Abstract
Transient receptor potential canonical (TRPC) channels constitute a group of receptor-operated calcium-permeable nonselective cation channels of the TRP superfamily. The seven mammalian TRPC members, which can be further divided into four subgroups (TRPC1, TRPC2, TRPC4/5, and TRPC3/6/7) based on their amino acid sequences and functional similarities, contribute to a broad spectrum of cellular functions and physiological roles. Studies have revealed complexity of their regulation involving several components of the phospholipase C pathway, Gi and Go proteins, and internal Ca2+ stores. Recent advances in cryogenic electron microscopy have provided several high-resolution structures of TRPC channels. Growing evidence demonstrates the involvement of TRPC channels in diseases, particularly the link between genetic mutations of TRPC6 and familial focal segmental glomerulosclerosis. Because TRPCs were discovered by the molecular identity first, their pharmacology had lagged behind. This is rapidly changing in recent years owning to great efforts from both academia and industry. A number of potent tool compounds from both synthetic and natural products that selective target different subtypes of TRPC channels have been discovered, including some preclinical drug candidates. This review will cover recent advancements in the understanding of TRPC channel regulation, structure, and discovery of novel TRPC small molecular probes over the past few years, with the goal of facilitating drug discovery for the study of TRPCs and therapeutic development.
Keywords: Calcium signaling, Drug discovery, Heterotrimeric G proteins, Nonselective cation channels, Phospholipase C, Receptor-operated channels
1. Introduction
1.1. The discovery and classification of TRPCs
The transient receptor potential (TRP) ion channels are named after the founding member of this superfamily that underlies the trp phenotype of the Drosophila phototransduction mutant that loses the sustained response to light stimulus (Cosens & Manning, 1969). Molecular cloning of the disrupted gene later revealed the encoded product to be a membrane protein that shares limited sequence homology with voltage-gated Na+ and Ca2+ channels (Montell & Rubin, 1989; Wong et al., 1989). However, it was not until 1992 when the channel function of the fly TRP protein was first demonstrated (Hardie & Minke, 1992) and this was followed by reconstituting the ion channel function of a closely related Drosophila homology, TRP-Like (TRPL) (Phillips, Bull, & Kelly, 1992) in heterologous systems (Hu et al., 1994; Vaca, Sinkins, Hu, Kunze, & Schilling, 1994). In 1995, the first mammalian TRP homolog (TRPC1) was reported without functional demonstration (Wes et al., 1995; Zhu, Chu, Peyton, & Birnbaumer, 1995). In the following year, five more related mammalian sequences (TRPC2–6) were revealed with the functionality of TRPC1 and TRPC3 implicated in receptor- or store-operated Ca2+ entry (Zhu et al., 1996). Finally, the last member, TRPC7, was reported three years later (Okada et al., 1999). In the meantime, many distantly related TRP homologous were also uncovered between 1997 and 2003, expending the superfamily to 28 mammalian members and six subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin). In invertebrates, there is yet another subfamily, TRPN (Drosophila NOMPC), which has no mammalian members (Montell et al., 2002).
The TRP channels are mostly Ca2+-permeable non-selective cation channels with few exceptions. For example, TRPV5 and TRPV6 are highly Ca2+ selective while TRPM4 and TRPM5 are Ca2+ impermeable. The majority of the TRP channels function at the plasma membrane (PM), but a few of them mainly work on membranes of intracellular organelles, such as endosomes and lysosomes (Dong et al., 2008; Dong et al., 2010). Unlike other TRP subfamilies, which were discovered based on functional screening or genetic linkage to disease, the mammalian TRPC members were identified strictly because of their sequence homology with the prototypical Drosophila TRP and TRPL proteins and all of them share about 30–35% amino acid sequence identity with TRP and TRPL across almost the entire length, rather than in just limited regions. Therefore, functionally, the mammalian TRPC members are also similar to the Drosophila TRP and TRPL in that they are all activated downstream from receptors that signal through phospholipase C (PLC) (Trebak, Vazquez, Bird, & Putney Jr, 2003; Tian et al., 2014; Bavencoffe, Zhu, & Tian, 2017). However, unlike the restricted expression in photoreceptors of the insect channels, mammalian TRPC channels are widely expressed in numerous cell types of many different tissues, displaying tremendous diversity in expression patterns and functions.
Although TRPC channels had been considered as the top molecular candidates that mediate capacitative or store-operated Ca2+ entry in the early days. This idea has run out of fashion after the identification of STIM1 and Orai1 in 2005–2006 (Feske et al., 2006; Liou et al., 2005; Vig et al., 2006; Zhang et al., 2005; Zhang et al., 2006), which encode the sensor that detects Ca2+ depletion from the endoplasmic reticulum (ER) store and the PM channel that mediates the Ca2+-release-activated Ca2+ (CRAC) current, respectively. Although evidence continues to accumulate for store-, or STIM-, or even Orai-operated or dependent TRPC channel function (see later), it is clear that TRPC proteins most likely do not participate in the formation of the highly Ca2+-selective CRAC channel. Rather, these proteins form nonselective cation channels with variable Ca2+ permeabilities and complex regulatory mechanisms that allow them to sense changes in various aspects of PLC signaling, including but not limited to the filling state of the ER Ca2+ store. The activation of TRPC channels mainly leads to Na+ and Ca2+ influx, causing two major consequences: membrane depolarization and cytosolic Ca2+ concentration ([Ca2+]c) elevation, both having important impacts on cellular function. Based on sequence homology, the seven mammalian members of the TRPC subfamily (TRPC1–7) fall into four subgroups: TRPC1, TRPC2, TRPC4/5, and TRPC3/6/7. Since TRPC2 is a pseudogene in humans, this review mainly focuses on recent studies of TRPC1, TRPC4/5 and TRPC3/6/7.
1.2. Tissue distribution and multimerization of TRPCs
Due to the lack of good quality antibodies, the tissue/cell type distributions of TRPC isoforms have mainly been examined based on mRNA (Fowler, Kyriaki, Ozkan, Phillips, & Cooper, 2007; Li, Xu, & Montell, 1999; Riccio et al., 2002). In addition, functional studies have yielded ample useful information about the contributions of individual TRPC isoforms in physiology and pathophysiology of certain tissue/cell types. These include studies using isoform specific Trpc knockout mice (Freichel et al., 2005), naturally occurring TRPC mutations in humans and rodents (Becker et al., 2009; Reiser et al., 2005; Winn et al., 2005), and some of the newly developed small molecular TRPC agonists and antagonists (Just et al., 2018; Seo et al., 2014; Yang et al., 2015; Zhou et al., 2017). Some of these studies will be discussed in greater details in the later part of this review. Here, we briefly summarize the findings from studying the expression of TRPC mRNAs, and in some cases proteins, as well as the interactions among different TRPC isoforms.
TRPC1 exhibits extensive expression in different tissues (Wes et al., 1995; Zhu et al., 1995). It is generally thought that TRPC1 may not form homomeric channels, but it serves as an auxiliary subunit in heteromeric channels containing other TRPC subtypes (Lintschinger et al., 2000; Sours-Brothers, Ding, Graham, & Ma, 2009; Strübing, Krapivinsky, Krapivinsky, & Clapham, 2001), or even more distantly related TRPP2 (Tsiokas et al., 1999), TRPV4 (Ma et al., 2010), and TRPV6 (Schindl et al., 2012). Functionally, the best characterized effect of TRPC1 on the biophysical properties of the heteromeric channels is the marked reduction of the unitary conductance of TRPC4 and TRPC5, especially at negative potentials, which also leads to a change in the current-voltage (I-V) relationship when comparing between TRPC1-TRPC4/5 heteromers and TRPC4 (or C5) homomers (Strübing et al., 2001). In neurons, TRPC1 is often found to heteromultimerize with TRPC4, or TRPC4 and TRPC5 together (Bröker-Lai et al., 2017; Phelan et al., 2013; Stroh et al., 2012).
TRPC2 is found to be highly expressed in vomeronasal organs of rodents (Liman, Corey, & Dulac, 1999) and pivotal for sex discrimination and male-male aggression (Leypold et al., 2002; Stowers, Holy, Meister, Dulac, & Koentges, 2002). Its functions in erythroid cells (Chu et al., 2004) and sperm acrosome reaction during fertilization (Jungnickel, Marrero, Birnbaumer, Lémos, & Florman, 2001) in mice have also been reported. These functions are likely carried out by other genes in people since TRPC2 is a pseudogene in humans (Zhu et al., 1996).
TRPC3 has been shown to be a multifunctional cellular sensor with a broad range of physiological/pathological functions. It is expressed in many tissues, but compared to brain, the expression levels are relatively low in peripheral tissues (Zhu et al., 1996). In the brain, TRPC3 is most prominently expressed in pituitary gland and Purkinje cells of the cerebellum (Hartmann et al., 2008; Riccio et al., 2002). TRPC3 expression has also been shown in heart (Goel, Zuo, Sinkins, & Schilling, 2007) and lungs of patients with idiopathic pulmonary arterial hypertension (IPAH) but not that of healthy individuals (Yu et al., 2004). TRPC3 is closely related to TRPC7 and to a lesser degree also TRPC6. These three subgroup members can form either homotetrameric channels or heterotetrameric channels by complexing among themselves in both native and heterologous expression systems (Trebak et al., 2003). It was shown that in rat brain synaptosomes, TRPC3 forms complexes with TRPC6 and C7 but not other TRPC members (Goel, Sinkins, & Schilling, 2002) and in heterologous systems, the expressed TRPC3 only interacts with TRPC6 and C7 but not TRPC1/C4/C5 (Hofmann, Schaefer, Schultz, & Gudermann, 2002). However, there is also evidence that TRPC3 forms a complex with TRPC1 (Liu, Bandyopadhyay, Singh, Groschner, & Ambudkar, 2005) or TRPC4 (Poteser et al., 2006). Therefore, the ability of TRPC3 to heteromerize with other TRPC members may be highly variable in different systems and under different conditions.
The mRNA of TRPC4 was found to be highly abundant in the corticolimbic regions of the brain (Fowler et al., 2007). In addition, it is also present in midbrain dopaminergic neurons of the ventral tegmental area and the substantia nigra (Illig, Varnell, Ostertag, Klipec, & Cooper, 2012). TRPC4 mRNA is also highly enriched in bones (Riccio et al., 2002). However, many of the well-characterized functions of TRPC4 are outside of these areas, suggesting broad expression and function (Fu, Gao, Shen, & Zhu, 2015; Mederos et al., 2018).
The mechanism of TRPC4 channel assembly had been studied using glutathione S-transferase pull-down, co-immunoprecipitation of expressed channel fragments and chimera with TRPC6 (Lepage et al., 2006). N- to N-terminal interactions at regions encompassing residues E87–H172 and D254–P304 and a N- to C-terminal interaction involving residues I627–S952 were identified. The multimerization of TRPC4 has also been studied using deletion mutations followed by patch-clamp recording to evaluate the channel function and Förster resonance energy transfer (FRET) to assess protein-protein interaction. It was found that the assembly of TRPC4 tetramers requires regions downstream of amino acid 99 (Ala) at the N-terminus and upstream of residue 730 (Gly) at the C-terminus (Myeong, Kwak, Hong, Jeon, & So, 2014). In addition, while the same N-terminal coiled-coil domain is crucial for heteromultimerization between TRPC1 and C4 or TRPC1 and C5, slightly different TRPC1 C-terminal regions are involved in the assembly with C4 and C5 (Myeong et al., 2016).
