (0.4, 1.1) | 0.1054 | ||
| Treatment with AED during hospitalization | 827 (39.8%) | 53 (68.8%) | 3.3 (2.0, 5.5) | <0.0001 | ||
| GCS at presentation, Median (IQR) | 15 (13, 15) | 14 (9.5, 15) | 0.90 (0.85, 0.95) | 0.0003 | ||
| Volume of hematoma (cc), Gmean (95% CI) | 8.5 (8.1, 8.9) | 22.3 (17.5, 28.4) | 2.4 (1.8, 3.1) | <0.0001 | 1.6 (1.2, 2.2) | 0.0007 |
| Lobar location of ICH | 569 (28.0%) | 47 (61.8%) | 4.2 (2.6, 6.7) | <0.0001 | 2.9 (1.7, 4.9) | <0.0001 |
| Temporal lobe ICH | 195 (9.6%) | 12 (15.8%) | 1.8 (0.9, 3.3) | 0.0795 | ||
| Procedures | ||||||
| Intraventricular catheter/ ventriculostomy/ shunt placement | 355 (17.1%) | 21 (27.3%) | 1.8 (1.1, 3.0) | 0.0225 | ||
| Surgical evacuation of hematoma | 161 (7.8%) | 24 (31.2%) | 5.4 (3.2, 9.0) | <0.0001 | 2.5 (1.3, 4.5) | 0.0041 |
| Early seizure during hospitalization | 135 (6.5%) | 13 (16.9%) | 2.9 (1.6, 5.4) | 0.0007 | ||
| Length of stay (days), Median (IQR) | 8 (5, 16) | 13 (7, 28) | 1.01 (1.01, 1.02) | 0.0017 |
Discussion
In our analysis of a large, multi-ethnic, prospective cohort, we found that the risk of developing late seizures after ICH is increased with lobar hemorrhage, large volume of hematoma, surgical evacuation, and younger age.
Multiple prior studies have established lobar (cortical) hemorrhage as a risk factor for early and late seizure development.[1,8,9,13,18] The association may be attributed to the increase in neuronal excitability and disinhibition after the alteration of the cortical structure, as evidenced by research in cellular and molecular mechanisms of epileptogenesis.[19] Various models with cortical injury reveal excessive excitatory circuits and loss of functional inhibitory inputs leading to epilepsy.[20] Volume of hematoma was also a significant factor in seizure development, consistent with several previous reports.[13,21]
Surgical hematoma evacuation was found to be an independent risk factor for seizure development in this study. While patients with larger hematoma volumes and cortically located hematomas are more likely to undergo surgery in general, the surgical evacuation itself was identified as a seizure risk factor after adjusting for multiple variables. This is an under-recognized factor, because some of the largest studies on seizures after ICH did not include surgical evacuation as a variable when evaluating seizure risk after ICH.[1,2,13] Studies that did investigate post-ICH surgical treatment found a high rate of seizures in these patients.[8,22] An open surgery can be expected to add neuronal injury to an already damaged area, increasing the risk of seizure. There is also evidence that surgery reduces mortality but increases the proportion of patients with poor functional outcome.[23] This could effectively increase the number of patients who survive to have late seizures. The result of the present study should not prevent surgeons from performing life-saving hematoma evacuations, but rather allow physicians and patients to be aware and prepared for an increased risk of late seizure.
It remains uncertain why younger age is associated with a higher risk of late seizure. This result is consistent with previous population studies.[8,13,21] It can be postulated that a greater survival in the young and higher mortality in the elderly are contributing factors.[24] Indeed, in our analysis we found that the mean age of patients who died during follow up was higher than the overall mean age, as expected. Furthermore, older patients have less motor seizures[25] and more competing diagnoses such as cognitive dysfunction, syncope, and medication adverse effects,[26] leading to underestimation of the true seizure incidence in this age group. Younger patients may have a more robust inflammatory response after a hemorrhage, resulting in greater neuronal excitability and potential for epileptogenesis.
There has been debate over early seizure as a risk factor for late seizure. Haapaniemi et al. reported an association between early seizure and late seizure, and they included early seizure in the CAVE score for estimating the risk of late seizure.[13] In contrast, several other population studies have shown a lack of such an association, but due to differences in patient populations, inclusion criteria, and analysis methods, a consensus has not been established.[1,2,10,27] In our study, we did not find an independent association of early seizure with late seizure risk. One hypothesis to explain this finding is that the majority of patients with early seizures were started on one or more AED, and were thereby effectively prevented from having late seizures. Another hypothesis is a difference in the epileptogenesis process; early seizure is caused by a space- occupying lesion and direct cellular dysfunction from blood products, while late seizure is caused by gliotic scarring, neuronal reorganization, and neurodegeneration, with the hemorrhage no longer present.[1,28] Therefore, although many patients with early seizures have subsequent late seizures due to common clinical characteristics, early seizures may not necessarily be an independent risk factor for late seizures.