TRPC5 is mainly expressed in brain tissues (Fowler et al., 2007; Okada et al., 1998; Philipp et al., 1998; Riccio et al., 2002), where functions in neurite growth, neurotransmission and learning have been clearly indicated (Bröker-Lai et al., 2017; Greka, Navarro, Oancea, Duggan, & Clapham, 2003; Phelan et al., 2013; Riccio et al., 2009). In some cases, TRPC5 exhibits similar functions as TRPC1/C4 (Bröker-Lai et al., 2017; Riccio et al., 2009; Riccio et al., 2014), but in other cases, the functions appear to be distinct (Phelan et al., 2013; Stroh et al., 2012). Like in the case of TRPC4, despite the low transcript levels found in early studies, TRPC5 has also been implicated to play important functions in peripheral tissues, including kidney (Zhou et al., 2017), the cardiovascular system (Lau et al., 2016), and cancer (Ma et al., 2012). Particularly, TRPC5 was found to serve a key role in sensing pressure at the aortic baroreceptors and thereby help stabilize blood pressure (Lau et al., 2016). In carotid arteries, TRPC5 serves a pivotal role in endothelium-dependent contraction (Liang et al., 2019). Uniquely, TRPC5 has been shown to underlie the upregulation of chemotherapy-induced multidrug resistance by promoting expression of p-glycoprotein and ATP-binding cassette subfamily B member 1 (ABCB1) in different types of cancers (Ma et al., 2012; Wang et al., 2015). In addition, TRPC5 not only promotes the formation of extracellular vesicles from breast cancer cells but is also contained in these circulating vesicles, from which it invades nonresistant cells to confer chemoresistance by stimulating p-glycoprotein expression (Ma et al., 2014). Moreover, a role for TRPC5 in angiogenesis has also been recently reported (Zhu et al., 2019).
TRPC6 is highly expressed in placenta, heart, lung, pancreas, kidney, and many areas of the brain (Riccio et al., 2002). In the nervous system, TRPC6 exhibits wide expression in extrinsic fibers innervating intrinsic cardiac ganglia (Calupca, Locknar, & Parsons, 2002), olfactory epithelium neurons (Elsaesser, Montani, Tirindelli, & Paysan, 2005), retinal ganglion cells (Warren, Allen, Brown, & Robinson, 2006), and many regions of the brain, such as the cortex, hippocampus, substantia nigra, and cerebellum (Sun, Sukumaran, Bandyopadhyay, & Singh, 2014). In the kidney, TRPC6 is found in glomeruli, specific tubular cells of the cortex and both the outer and inner medulla, as well as podocytes (Goel, Sinkins, Zuo, Estacion, & Schilling, 2006). In the lung, it is found in human airway smooth muscle and both undifferentiated and differentiated bronchial epithelial cells (Corteling et al., 2004).
In mice, TRPC7 mRNA is highly abundant in the heart, lung, and eye and detectable at lower levels in the brain, spleen, and testis (Okada et al., 1999). In human samples, TRPC7 mRNA is prominently found in the kidney, pituitary gland, and multiple regions of the brain (Riccio et al., 2002). It has been common that results about TRPC mRNA expression from different studies do not match. This could result from differences in sample preparation, e. g. some of the human tissue samples were from tumor instead of normal tissues, methods used for mRNA detection, Northern blotting vs. RT-PCR, species and age differences, and etc.
2. Structure and functional regulation
2.1. High resolutions structures of TRPCs
High resolution structures of TRPCs were not available until 2018. Owning to the great advancement in single-particle cryogenic electron microscopy (cryo-EM) technology, high-resolution structures of many previously unattainable receptor classes, including that of TRPCs, have been resolved to near 3Å (Fig. 1). Compare to traditional X-ray crystallography, cryo-EM does not require crystallization and/or diffraction, and only a small amount of protein/protein complex is required. Therefore, structure determination of membrane proteins, particularly various ion channels, is greatly facilitated [see recent review by Groschner & Tiapko, 2018]. The current cryo-EM structures include homomeric TRPC3, TRPC4, TRPC5 and TRPC6 (Duan et al., 2019; Fan et al., 2018; Vinayagam et al., 2018; Duan et al., 2018; Tang et al., 2018; Azumaya, Sierra-Valdez, Cordero-Morales, & Nakagawa, 2018; Sierra-Valdez, Azumaya, Romero, Nakagawa, & Cordero-Morales, 2018). The overall architectures of these TRPCs are similar, all showing tetrameric structures with six transmembrane α helices in each subunit and the cytoplasmic N-termini surrounding the C-termini. The first four transmembrane α helices (S1–S4) form a voltage sensor-like domain similar to the voltage sensor domains of voltage-gated K+, Na+ and Ca2+ channels, and the last two α helices (S5–S6) form a structurally conserved ion conducting or pore domain common to all TRP channels, voltage-gated channels, inwardly rectifying K+ channels, and bacterial K+ and Na+ channels. In the tetrameric structures, the voltage sensor-like domain of one protomer is domain-swapped to interact with the pore domain of the neighboring protomer and the four pore domains join at the center of the tetrameric complex to form the ion conducting pore. This arrangement is also very common in TRP channels and voltage-gated ion channels.
Fig. 1. Structures of TRPC channels.
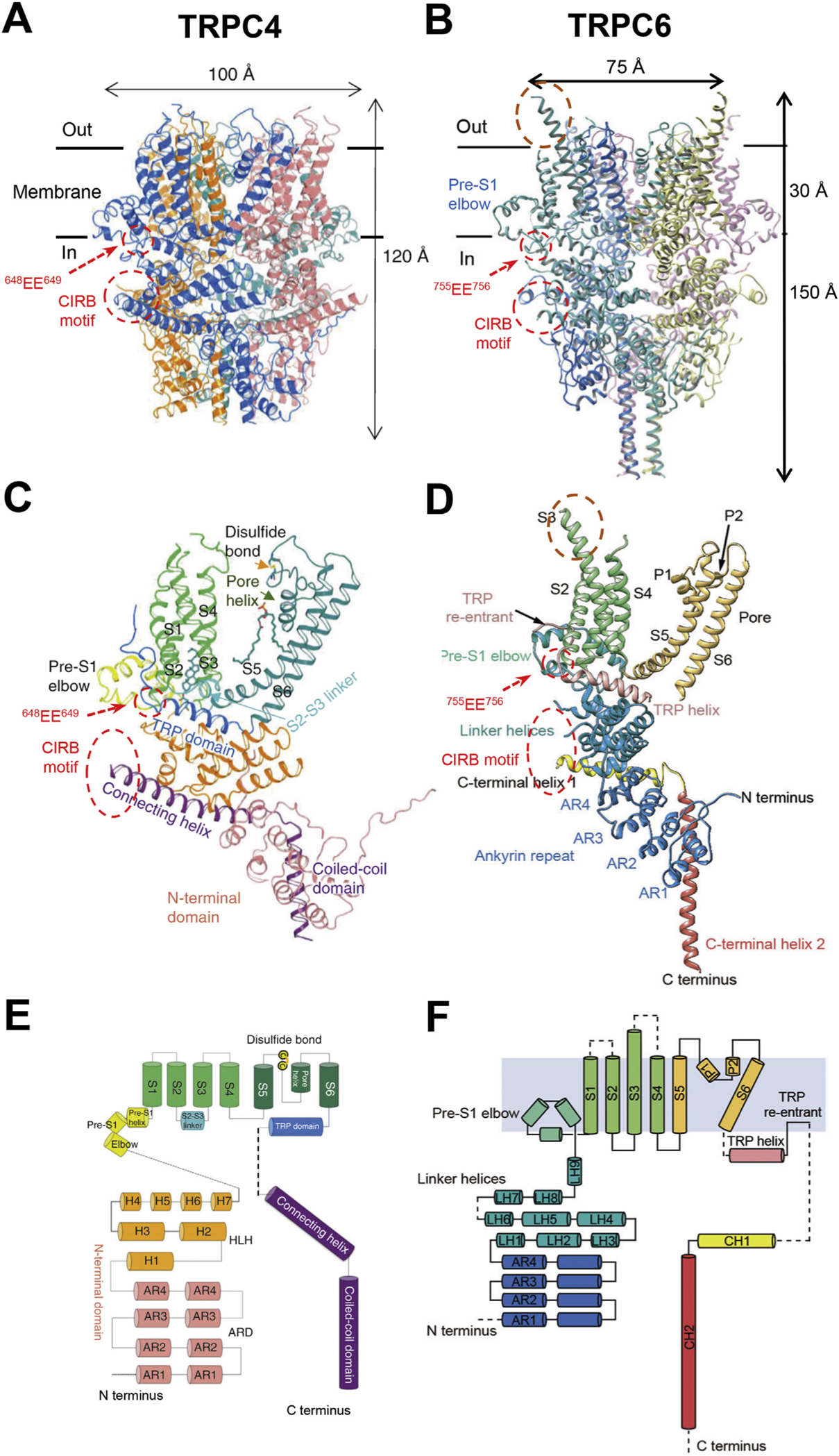
A & B, cryo-EM structures of C terminus truncated mouse TRPC4 (aa 1–758) (A) and full-length human TRPC6 (aa 1–931) (B), as reported by Duan et al. (2018) and Tang et al. (2018), respectively. The transmembrane regions are defined by the horizontal black lines with the thickness of about 30 Å. Areas of the Calmodulin- and IP3 receptor-binding (CIRB) motifs and the two conserved acidic residues (EE) critical for regulation by STIM1 are indicated by the dashed red circles. The extracellular protrusion of S3 transmembrane helix in TRPC6 is encircled by brown dashed line. Note the missing structures between the TRP re-entrant loop and CIRB motif in both examples. C & D, ribbon diagrams of single subunits of TRPC4 (C) and TRPC6 (D). TRP domain (in C) is equivalent to TRP helix (in D); Connecting helix and coiled-coil domain (in C) are equivalent to C terminal helices 1 and 2 (in D), respectively. E & F, topology and domain organization of TRPC4 (E) and TRPC6 (F) single subunits. Cylinders indicate α helices; dashed lines highlight unresolved structures.
The ion conducting pore is formed by the S5 and S6 helices and an intervening pore (P) loop in a symmetric arrangement from all four protomers. The narrowest part or channel gate is found at the bundle near the C-terminal ends of the four S6 helices and for nearly all the TRPC structures, they include isoleucine (I), asparagine (N), and glutamine (Q) from the highly conserved sequence VLLNMLIAMXNXSXQ, except for TRPC3 in which L654 and I658, one residue before the commonly found I and N, respectively, were shown to form the most restricted path (Fan et al., 2018). The radiuses of the lower gates of the resolved TRPC structures are all <1 Å, indicating closed conformations. The narrowest part of the selectivity filter was formed by a conserved glycine, which is three residues apart from the glutamate (E630 in TRPC3 and E686 in TRPC6) situated in the entrance of the selectivity filter and shown for TRPC3 to be critical for the Ca2+ permeability of the channel (Poteser et al., 2011). Interestingly, this residue is substituted by an asparagine in TRPC4 (N580) and TRPC5 (N584), for which TRPC5-N584 has been shown to be important for the Ca2+ permeability and Gd3+ sensitivity [Chen et al., 2017; Duan et al., 2019].
All TRPC structures contain a three helical region designated as pre-S1 elbow and pre-S1 helix before the transmembrane S1 helix (Fig. 1). This region is halfway embedded in the membrane, with its N-terminal end exposed to the cytoplasmic side to connect with a long stretch of tightly folded linker helices located at the proximal N-terminus of each protomer. Immediately before the linker helices are the four ankyrin-like repeats that form the outskirt of the cytoplasmic architecture, which completely surrounds the four helical bundle composed of the second C-terminal helix (CH2) as designated by some (Azumaya et al., 2018; Tang et al., 2018), which is also referred to coiled-coil (Duan et al., 2018; Duan et al., 2019) or pore helix (Fan et al., 2018) domains by other groups, from the four protomers running in parallel. The CH2 domain is preceded by CH1 (also known as connecting helix (Duan et al., 2018, Duan et al., 2019) or rib helix (Vinayagam et al., 2018)) via a short loop that crosses over the adjacent protomer such that the CH2 domain is in close contact with the ankyrin repeats of the same protomer. The knots at the crossovers and the four helical bundle formed by CH2 may help stabilize the tetrameric structure. On the other hand, the CH1 domain runs near perpendicularly (~100–120°) to CH2, but in parallel with the membrane, into a cavity between the linker helices and ankyrin repeats of a neighboring protomer. Separated by the linker helices, the CH1 domain also runs anti-parallelly with the TRP domain, a well-conserved region in all TRP channels thought to be critical for channel gating. Between the TRP and CH1 domain is a TRP re-entrant helix, which is halfway embedded into the membrane and in some cases looks more like a short open loop rather than a helix (Duan et al., 2019; Tang et al., 2018), and a stretch of unresolved region exposed to the cytoplasm (dashed lines in Fig. 1E, F).
The C-terminal end of the unresolved region and the beginning of CH1 contain the characteristic CIRB (Calmodulin and IP3 Receptor Binding) motif found in all TRPC isoforms, including the invertebrate TRP and TRPL (Tang et al., 2001; Zhang et al., 2001). Interestingly, this motif also interacts with phosphoinositides and inositol polyphosphates (TRPC6) (Kwon, Hofmann, & Montell, 2007), Gαi/o proteins (TRPC4/5) (Jeon et al., 2012), and SEC14 and Spectrin Domain Containing 1 (SESTD1), a Ca2+-dependent phospholipid/cytoskeleton-binding protein (TRPC4/5) (Miehe et al., 2010). The fact that it is exposed to the cytoplasmic environment gives the CIRB motif the opportunity to interact with different partners, making it a hotspot for regulation (Fig. 1A–D). As the major portion of the CIRB sequence is actually included in CH1, it is possible that the binding of CIRB motif by its protein partner presses the CH1 domain like a lever to cause conformation changes near the lower gate. Open structures and structures with CIRB motif binding partners will likely reveal important insights into how relative motions between CH1, TRP domain, and S4–S5 linker, which likely interacts with the TRP domain, are involved in TRPC channel gating. Notably, at the exposed side connected to the unresolved region, the CH1 domain begins ~3–4 residues (one turn) earlier in TRPC4/5 than in TRPC3/6 [compare Vinayagam et al., 2018; Duan et al., 2019; with Tang et al., 2018; Azumaya et al., 2018]. This may give rise to the wider overall shape of TRPC4/5 (100 to 105 Å in diameter) (Duan et al., 2018; Vinayagam et al., 2018) and the skinner look of TRPC3/6 (75–85 Å in diameter) (Fan et al., 2018; Tang et al., 2018) (Fig. 1A, B).
The presence of the pre-S1 elbow and the TRP re-entrant helix makes TRPC structures resemble TRPMs and NOMPC more than TRPA and TRPVs (Jin et al., 2017; Guo et al., 2017; Autzen et al., 2017; Yin et al., 2017; Huang, Winkler, Sun, Lü, & Du, 2018; Wang et al., 2018). It was found that the pre-S1 elbow and pre-S1 helix pull the intracellular half of the S1 transmembrane helix away from the pore center, creating a window to expose the intracellular half of S4 helix and the S4–S5 linker to the lipid environment (Fan et al., 2018). This may be important for lipid gating. However, although densities for lipids were found in the TRPC3 structure, the resolution was not high enough to determine their identity (Fan et al., 2018). One of these lipids indeed interacts with pre-S1 elbow, S1, S4 and S4–S5 linker of TRPC3 and a mutation here (T561A on S4) has been reported to enhance TRPC3 channel function, causing cerebellar ataxia in mice (Becker et al., 2009). The second lipid was found to be wedged between the P loop and S6 of the neighboring protomer. Because G640 on S6, which interacts directly with the hydrocarbon tail of the lipid, was showed to be critical for lipid gating of TRPC3 (Lichtenegger et al., 2018), this site may be accessed by lipids from the extracellular side to activate the channel. Lipid densities were also found in other studies of TRPC structures and these were identified as cholesteryl hemisuccinate and phosphatidic acid (Duan et al., 2018; Tang et al., 2018; Vinayagam et al., 2018).
Unique to TRPC4 and C5 are the presence of an extracellular disulfide bond between two cysteines that are five residues apart located within the linker between S5 and the pore helix of the same protomer (Fig. 1C, E) and an intracellular cation (most likely Na+) binding site formed by two residues (E417, Q420 of C4; E418, E421 of C5) at the last two helical turns of S2 and another two (N435, D438 of C4; N436, D439 of C5) at the beginning two helical turns of S3 (Fig. 2). While the disulfide bond has been shown to be pivotal for redox sensing of TRPC4 and C5 (Duan et al., 2018; Xu et al., 2008), the functional significance of the Na+ binding site remains undefined. Ironically, in whole-cell voltage clamp recordings, TRPC4 and C5 have often exhibited larger inward currents when the cell was bathed in the Cs+- than Na+-based bath solutions (Jeon et al., 2008; Sung et al., 2011). This feature has been utilized to reveal constitutive channel activities that were otherwise undetectable in the normal Na+-based physiological solution (Jeon et al., 2012; Jeon, Thakur, Tian, So, & Zhu, 2016). It may be possible that the Na+ binding site mediates inhibition of TRPC4/5 by Na+ flowed into the cell through the open channels.
Fig. 2. Intracellular Na+binding sites identified from TRPC4 (left) and TRPC5 (right) cryo-EM structures.
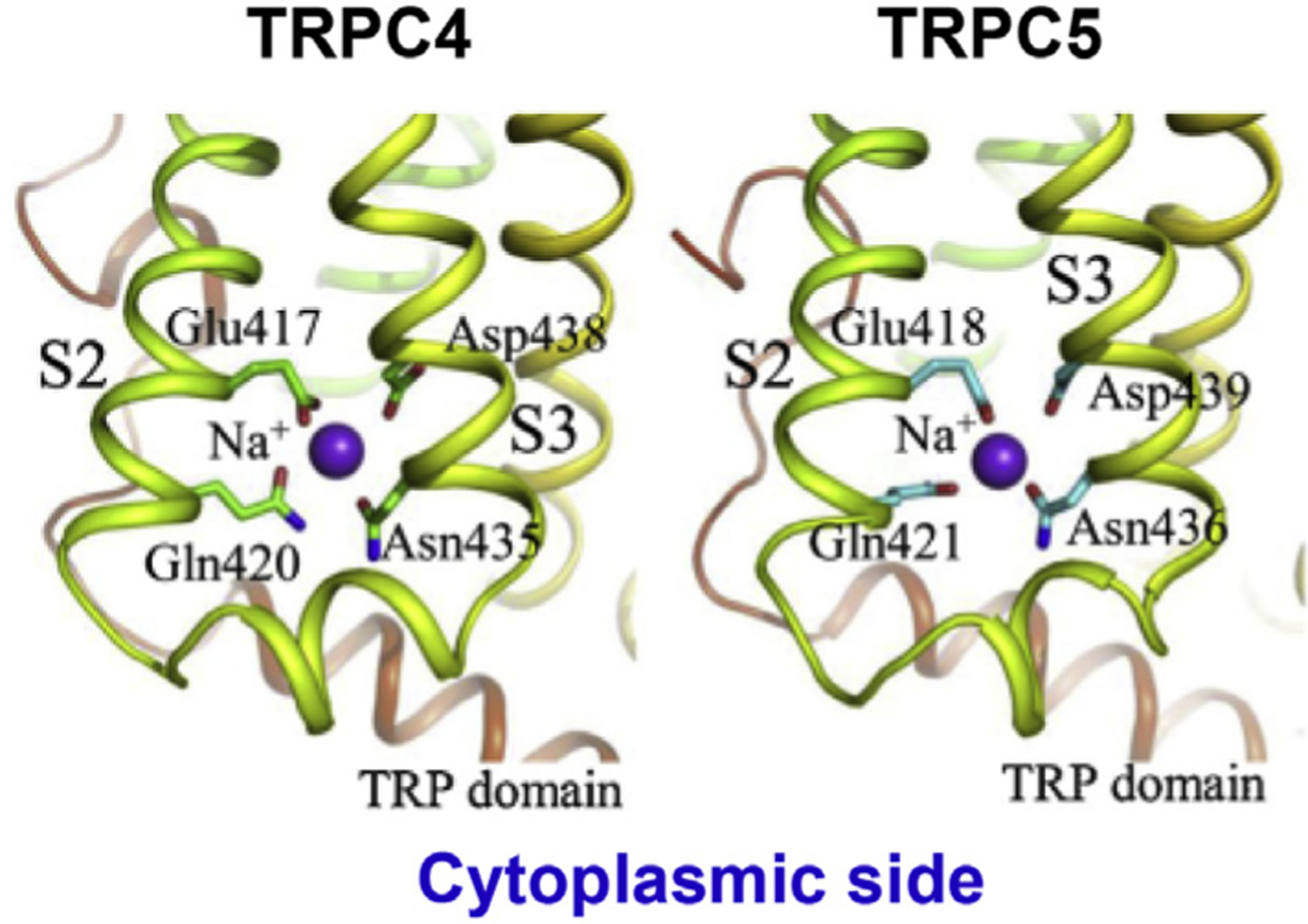
S2 and S3 depict the second and third transmembrane helices, respectively. Adopted from Li et al. (2019) with modifications.
Unique to TRPC3 and C6 is the extended S3 helix at the extracellular side, which extends about four helical turns longer than that of TRPC4 and C5 (Fig. 1B, D). This protrusion from the membrane surface may help support an extracellular pocket potentially targetable by small molecules (Fan et al., 2018; Tang et al., 2018). Clearly, much remained to be learned from these structures. It is anticipated that these high-resolution structures will greatly facilitate the study of structural and functional relationships of TRPCs and the development of drugs targeting these channels.