Currently major guidelines do not recommend the use of prophylactic AEDs in patients with spontaneous ICH.[29,30] There is no evidence that AEDs improve outcomes.[31,32] However, it is notable that no study has looked at selective treatment allocation based on risk assessment. Prophylactic AED use could be beneficial in a select group of patients that are at high risk for late seizure development, by reducing seizure-related morbidity and recurrent hospitalization. The CAVS score is an easy and reliable risk assessment tool that can be used in future randomized controlled trials to answer this question.
The main strengths of the study include a large, multi-ethnic population and prospective enrollment of patients. To our knowledge, this is the largest study on ICH and late seizures reported so far. In addition, by study design, there was equal power to detect differences by race/ethnicity. There are limitations to this study. The incidence of late seizure was lower in our study (3.1%) compared with the incidence reported in similar studies, ranging from 2.6% to 9.5%.[1,2,13,21,33] The rate of early seizure in our study is comparable to other studies. This may reflect a more liberal use of AED prophylaxis at the time of discharge.[34,35] Also, as the data on seizures was gathered from self-reported interviews, the incidence of late seizure may have been underestimated. We did not have data on subclinical electrographic seizures. Another limitation of the study was the shorter follow up period compared to the original CAVE paper. In addition, the type and description of seizure was not available, and EEG confirmation was rarely available. A causal relationship cannot be assessed due to the methodologic limitation of an observational study.
Conclusion
The CAVE score is valid and useful in predicting the risk of seizure in a multi-ethnic population after ICH. The CAVS score, including cortical hemorrhage, younger age, greater volume of hematoma, and surgical hematoma evacuation, has similar predictive value while representing the current practice of AED use. Further investigation is warranted to identify the potential benefit of prophylactic AEDs for patients with a high CAVS score.
Highlights.
Risk factors for late seizure after ICH: cortical location, younger age, large hematoma volume, and surgical evacuation
Early seizure does not independently increase late seizure risk
CAVE score is validated in a multi-ethnic/racial cohort
CAVS has a similar predictive value and includes surgical evacuation, an independent risk factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bladin CF, Alexandrov AV, Bellavance A et al. , Seizures After Stroke: A Prospective Multicenter Study. Arch Neurol 2000;57(11): 1617–22. [DOI] [PubMed] [Google Scholar]
- [2].Rossi C, De Herdt V, Dequatre-Ponchelle N et al. , Incidence and predictors of late seizures in intracerebral hemorrhages. Stroke 2013;44(6): 1723–5. [DOI] [PubMed] [Google Scholar]
- [3].Madžar D, Kuramatsu JB, Gollwitzer S et al. , Seizures among long-term survivors of conservatively treated ICH patients: incidence, risk factors, and impact on functional outcome. Neurocrit Care 2014;21(2):211–9. [DOI] [PubMed] [Google Scholar]
- [4].Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 1993;34(4):592–6. [DOI] [PubMed] [Google Scholar]
- [5].Beghi E, Carpio A, Forsgren L et al. , Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010;51(4):671–5. [DOI] [PubMed] [Google Scholar]
- [6].Jennett B, Early traumatic epilepsy. Incidence and significance after nonmissile injuries. Arch Neurol 1974;30(5):394–8. [DOI] [PubMed] [Google Scholar]
- [7].Hesdorffer DC, Benn EKT, Cascino GD et al. , Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009;50(5): 1102–8. [DOI] [PubMed] [Google Scholar]
- [8].Qian C, Löppönen P, Tetri S et al. , Immediate, early and late seizures after primary intracerebral hemorrhage. Epilepsy Res 2014;108(4):732–9. [DOI] [PubMed] [Google Scholar]
- [9].Passero S, Rocchi R, Rossi S et al. , Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia 2002;43(10): 1175–80. [DOI] [PubMed] [Google Scholar]
- [10].Faught E, Peters D, Bartolucci A et al. , Seizures after primary intracerebral hemorrhage. Neurology 1989;39(8): 1089–93. [DOI] [PubMed] [Google Scholar]