2.2. Mechanism of activation of TRPC channels
2.2.1. The PLC pathway
2.2.1.1. Diacylglycerols.
Like the prototypical Drosophila TRP and TRPL, mammalian TRPC channels have been shown to be activated downstream from receptors that signal through PLC (Liu & Montell, 2015; Tian et al., 2014; Trebak et al., 2003). This may occur through activation of Gq/11-coupled receptors and receptor tyrosine kinases. However, the PLC pathway consists of many steps or constituents, including hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) at the PM, production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerols (DAG), activation of IP3 receptors at the ER membrane, Ca2+ store depletion, and [Ca2+]c increase. It has become clear that several of these steps are involved in TRPC channel gating (Fig. 3A). The first clue came from the study showing that both TRPC3 and C6 could be directly activated by diacylglycerols (Hofmann et al., 1999). Both the more natural 1-stearoyl-2-arachidonyl-sn-glycerol (SAG) and synthetic DAG analog 1-oleoyl-2-acetyl-sn-glycerol (OAG) were reported to be able to activate TRPC3 and C6 channels when applied either from the extracellular or cytoplasmic sides; however, they did not activate TRPC4 or C5 (Hofmann et al., 1999). Subsequently TRPC2 and TRPC7 were also shown to be activated by DAG (Lucas, Ukhanov, Leinders-Zufall, & Zufall, 2003; Okada et al., 1999). Only recently, it was shown that TRPC4/5 are also DAG-regulated channels, but the lipid sensitivity was masked by a multiple PDZ domain protein, Na+/H+ exchanger regulatory factor (NHERF) (Storch et al., 2017), which binds to the C-terminal ends for TRPC4/5 (Tang et al., 2000) (Fig. 3C). Upon removal of NHERF binding by phosphorylation of a threonine residue in the PDZ-binding motif of TRPC4/5 by protein kinase C, the DAG sensitivity appears, indicating a dynamic regulatory mechanism (Storch et al., 2017). Thus, DAGs appear to be direct activators of all mammalian TRPC channels.
Fig. 3. Mechanism of activation of TRPC channels.
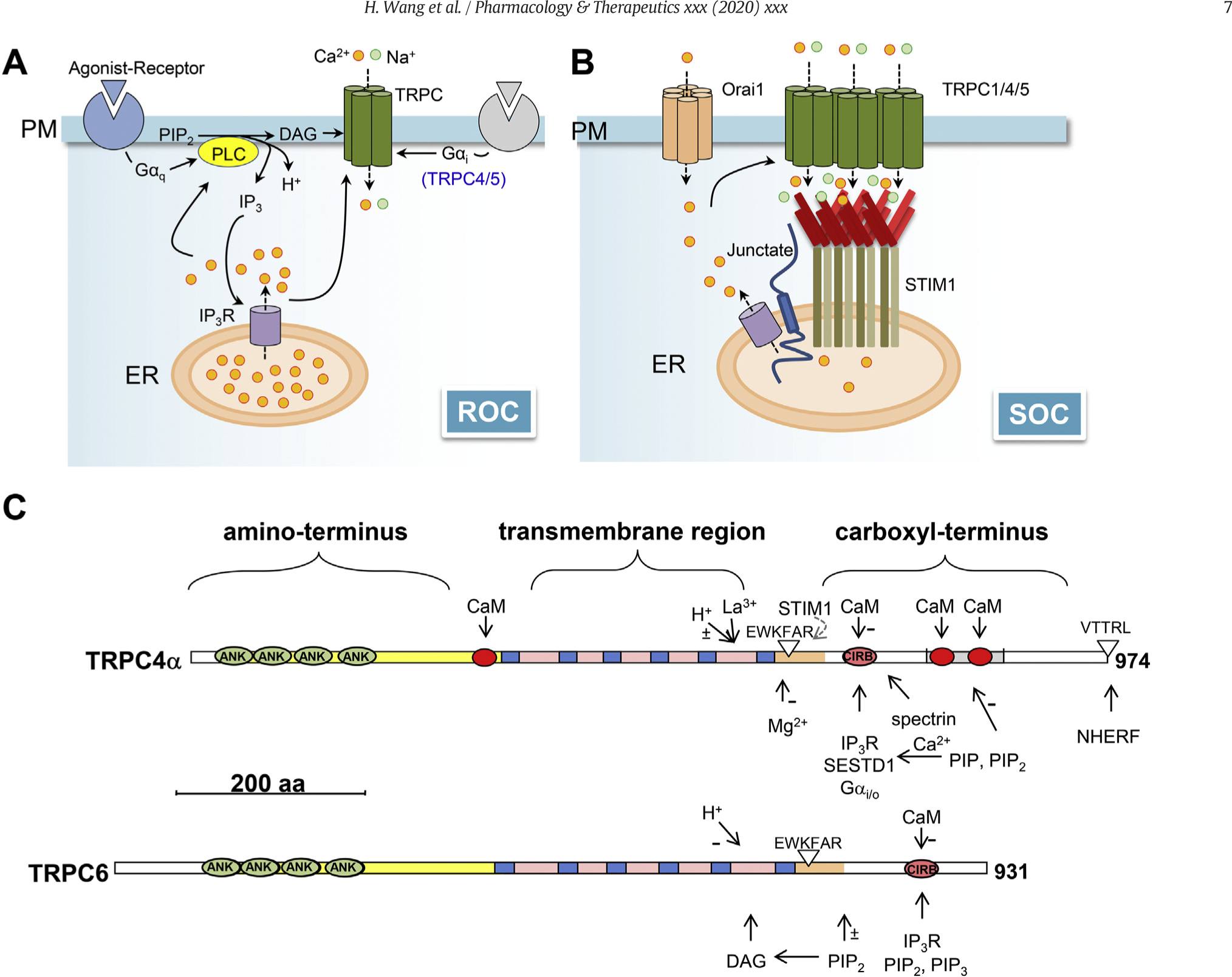
A, receptor-operated channel (ROC) activation of TRPCs. Independently of STIM1, receptor activation leads to PLC hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and production of inositol 1,4,5-trisphosphate (IP3), diacylglycerols (DAG), and protons (H+). All of them have been implicated in the regulation of TRPC channel function. In addition, IP3 activates IP3 receptors (IP3R) on the endoplasmic reticulum (ER) membrane to release stored Ca2+, causing [Ca2+]c to increase. Ca2+ regulates TRPC channels in both calmodulin (CaM)-dependent and -independent manners. Specifically for TRPC4 and C5, the activation of Gi/o proteins also triggers channel activation in conjunction with PLC stimulation. B, store (or STIM)-operated channel (SOC) activation of TRPCs. TRPC1/4/5 have been shown to be directly activated by STIM1 (see text for details), which senses ER Ca2+ store depletion to oligomerize and move towards the plasma membrane (PM). This may be facilitated by junctate, which interacts with IP3Rs, TRPCs, and STIM1, as well as by Ca2+ influx mediated by Orai1. C, linear representation of selected key structure features of TRPC4α (upper) and TRPC6 (lower). Ankyrin-like (ANK) repeats, transmembrane segments (blue boxes), and CaM-binding sites (red circles) are labeled. EWKFAR indicates the TRP motif. VTTRL indicates the PDZ-binding domain. Experimental evidence for some of the features indicated in TRPC4α has only been reported for TRPC5. −, experimental evidence exists for negative regulation; ±, experimental evidence exists for both positive and negative regulation. Binding motifs for NHERF (Tang et al., 2000), PIP2 and PIP3 (Kwon et al., 2007), SESTD1 (Miehe et al., 2010), Gi/oα-GTP (Jeon et al., 2012), STIM1 (Zeng et al., 2008), spectrin (Odell, Van Helden, & Scott, 2008), and Calmodulin- and IP3 receptor-binding (CIRB) site (Zhang et al., 2001; Tang et al., 2001) are indicated.
However, comparing to receptor stimulation, DAGs, often by the use of OAG because of its easier access and better solubility in aqueous solutions than naturally occurring DAGs, do not appear to recapitulation all features of TRPC channel activation, at least in electrophysiological experiments (Albert & Large, 2003; Shi et al., 2004). The magnitude of OAG-evoked current tends to be smaller and the kinetics of its activation are slower than TRPC currents induced via receptor stimulation. Presumably, the solubility issue and side of application of the lipid, i. e. OAG is typically applied extracellularly but the endogenously produced DAGs should first appear in the inner leaflet of PM following the hydrolysis of PIP2, could account for some of the differences between TRPC channel activation by OAG and receptor agonists. Recently, DAG photoswitches have been developed to allow equilibration of the lipids with the membrane in both leaflets before activation by light (Lichtenegger et al., 2018). This may help overcome some of the above issues. Combining mutagenesis and functional analysis using different DAG analogs including one of the DAG photoswithches, OptoDArG (Table 1), the authors demonstrated that DAG may gain access to the channel through a subunit-joining fenestration towards a conserved glycine residue behind the selectivity filter of TRPC3 (Lichtenegger et al., 2018).
Table 1.
Small molecule TRPC3, C6, C7 agonists.
| Namea | Structure | Target | Concentration or EC50 | Reference |
|---|---|---|---|---|
| Pyrazolopyrimidines 4n | 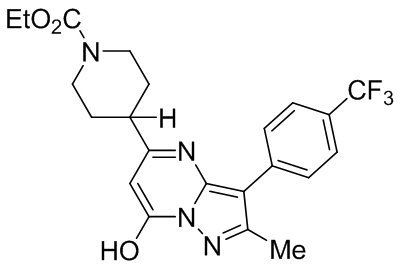 |
TRPC3 TRPC6 TRPC7 |
EC50, 19 nM EC50, 1.39 μM EC50, 90 nM |
Qu et al., 2017 |
| GSK1702934A | 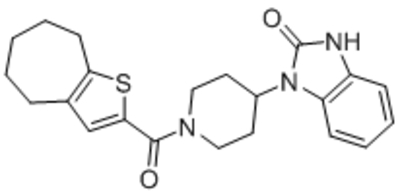 |
TRPC3 TRPC6 |
EC50, 80 nM EC50, 440 nM |
Xu et al., 2013 |
| OptoBI-1 | 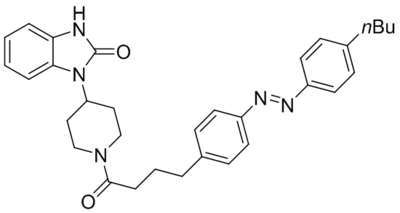 |
TRPC3 TRPC6 TRPC7 photoswitch activation |
Tested at 10–20 μM | Tiapko et al., 2019 |
| OptoDArG | 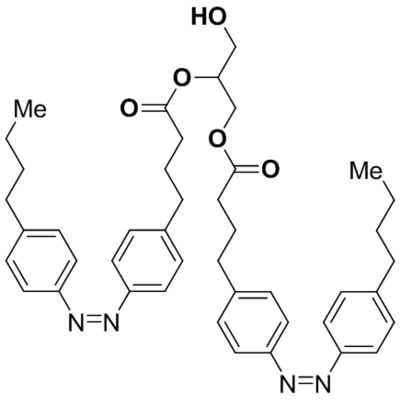 |
TRPC3 TRPC6 photoswitch activation |
Tested at 30 μM | Lichtenegger et al., 2018 |
|
Hyperforin (IDN5522) |
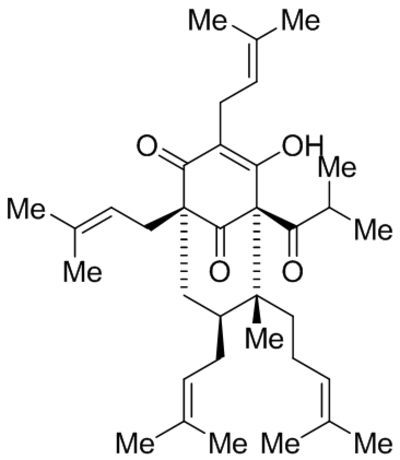 |
TRPC6 activation and increasing expression | EC50, 1.51 μM |
Leuner et al., 2007; Leuner et al., 2010; Thiel & Rossler, 2017 |
| Tetrahydrohyperforin (IDN5706), | 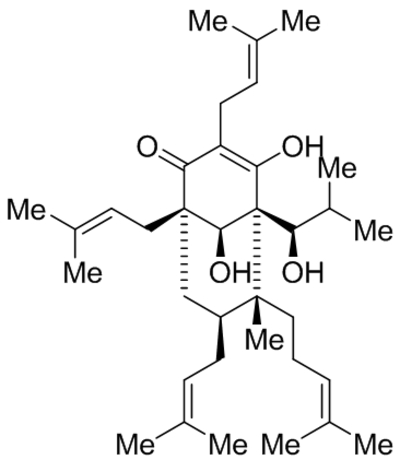 |
TRPC3 TRPC6 TRPC7 |
EC50, 0.5 μg/mL (~1 μM) |
Montecinos-Oliva et al., 2014 |
| Hyp9 | 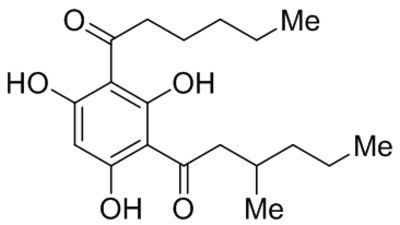 |
TRPC6 | EC50, 1.26 μM | Leuner et al., 2010 |
| Calycosin (CAL) |  |
Upregulated TRPC6 expression | 20 mg/kg body weight (in vivo), 60 μM (in vitro) | Guo et al., 2017a |
| PPZ1 |  |
TRPC3 TRPC6 TRPC7 |
EC50, 57 μM EC50, 67.3 μM EC50, 45.9 μM |
Sawamura et al., 2016 |
| PPZ2 | 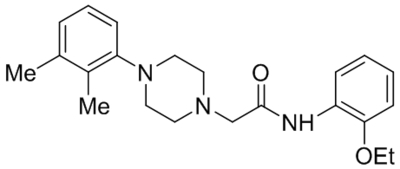 |
TRPC3 TRPC6 TRPC7 |
EC50, 10.2 μM EC50, 8.4 μM EC50, 2.9 μM |
Sawamura et al., 2016 |
| (+)-Conocarpan | 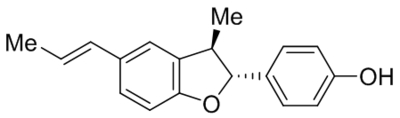 |
TRPC6 | EC, 6.01 μM | Yang et al., 2019 |
| C20 | 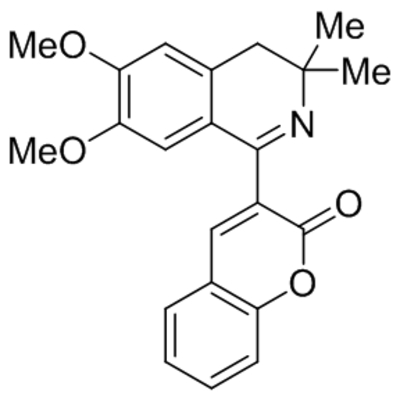 |
Positive allosteric TRPC6 modulator | EC50, 2.4 μM | Hafner et al., 2019 |
| Guanabenz | 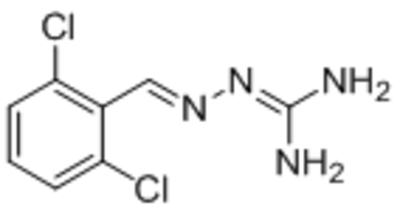 |
Activated TRPC6 | EC50, 100 μM | Bertrand et al., 2015 |
| 20-Hydroxyeicosa-tetraenoic acid (20-HETE) | 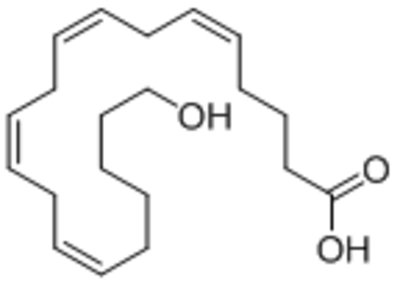 |
Activated TRPC6 and increased surface expression | EC50, 0.8 μM | Basora et al., 2003; Roshanravan et al., 2016 |
| Astragaloside IV (AS-IV) | 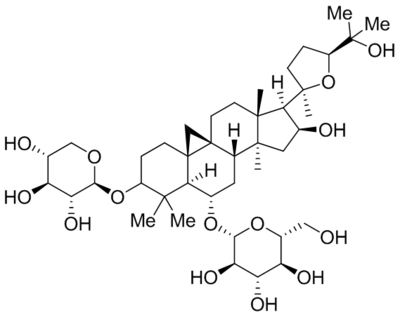 |
Prevented TRPC6 down regulation | 2.5 mg/kg body weight/day (in vivo) | He et al., 2018 |
| Lysophosphatidyl-choline (LysoPC) | 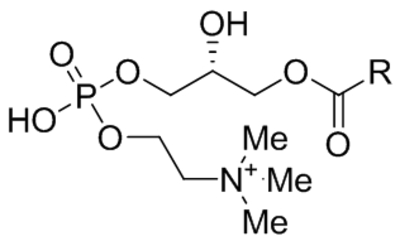 |
Activated TRPC6 | 10 μM | Chaudhuri et al., 2008 |
| Zeranol | 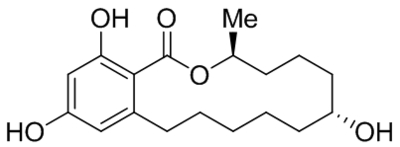 |
Increased TRPC3 expression | 10 nM | Zhu et al., 2016a |
| Aflatoxin B1 | 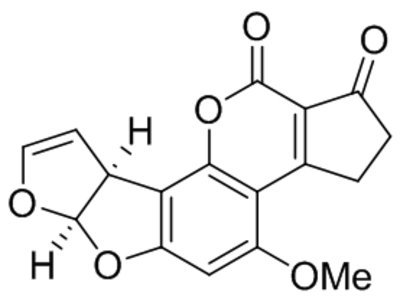 |
Increased TRPC3 expression | 1 nM | Zhu et al., 2016b |
Compounds with selectivity information and likelihood of direct interaction with the channel are highlighted in bold letters and shaded rows.
2.2.1.2. IP3 and IP3 receptors.
On the other hand, numerous studies have shown the involvement of other constituents of the PLC pathway in TRPC channel activation. These include IP3, the other product of PIP2 hydrolysis, which might enhance TRPC7 activation (Shi et al., 2004) or activate TRPC3 and C5 through IP3 receptors (Kanki et al., 2001; Kiselyov et al., 1998). Indeed, IP3 receptors were shown to be physically associated with TRPCs (Boulay et al., 1999; Kiselyov, Mignery, Zhu, & Muallem, 1999) and direct binding between the N-terminus of the IP3 receptor and the C-terminal CIRB motif of the TRPC has been demonstrated for all three IP3 receptors and all TRPC subtypes (Tang et al., 2001; Zhang et al., 2001) (Fig. 3C). Functionally, TRPC3, TRPC4, and endogenous TRPC1-containing channels were activated by IP3 receptors but inhibited by Ca2+-calmodulin (CaM), which competes with the IP3 receptors for binding to the CIRB motif in a mutually exclusive manner (Kiselyov et al., 1998; Tang et al., 2001; Vaca & Sampieri, 2002; Zhang et al., 2001).
To make the matter more complicated, TRPC2, C3 and C5, but not C1, were reported to interact with Junctate (Fig. 3B), an ER transmembrane protein that also binds to IP3 receptors (Stamboulian et al., 2005; Treves et al., 2004; Treves et al., 2010). Junctate has more recently been found in the complex where STIM1 interacts with Orai1 at the ER-PM junction (Srikanth et al., 2012), Intriguingly, the overexpression of junctate led to an increase in the number of ER-PM junctions that contained IP3 receptors and TRPC3 and this effect was further enhanced by overexpressing TRPC3 (Treves et al., 2010). With the ability to sense Ca2+ contents in the ER lumen and to bridge ER-PM interactions, junctate is thus thought to be an important player in store-operated Ca2+ entry (Srikanth et al., 2012; Treves et al., 2004).
2.2.1.3. Protons.
The hydrolysis of PIP2 by PLC also produces protons (H+), which lead to local acidification at the cytoplasmic side of the PM. It was estimated that at the physiological pH of 7.2, the breakdown of each molecule of PIP2 yields 0.8 proton (Huang et al., 2010). The effect of intracellular acidification has been studied in Drosophila photoreceptors for endogenous TRP and TRPL channels. For both, this often unappreciated by-product of PIP2 hydrolysis was found to facilitate channel activation (Huang et al., 2010), although it was suggested in a later study that the acidification may exert its effect through a scaffolding protein, INAD, that holds PLC and TRP channels in a complex in the rhabdomere of the insect’s photoreceptor (Liu et al., 2011). It was reported recently that the decrease of intracellular pH also facilitates activation of TRPC4 channels (Wang et al., 2018). Therefore, it is possible that H+, as a product of PIP2 hydrolysis and a general second messenger of PLC signaling, also contributes to activation of most, if not all, TRPC channels.
2.2.1.4. PIP2.
Not only are the products of PLC activity but also the substrate, PIP2, is important for TRPC channel gating. The effects of PIP2 on TRPC activation are complex. On one hand, the channels may be somewhat inhibited by the basal levels of PIP2, as well as its precursors, phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol (PI) on the PM. Thus, the breakdown of PIP2 by PLC, which also lowers PIP and PI levels because of rapid conversions of PI to PIP and PIP to PIP2 by kinases, should relieve the block and allow channel activation. On the other hand, some levels of PIP2 may be needed to support TRPC channel activity, given that the production of DAG, IP3 and H+ is dependent on the substrate, although this may not be the only reason for the PIP2 dependence. It was shown that TRPC5 activation was enhanced by hydrolyzing PIP2 with PLC or inhibiting the kinase that phosphorylates PIP to PIP2 but suppressed by dephosphorylating PIP2 to PIP via a phosphatase. However, direct application of PIP2 to the cytoplasmic side of inside-out patches enhanced TRPC5 function (Trebak et al., 2009). In whole-cell recordings, infusing PIP2 into the cell via the patch pipette, which would enhance the resting PIP2 level, suppressed activation of TRPC4α, not TRPC4β, by receptor agonist (Otsuguro et al., 2008). Here, the β isoform lacks 84 amino acids at the C-terminus as compared to the full-length TRPC4α isoform because of an alternative intron acceptor site. Thus, a PIP2-inhibitory site may be located within the 84 amino acids missing in TRPC4β. However, this apparently is not the only site and/or action of PIP2, as the similar manipulation delayed desensitization of TRPC4β and TRPC5 currents (Kim et al., 2014; Kim, Kim, Jeon, Kim, & So, 2008). In experiments where endogenous PIP2 levels were reduced by overexpressing constitutively active Gαq protein or phosphatase that dephosphorylates PIP2 to PIP, the activation of TRPC4β was completely inhibited (Kim et al., 2014) and the G protein effect was rescued by intracellular dialysis of PIP2 (Jeon et al., 2012), highlighting the dependence of TRPC4 activation on PIP2.