- [11].Doria JW, Forgacs PB, Incidence, Implications, and Management of Seizures Following Ischemic and Hemorrhagic Stroke. Curr Neurol Neurosci Rep 2019; 19(7):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chi NF, Kuan YC, Huang YH et al. , Development and validation of risk score to estimate 1-year late poststroke epilepsy risk in ischemic stroke patients. Clin Epidemiol 2018;10:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Haapaniemi E, Strbian D, Rossi C et al. , The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014;45(7): 1971–6. [DOI] [PubMed] [Google Scholar]
- [14].Cordonnier C, Leys D, Dumont F et al. , What are the causes of pre-existing dementia in patients with intracerebral haemorrhages. Brain 2010;133(11):3281–9. [DOI] [PubMed] [Google Scholar]
- [15].Woo D, Rosand J, Kidwell C et al. , The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study protocol. Stroke 2013;44(10):el20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke 1990;21(4):637–76. [DOI] [PubMed] [Google Scholar]
- [17].Broderick JP, Brott T, Tomsick T et al. , Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg 1993;78(2): 188–91. [DOI] [PubMed] [Google Scholar]
- [18].De Reuck J, Hemelsoet D, Van Maele G, Seizures and epilepsy in patients with a spontaneous intracerebral haematoma. Clin Neurol Neurosurg 2007;109(6):501–4. [DOI] [PubMed] [Google Scholar]
- [19].Li H, Prince DA, Synaptic activity in chronically injured, epileptogenic sensory-motor neocortex. J Neurophysiol 2002;88(1):2–12. [DOI] [PubMed] [Google Scholar]
- [20].Prince DA, Parada I, Scalise K et al. , Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia 2009;50(Suppl. 2): 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang T-M, Lin W-C, Chang W-N et al. , Predictors and outcome of seizures after spontaneous intracerebral hemorrhage. Clinical article. J Neurosurg 2009; 111(1):87–93. [DOI] [PubMed] [Google Scholar]
- [22].Garrett MC, Komotar RJ, Starke RM et al. , Predictors of seizure onset after intracerebral hemorrhage and the role of long-term antiepileptic therapy. J Crit Care 2009;24(3):335–9. [DOI] [PubMed] [Google Scholar]
- [23].Löppönen P, Tetri S, Juvela S et al. , A population based study of outcomes after evacuation of primary supratentorial intracerebral hemorrhage. Clin Neurol Neurosurg 2013;115(8):1350–5. [DOI] [PubMed] [Google Scholar]
- [24].Rådholm K, Arima H, Lindley RI et al. , Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing 2015;44(3):422–7. [DOI] [PubMed] [Google Scholar]
- [25].Kellinghaus C, Loddenkemper T, Dinner DS, Seizure semiology in the elderly: a video analysis. Epilepsia 2004;45(3):263–7. [DOI] [PubMed] [Google Scholar]
- [26].Verellen RM, Cavazos JE, Pathophysiological considerations of seizures, epilepsy, and status epilepticus in the elderly. Aging Dis 2011;2(4):278–85. [PMC free article] [PubMed] [Google Scholar]
- [27].Serafini A, Gigli GL, Gregoraci G et al. , Are Early Seizures Predictive of Epilepsy after a Stroke? Results of a Population-Based Study. Neuroepidemiology 2015;45(1):50–8. [DOI] [PubMed] [Google Scholar]
- [28].Hunt RF, Boychuk JA, Smith BN, Neural circuit mechanisms of post-traumatic epilepsy. Front Cell Neurosci 2013;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hemphill JC, Greenberg SM, Anderson CS et al. , Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46(7):2032–60. [DOI] [PubMed] [Google Scholar]
- [30].Steiner T, Al-Shahi Salman R, Beer R et al. , European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014;9(7):840–55. [DOI] [PubMed] [Google Scholar]
- [31].Zandieh A, Messé SR, Cucchiara B et al. , Prophylactic Use of Antiepileptic Drugs in Patients with Spontaneous Intracerebral Hemorrhage. J Stroke Cerebrovasc Dis 2016;25(9):2159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Spoelhof B, Sanchez-Bautista J, Zorrilla-Vaca A et al. , Impact of antiepileptic drugs for seizure prophylaxis on short and long-term functional outcomes in patients with acute intracerebral hemorrhage: A meta-analysis and systematic review. Seizure 2019;69:140–6. [DOI] [PubMed] [Google Scholar]
- [33].Biffi A, Rattani A, Anderson CD et al. , Delayed seizures after intracerebral haemorrhage. Brain 2016;139(Pt 10):2694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Beghi E, Efficacy and tolerability of the new antiepileptic drugs: comparison of two recent guidelines. Lancet Neurol 2004;3(10):618–21. [DOI] [PubMed] [Google Scholar]
- [35].Sheth KN, Martini SR, Moomaw CJ et al. , Prophylactic Antiepileptic Drug use and Outcome in the Ethnic/Racial Variations of Intracerebral Hemorrhage Study. Stroke 2015;46(12):3532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]