More recently, it was shown that TRPC4 (both α and β isoforms) activation is absolutely dependent on PLCδ1 and Gi/o protein signaling (Thakur et al., 2016). As such, PIP2 serves not only as the substrate but also the membrane anchor of this PLC isozyme. It is worth pointing out that PLCδ1 may represent the prototypical PLC isozyme because it is the only family found in lower eukaryotes like yeast and slime molds (Meldrum, Parker, & Carozzi, 1991; Rhee & Bae, 1997). PLCδ1 is solely activated by Ca2+ while other mammalian PLC isozymes, which were likely evolved from PLCδ1, contain additional expanded domains for more specific regulations. Thus, it is possible that PLCβ’s and PLCγ’s contribute to TRPC4 activation because of their coupling to receptor signaling and their activation is propagated to PLCδ1 owning to [Ca2+]c elevation. It is also interesting to note that in the same study by Thakur et al. (2016), manipulations that lowered PIP2 levels in the cell also made it easier for TRPC4 to be activated by low concentrations of receptor agonist, again indicating a tonic inhibition by the phosphoinositide. The dual effects of PIP2 on channel activation may explain the seemingly conflicting results found about carbacholevoked native TRPC4-like currents in ileal myocytes where intracellular dialysis of PIP2 was reported to either accelerate (Otsuguro et al., 2008) or slow down current desensitization (Kim et al., 2008). Presumably, some variations in experimental conditions between the two studies, e. g. species used (guinea pigs vs. mice), sample preparations, compositions of the pipette and bath solutions, could impact the resting PIP2 contents and/or state of the channel and thereby account for the different results.
For other TRPC channels, PIP2 dependence has been reported for native channels in vascular smooth muscle cells likely composed of TRPC1/C5 heteromers, where TRPC1 appeared to confer the PIP2 regulation (Shi et al., 2012). In heterologous systems, PIP2 activated overexpressed TRPC6 and C7 in inside-out patches (Lemonnier, Trebak, & Putney Jr, 2008), but depleting PIP2 in rabbit mesenteric artery smooth muscle cells augmented endogenous TRPC6-like activity (Albert, Saleh, & Large, 2008). With the use of membrane depolarization in whole-cell recordings to dephosphorylate PIP2 through voltage-sensitive phosphatases, PIP2 was found to be essential in supporting TRPC3, C6, and C7 function, and the three TRPC members exhibited differences in the sensitivity to PIP2 (Imai, Itsuki, Okamura, Inoue, & Mori, 2012). It was also shown that although both DAG and PIP2 are important for the activation of TRPC6 and C7 channels, channel inactivation is correlated with the loss of PIP2, but not DAG (Itsuki et al., 2014). In Drosophila photoreceptor cells, the light-induced activation of TRP and TRPL channels was mediated by phosphoinositide hydrolysis and intracellular pH decrease; however, direct application of PIP2 to the cytoplasmic side of inside-out patches excised from insect S2 cells overexpressing TRPL also strongly increased channel function (Huang et al., 2010). Therefore, the phosphoinositides seem to exert complex effects on TRPC channels, including both facilitation and inhibition, as well as specific aspects of channel gating. The relative levels and number and positions of phosphates, i. e. PI, PIP, or PIP2, as well as their cluster sizes and distribution patterns on PM, as they relate to TRPC channels, are all important questions that warrant further investigation.
2.2.1.5. Ca2+.
The rise in [Ca2+]c affects different TRPC subtypes differently. As a part of the PLC signaling, the Ca2+ signal first arises from IP3-induced ER Ca2+ release; this probably provides the initial trigger to facilitate the activation of TRPC4 and C5. Then Ca2+ influx through the activated TRPC channels, as well as Orai channels that are activated as a result of ER Ca2+ store depletion (Cheng, Liu, Ong, & Ambudkar, 2008; Feske et al., 2006; Vig et al., 2006), may further enhance the TRPC activity. The effect of intracellular Ca2+ on TRPC4 and C5 activation had first been shown by whole-cell patch clamp recordings using the intracellular solutions that contained either EGTA or BAPTA as the Ca2+ buffer with the free [Ca2+]c chelated at desired levels (Schaefer et al., 2000). Between the two Ca2+ chelators, EGTA binds to Ca2+ about 100–1000 times slower than BAPTA and therefore allows Ca2+ level near the source of Ca2+ generation (i. e. Ca2+-permeable channels) to increase to a higher amplitude and to last for a longer time period than BAPTA. Thus, a comparison between results obtained with EGTA- vs. BAPTA-buffered solutions helps reveal the effect of near membrane Ca2+ dynamics on the channel, especially when Ca2+ entry serves a major role in channel activation and/or desensitization. On the other hand, the activation of TRPC4 and C5 is heavily influenced by extracellular Ca2+ (Jung et al., 2003). Thus, in experiments where Ca2+ effect was observed by changing Ca2+ levels in the bath solution, it is difficult to distinguish whether the side of action for Ca2+ is extracellular, representing a direct effect, or intracellular, which is secondary to Ca2+ entry via the open channels. The situation may be further complicated by the presence of additional Ca2+ entry mechanism, for instance, voltage-gated Ca2+ channels (VGCCs) and Orai channels (see later). By increasing [Ca2+] right underneath the PM, these channels also affect TRPC gating (Tian et al., 2014; Gross et al., 2009; Cheng et al., 2008).
Direct effect of intracellular Ca2+ has at least been demonstrated for heterologously expressed homomeric TRPC5 channels, where direct intracellular infusion of pipette solutions that contained higher than resting Ca2+ level (≥300 nM) or flash photolysis of preloaded caged Ca2+ triggered channel activation (Gross et al., 2009). In a separate study, the intracellular Ca2+ dependence of TRPC5 was studied in the context of stimulation of the co-expressed M1 muscarinic receptor. It was shown that 1 μM cytosolic Ca2+ was needed to strongly potentiate the receptor-operated TRPC5 activation (Blair, Kaczmarek, & Clapham, 2009). For TRPC4β activated through the co-expressed μ opioid receptor (μOR), high cytosolic Ca2+ concentrations are also necessary, with an estimated EC50 of ~12 μM (Thakur et al., 2016). Thus, the sensitivity to Ca2+ appears to be different for TRPC4 and C5 and dependent on the mode of activation and other experimental conditions.
A CaM binding site downstream from the conserved CIRB motif has been reported to be critical for accelerating TRPC5 activation by Ca2+-CaM (Ordaz et al., 2005). Multiple Ca2+-CaM sites are also found at equivalent region of TRPC4α (Tang et al., 2001; Trost, Bergs, Himmerkus, & Flockerzi, 2001) despite sequence divergency in this area of TRPC4α and C5 (Zhu, 2005). However, since the area is excluded in TRPC4β, the Ca2+ dependence of TRPC4β activation must not be conferred by these binding sites. One possibility is that the Ca2+ dependence could be conferred by PLCδ1, the specific PLC isozyme required for the Gi/o-mediated TRPC4 activation (Thakur et al., 2016). Interestingly, the potentiation of TRPC6 activity by cytosolic Ca2+ was shown to be dependent on CaM-dependent kinase II (CaMKII) phosphorylation at Thr-487 (Shi et al., 2004; Shi et al., 2013). Furthermore, rapid translocation of TRPC5 from intracellular vesicles to PM in response to receptor stimulation has been observed (Bezzerides, Ramsey, Kotecha, Greka, & Clapham, 2004) and it may be quite common among TRPCs. This may also contribute to the Ca2+-dependence of channel activation since membrane fusion is triggered by Ca2+.
Ca2+ dependent inhibition has also been reported for TRPCs. Under similar experimental conditions, TRPC7 only displayed inhibition by Ca2+ whereas TRPC6 exhibited both potentiation and inhibition (Shi et al., 2004). Likewise, receptor agonist-evoked TRPC3 current was strongly inhibited by the rise in [Ca2+]c (Thyagarajan et al., 2001). The mechanisms for the Ca2+-dependent inhibition may involve both CaM-dependent and CaM-independent processes (Polat et al., 2019; Shi et al., 2004) with the IC50 of Ca2+ inhibition of TRPC7 being ~10 time lower than that of TRPC6. Single channel studies revealed that millimolar extracellular Ca2+ also suppressed the unitary conductance of TRPC6 and C7 at negative potentials, again with sensitivity higher for TRPC7 than C6 (Shi et al., 2004). A recent study indicates that CaM mediates Ca2+-dependent inactivation of TRPC6 by altering the assembly of the C-terminal coiled-coil domain, where gain-of-function TRPC6 mutations have been found from patients with focal segmental glomerulosclerosis (FSGS) (Polat et al., 2019). For TRPC4 and C5, Ca2+ was shown to either inhibit channel activation or accelerate current inactivation at concentrations higher than that needed to support the channel opening (Gross et al., 2009; Ordaz et al., 2005; Thakur et al., 2016). While the activation of TRPC1 in human submandibular gland (HSG) cells is facilitated by Ca2+ influx mediated by Orai1-containing channels (Cheng et al., 2008), Ca2+ also inhibits TRPC1 function through CaM binding to a site at the very C-terminal end of TRPC1 protein (Singh, Liu, Tang, Zhu, & Ambudkar, 2002). Thus, for several TRPC subtypes, Ca2+ exert both stimulatory and inhibitory effects, utilizing diverse mechanisms including direct Ca2+ binding, PLCδ, CaM, CaMKII, and presumably others, such as vesicular trafficking. The resulting Ca2+ sensitivities differ greatly among different channels and experimental conditions, providing a delicate system to fine tune the channel function.
2.2.2. Gi/o signaling
The pertussis toxin (PTX)-sensitive Gi/o proteins exert a unique effect on the activation of TRPC4 and C5 (Fig. 3A). The knowledge on the involvement of Gi/o protein signaling in receptor-operated activation of TRPC4-containing channels can be dated back to more than 20 years when the endogenous muscarinic cation current (mICAT) in intestinal smooth muscle cells were found to be codependent on both M2 (Gi/o-coupled) and M3 (Gq/11-coupled) muscarinic receptors (Zholos & Bolton, 1997). It was shown later that mICAT is mainly mediated by channels composed of TRPC4 (Tsvilovskyy et al., 2009). Importantly, mICAT was inhibited by the pre-treatment of cells with PTX (Pucovský, Zholos, & Bolton, 1998). Similarly, the PTX treatment also suppressed current development of heterologously expressed TRPC4 induced through intracellular infusion of GTPγS (Otsuguro et al., 2008). Furthermore, the knockdown of Gαi1, but not that of Gαq/11, in Xenopus oocytes strongly inhibited the activation of TRPC5 expressed in these cells (Tabata et al., 2002). In mammalian expression systems, the expressed TRPC5 was activated through stimulation with sphingosine-1-phosphate (S1P) or oxidized phospholipids and these activities were all inhibited by PTX (Al-Shawaf et al., 2010; Xu et al., 2006).
In principle, Gi/o proteins could induce PLCβ activation through Gβγ dimers freed from the heterotrimeric complex as a part of G protein activation. However, an early study using pipette dialysis of antibodies against different G protein subunits showed that inhibiting Gαo, rather than Gβ, suppressed the development of mICAT (Yan et al., 2003). Also, although TRPC4 and C5 are readily activated via stimulation of overexpressed Gq/11-coupled histamine and muscarinic receptors (Kim et al., 2014; Obukhov & Nowycky, 2004; Schaefer et al., 2000), stimulation of the endogenous muscarinic receptor (likely M3) with carbachol in HEK293 cells overexpressing TRPC4 alone evoked very small, if any, current (Thakur et al., 2016). Intriguingly, the same manipulation effectively activated TRPC3, C5, C6, and C7 currents in the same expression system, as well as robust [Ca2+]c increase through IP3-mediated ER Ca2+ release in untransfected HEK293 cells, demonstrating efficient coupling to Gq/11-PLCβ. The discrepancy between endogenous and overexpressed receptors was resolved by pre-treating the cells with PTX, which abolished TRPC4α and TRPC4β activation by the overexpressed M1, M3, and M5 receptors, suggesting that all of them were promiscuously coupled to Gi/o proteins when overexpressed. Importantly, the PTX treatment also suppressed TRPC5 currents, evoked through either endogenous or overexpressed M3 receptors, by at least 50% (Thakur et al., 2016) while having no effect on the activation of TRPC3, C6 and C7 (M. X. Zhu, unpublished observation). Also interesting is that when overexpressed in HEK293 cells, the Gi/o-coupled μOR activated PLC very poorly, but it mediated robust TRPC4 activation (Thakur et al., 2016). Therefore, the receptor-operated TRPC4 activation is not mediated through Gq/11-PLCβ or Gi/o-Gβγ-PLCβ signaling; rather it exhibits an absolute dependence on Gi/o proteins. In this regard, TRPC5 is actually different from TRPC4 in that it may be activated by either Gq/11 or Gi/o.
The Gi/o-mediated TRPC5 activation has been suggested to occur through stimulation of Ca2+-independent group 6 (GVI) phospholipase A2 (iPLA2) and the resultant production of lysophospholipids (AL-Shawaf et al., 2011). However, the iPLA2 inhibitor used in this study, bromoenol lactone, has been found to block TRPC5 channels independently of iPLA2 (Chakraborty et al., 2011). In any case, lysophospholipids may have complex effects on TRPC and other channels, as well as properties of the membrane from where these channels are recorded. Alternatively, Jeon and colleagues had examined the effects of different G protein subunits on TRPC4 and C5 activation through overexpression of Gβγ or constitutively active Gα mutants. They showed that coexpression of constitutively active Gαi2, Gαi3, or Gαo, but not Gαi1 or Gβγ, led to TRPC4 activation in the absence of any stimulus, with Gαi2 being the most potent. For TRPC5, Gαi3 may be more preferred (Jeon et al., 2012). With the combined approach of co-immunoprecipitation and functional evaluation of deletion mutants, the critical site of TRPC4 for Gαi2 regulation was found to overlap with the CIRB motif (Jeon et al., 2012). Given that in the high resolution TRPC4 structures, the CIRB motif is largely exposed at the end of the extended lever formed by CH1 helix (see above), it is possible that the interaction with Gαi/o at this region induces an allosteric change that pulls the lower gate to open. Further evidence for the involvement of Gαi/o-GTP, rather than Gβγ, in the receptor-operated activation of TRPC4 came from studying the effects of Gi/o-regulatory proteins that contain either GTPase-activating protein (GAP) or guanine-nucleotide-dissociation inhibitor (GDI) domains. It was shown that although Gi/o-mediated TRPC4 activation can be inhibited through both GAP and GDI functions of Regulators of G protein Signaling (RGS) proteins in an additive fashion, only the GAP, but not GDI, function can accelerate current desensitization (Jeon et al., 2016).
2.2.3. Store- or STIM-operated activation
Because of the history of mammalian TRPC discovery, a large volume of early studies were devoted to establishing the link between ER Ca2+ store depletion and TRPC channel activation. The concept of store-operated or capacitative Ca2+ entry was first proposed by James Putney Jr. over 30 years ago (Putney Jr., 1986), with the idea that in order for the cell to maintain its Ca2+ reserve, the loss of ER Ca2+ storage as a result of Ca2+ mobilization due to receptor activation should trigger a mechanism that brings Ca2+ into the cell from extracellular space. This mechanism requires the presence of a Ca2+-permeable channel on the PM that opens in response to ER Ca2+ depletion. In subsequent years, several store- or receptor-operated ionic currents with varying properties were discovered in electrophysiological studies. Among them, the highly Ca2+ selective CRAC current (ICRAC) first described in T-lymphocytes and mast cells, with single channel conductance too small to be detectable, was considered as the most typical store-operated channel (Hoth & Penner, 1992; Lewis & Cahalan, 1989). Although other channels have been described (Fasolato, Hoth, Matthews, & Penner, 1993; Vaca & Kunze, 1993), their ion selectivity, single channel conductance, and mode of activation tended to vary so much that no consensus could be drawn as to how to categorize them. Strictly speaking, store depletion is usually an inevitable component of PLC signaling. Thus, if a Ca2+-permeable channel becomes activated as a result of PLC signaling, i. e. being receptor-operated, then it should be able to fulfill the role of store refilling. However, there have been growing appreciations of unique functions carried out by different Ca2+ entry pathways, including local Ca2+ signaling and temporal patterns (Berridge, 1995). Thus, having multiple and diverse channel types involved would be advantageous for signaling and cell function.
The original drive for the search of mammalian homologs of Drosophila TRP and TRPL indeed followed the hypothesis that store-operated Ca2+ entry might be mediated by such channels, given the close similarity between the insect’s phototransduction and mammalian PLC pathway (Hardie & Minke, 1993). Thus, the initial functional characterization of the newly identified TRPCs had always included a Ca2+-free and Ca2+-readdition protocol while changes in [Ca2+]c were monitored by the pre-loaded fluorescence Ca2+ indicator dye, e. g. Fura-2 (Zhu et al., 1996; Zhu, Jiang, & Birnbaumer, 1998). Cells were stimulated with either a receptor agonist to trigger Gq/11-PLCβ signaling or thapsigargin (TG), an inhibitor of the sarco/endoplasmic reticulum ATPase (SERCA), to induce internal Ca2+ store depletion independently of receptor activation. While the receptor agonist had always elicited an increase in the extracellular Ca2+-dependent [Ca2+]c rise associated with TRPC overexpression, evidence for TG-induced activation was only obtained for TRPC1, C3, C4, and C5 (Liu et al., 2000; Philipp et al., 1996; Philipp et al., 1998; Thyagarajan et al., 2001; Zhu et al., 1996; Zhu et al., 1998), but not for TRPC6 and C7 (Boulay et al., 1997; Okada et al., 1999). For TRPC3 and C5, there are also reports suggesting the non-capacitative nature of the channels (Okada et al., 1998; Zhu et al., 1998).
Electrophysiological recording results have all agreed that the expressed TRPC channels do not recapitulate the key features of ICRAC, especially with respect to ion selectivity and single channel conductance (Hurst, Zhu, Boulay, Birnbaumer, & Stefani, 1998; Liu et al., 2000; Okada et al., 1998; Zitt et al., 1996). Therefore, despite the numerous reports on the effects of knocking down or knocking out TRPC expression or using dominant negative TRPC constructs to suppress store-operated Ca2+ entry (Yuan et al., 2009), the contribution of TRPCs in this pathway remains controversial, although it is generally agreed that all TRPCs can function as receptor-operated Ca2+-permeable nonselective cation channels. Possibility exists that store sensitivity may be acquired through heteromultimerization between different TRPC isoforms or a TRPC with other auxiliary proteins (Vazquez, Lievremont, St J Bird, & Putney, 2001; Zhu et al., 1998). However, even with TRPC heteromers, the biophysical properties are still quite different from that of the CRAC channel (Lintschinger et al., 2000; Strübing et al., 2001). Only in one example, successful heterologous reconstitution of a CRAC-like current was achieved using TRPC in combination with a low level of Orai1 overexpression (Liao et al., 2008).
The discovery of STIMs and Orai’s had turned things around with the demonstration of ICRAC-like current formed by the heterologously expressed Orai1 and ER Ca2+ store depletion sensed and conveyed to the PM by STIM1 (Feske et al., 2006; Liou et al., 2005; Roos et al., 2005; Vig et al., 2006). Growing evidence suggests that STIM1 not only interacts with Orai1, but also TRPCs and physical and/or functional interplays also occur between Orai1 and TRPCs. It is likely that at least some TRPC channels can be STIM-operated. It was reported that STIM1 can be physically associated with TRPC1, C2, C4, C5 (Huang et al., 2006; Lopez, Salido, Pariente, & Rosado, 2006) and C6 (Albarran et al., 2014; Boustany et al., 2010), but not C3 and C7, in response to Ca2+ store depletion at least in some cases. STIM1 can also mediate heteromultimerization between TRPC1 and C3, making the latter STIM1 dependent (Yuan, Zeng, Huang, Worley, & Muallem, 2007). For TRPC1, the interaction with STIM1 may involve coiled-coil regions at N- and C-terminal regions of the channel (Ong & Ambudkar, 2017) and the STIM-Orai1-activating region (SOAR) of STIM1 (Lee et al., 2014). Thus, the SOAR domain of STIM1 is equally important for activating Orai and TRPC channels. In the full-length STIM1, however, the last two lysine residues may be especially critical for TRPC gating, as they form electrostatic interactions with the two conserved aspartates/glutamates (D639, D640 of TRPC1; E648, E649 of TRPC4) at TRPC C-terminus (Zeng et al., 2008), which are located right at the border of the TRP helix and TRP re-entrant as shown by the recent high resolution structures (Fig. 1A–D) (Duan et al., 2018; Tang et al., 2018; Vinayagam et al., 2018).
The ability of STIM1-SOAR domain to activate TRPC currents has been tested using excised inside-out patches, showing that the purified recombinant SOAR fragment directly activated single channel currents of TRPC1, C4 and C5, but not C3 and C6 (Asanov et al., 2015), despite the conservation of the two acidic residues in TRPC3 and C6 (Tang et al., 2018; Zeng et al., 2008). Combining the single channel recording technique with single molecule fluorescence imaging, the authors also demonstrated that two SOAR molecules are needed for one TRP tetramer and the activity was antagonized by four CaM molecules (Asanov et al., 2015).
Results of several studies support the idea that the interaction with STIM1 may switch the TRPC channel from store-independent to store-dependent gating (Asanov et al., 2015; Desai et al., 2015; Lee et al., 2014; Ong et al., 2007; Saul, Stanisz, Backes, Schwarz, & Hoth, 2014; Sundivakkam et al., 2012; Zeng et al., 2008). This might explain why in experiments with heterologous expression of TRPCs, which are typically carried out without co-expression of STIM1, the channels often behaved in a store-independent manner, with store-operation appearing only under strictly defined conditions (Philipp et al., 1998; Zhu et al., 1998). Therefore, as in the case of STIM1 gating Orai1, the stoichiometry between STIM1 and TRPC is also important for reconstituting store- or STIM1-operated TRPC channel gating (Fig. 3B). Plausibly, Orai1 and TRPCs may compete for binding to STIM1, and the outcome would determine whether the store-operated Ca2+ entry in the cell is carried out by CRAC channels formed by Orai1 or nonselective cation channels formed by TRPCs (de Souza, Ong, Liu, & Ambudkar, 2015; Desai et al., 2015; Lee et al., 2014; Saul et al., 2014).
The regulation by STIM1 sometimes may involve specific STIM1 isoforms or it may have additional consequences than channel gating. In human myotubes. a functional interaction between STIM1L (a splice variant of STIM1) and TRPC1/C4 promotes store-operated Ca2+ entry to enable fast repetitive Ca2+ transients and support myogenesis and muscle differentiation (Antigny et al., 2017). In the case of TRPC6, the interaction with STIM1 was reported to cause its translocation from PM to ER, reducing Ca2+ entry through the TRPC6-containing channels but increasing Ca2+ release from ER (Albarran et al., 2014).
Also, the relationship between Orai1 and TRPCs is not just competition. Orai1 may also be involved in the store-operated TRPC gating. With the endogenous Orai1 expression knocked down, both store-operated Ca2+ entry and TRPC1 currents were abolished and conversely, the overexpression of Orai1 augmented these activities (Cheng et al., 2008). The upregulation of Orai1 also enhanced store-operated Ca2+ entry through several other TRPC subtypes (Liao et al., 2007; Liao et al., 2008; Liao et al., 2009). It was proposed that Orai and TRPC proteins might co-assemble into a channel complex (Liao et al., 2008). Interestingly, the Ca2+ selectivity of an endogenous TRPC1/C4 channel was reduced by the knockdown of Orai1 in endothelial cells, along with the decrease in store-operated currents (Cioffi et al., 2012). This implies a different mechanism from the one that assumes Orai and TRPC to form separate channels independently regulated by STIM. It has also been suggested that STIM, Orai, and TRPC may coexist in a complex named as “store-operated calcium influx complex (SOCIC)”, where channels composed of both TRPC and Orai and those made of either TRPC or Orai separately could assemble and disassemble dynamically in response to different scenarios of store depletion and/or receptor activation (Vaca, 2010). In injured medial and neointimal proliferative vascular smooth muscle cells prepared from carotid artery, the STIM-Orai-TRPC complex also includes Homer1, a scaffolding protein known to be involved in the assembly of SOCIC. It was shown that the knockdown of Homer1 suppressed not only store-operated Ca2+ entry, but also proliferation and migration of vascular smooth muscles cells as well as neointima formation (Jia, Rodriguez, Williams, & Yuan, 2017). However, in contractile vascular smooth muscle cells freshly isolated from mesenteric artery, store-operated Ca2+ entry required TRPC1 but not Orai1 (Shi et al., 2017).
Importantly, regardless of the modes of activation and channels involved, store-operated channel gating occurs at specialized PM domains, likely lipid rafts, through interaction with polymerized STIM1 situated on the ER membrane (Vaca, 2010), making them all STIM-regulated channels. As described above, the ER-PM junctions also contain junctate, capable of interacting with both STIM1-Orai1 complex (Srikanth et al., 2012) and IP3 receptors /TRPCs (Stamboulian et al., 2005; Treves et al., 2004, 2010). On the other hand, in spite of the inter-dependence between TRPC and Orai proteins, ample studies have revealed distinct cellular and physiological functions between channels formed by Orai’s and TRPCs (Cheng, Ong, Liu, & Ambudkar, 2013; Ong, Jang, & Ambudkar, 2012). The interactions of TRPC, Orai and STIM proteins and their contributions to store- and receptor-operated Ca2+ entry will continue to be hot topics of future investigations.
3. Small molecular probes of TRPC
3.1. Physiological function and pathophysiological relevance
The TRPC channels have well-recognized roles in many cell signaling pathways that impact the function of diverse cells and tissues in physiology and diseases. Numerous studies have provided very rich information concerning the physiological significance and pathophysiological roles of individual TRPC isoforms. Many of these studies have been included in recent comprehensive review articles and book chapters that focus on specific organ/tissue system or disease, including cardiovascular system (Yue et al., 2015; Alonso-Carbajo et al., 2017; Xiao, Liu, Shen, Cao, & Li, 2017; Avila-Medina et al., 2018), with emphasis on vascular endothelial and smooth muscle cells (Beech, 2013; Earley & Brayden, 2015; Ampem et al., 2016; Grayson, Murphy, & Sandow, 2017), cardiac remodeling (Falcon et al., 2019), cardiac fibrosis (Numaga-Tomita et al., 2017), atrial fibrillation (Han & Li, 2018) and therapeutic angiogenesis (Moccia, Lucariello, & Guerra, 2018); skeletal muscles (Numaga-Tomita et al., 2019; Sauc & Frieden, 2017); lung and lung diseases (Smith, Ayon, Tang, Makino, & Yuan, 2016; Malczyk et al., 2017; Dietrich, Steinritz, & Gudermann, 2017); kidney and kidney diseases (Schlondorff, 2017; Staruschenko, Spires, & Palygin, 2019; Zhou & Greka, 2016); salivary gland physiology and dysfunction (Ambudkar, 2016), reproduction and sperm function (Götz, Qiao, Beck, & Boehm, 2017; Kumar et al., 2018); immune system and inflammation (Ramirez et al., 2018); and many different aspects of nervous systems and neurological diseases, e. g. neurological functions (Sun et al., 2014); neurotransmission and hormone sensing (Kelly, Qiu, & Ronnekleiv, 2018) as well as glucose sensing (Fioramonti, Chrétien, Leloup, & Pénicaud, 2017) in the hypothalamus, neural development (Feng, He, Li, & Wang, 2015; Tai & Jia, 2017), neurological diseases especially neurodegeneration (Pchitskaya, Popugaeva, & Bezprozvanny, 2018; Secondo, Bagetta, & Amantea, 2018; Wang et al., 2017), Alzheimer’s disease (Lu, He, & Wang, 2017); Parkinson’s disease (Sukumaran, Sun, Schaar, Selvaraj, & Singh, 2017), seizure and excitotoxicity (Zheng & Phelan, 2014), stroke (Zhang & Liao, 2015), brain white matter function and ischemia (Cornillot, Giacco, & Hamilton, 2019), and neuropsychiatric diseases (Griesi-Oliveira, Suzuki, & Muotri, 2017; Zeng, Tian, & Xiao, 2016). The involvement of TRPC channels in cancer proliferation, invasion, and chemoresistance is also nicely covered in recent reviews [He & Ma, 2016; Gaunt, Vasudev, & Beech, 2016; Jardin & Rosado, 2016; Li & Ding, 2017; Zhan & Shi, 2017]. Readers are referred to these articles for details about TRPC channel contribution in various physiological systems and cell types and their relevance to different types of diseases. It is important to point out that one of the major caveats of many of the previous research on TRPC channels was the lack of good pharmacology. Therefore, the conclusions were mainly based on genetic evidence through phenotype analysis of naturally occurring mutations or gene knockout or knockdown, examining expression and sometimes using blockers that are unspecific. Clearly the genetic based approaches suffer from compensatory changes that may not directly reflect the TRPC function.
3.2. TRPC drug discovery
Given the relatively widespread expression of TRPC channels in many tissues, their contributions to store- and/or receptor-operated Ca2+ entry, and their implications in diverse physiological functions in a wide range of systems, TRPCs have gained attention as potential therapeutic targets for treating cardiovascular disorders, endocrine diseases, neurological disorders, chronic kidney disease, pain, cancer and several other pathological conditions. As pointed out before, mammalian TRPC channels are activated downstream from receptors that signal through the PLC pathway, which involves either Gq/11 proteins or receptor tyrosine kinases. Nearly all second messengers associated with the PLC pathway exert some roles in TRPC channel activation. For example, diacylglycerols (DAGs) may directly activate all TRPC channels, despite that for TRPC4/5, this requires specific conditions (Hofmann et al., 1999; Okada et al., 1999; Storch et al., 2017); IP3 could activate TRPC3 and TRPC5 in an IP3 receptor-dependent manner, via perhaps a direct interaction between the N-terminus of the IP3 receptor and the CIRB motif of the TRPC (Kanki et al., 2001; Kiselyov et al., 1998; Tang et al., 2001; Zhang et al., 2001); elevation in [Ca2+]c either directly activates or potentiates the activation of TRPC1, C4, C5, or C6 (Shi et al., 2004; Cheng et al., 2008; Blair et al., 2009; Gross et al., 2009; Tian et al., 2014; Thakur et al., 2016), but further increased [Ca2+]c can also inhibit TRPC channel function (Ordaz et al., 2005; Shi et al., 2004; Singh et al., 2002; Thakur et al., 2016; Thyagarajan et al., 2001; Zhang et al., 2001). Moreover, the substrate of PLC, PIP2, also exerts complex effects on TRPC channels, including both some levels of dependence on the presence of PIP2 for channel activation and also some inhibition by the lipid (Imai et al., 2012; Kim et al., 2008; Lemonnier et al., 2008; Otsuguro et al., 2008; Shi et al., 2012; Thakur et al., 2016). Among these factors, only DAGs have been exploited in drug development to create new analogs that activate TRPC3 channels (Lichtenegger et al., 2018; Tiapko, Bacsa, de la Cruz, Glasnov, & Groschner, 2016). Furthermore, the receptor-operated activation of TRPC4/5 channels also includes Gi/o-coupled receptors and the PTX-sensitive Gi and Go proteins (Thakur et al., 2016).
Because the molecular identification of TRPC channels preceded their functional characterization, the pharmacology of TRPC channels had lagged behind, hindering the full understanding of their physiological and pathological contributions in native systems. In recent years, however, there has been an explosion of small molecular probes, including both natural and synthetic compounds, identified to exhibit specific activities on selective subtypes of TRPC channels, owning to the extraordinary efforts from both academic laboratories and pharmaceutical industry. To date, a series of validated TRPC probes have been developed to facilitate the exploration of the channels’ function in various native systems and the potential of these nonselective cation channels as therapeutic targets through synthetic small molecule inhibitors or activators. Herein, we summarize these novel probes and discuss their impact in the context of cardiovascular, pulmonary, neurological, and renal diseases and cancer, attempting to delineate a framework for further exploration of TRPC-based therapies. The names, structures, and effective concentrations of these molecules are shown in Tables 1–4. To facilitate future studies, we highlight those with reasonably good selectivity on specific TRPC subtypes or subgroups and likelihood of direct actions on the channel function. Users are advised to examine the original publications (referenced in the tables) for details.
Table 4.
Small molecule TRPC1, C4, C5 antagonists.
| Namea | Structure | Target | Concentration or IC50 | Reference |
|---|---|---|---|---|
| ML204 | 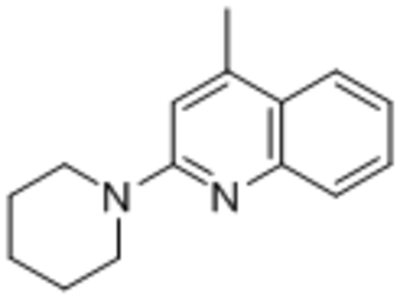 |
TRPC4 TRPC5 |
IC50, 0.96 μM | Miller et al., 2011 |
| M084 | 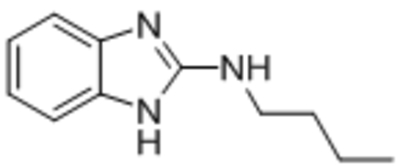 |
TRPC4 TRPC5 |
IC50, 10.3 μM IC50, 8.2 μM |
Zhu et al., 2015 |
| HC-070 | 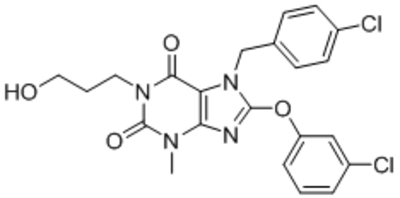 |
TRPC4 TRPC5 TRPC4–C1 TRPC5–C1 |
IC50, 46 nM IC50, 9.3 nM |
Just et al., 2018 |
| HC-608 (Pico145) | 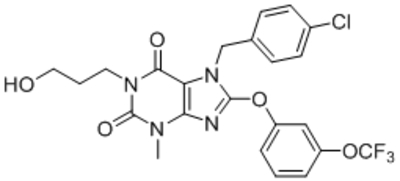 |
TRPC4 TRPC5 TRPC4–C1 TRPC5–C1 |
IC50, 32.5 nM IC50, 6.2 nM 9 – 1,300 pM |
Just et al., 2018; Rubaiy et al., 2017 |
| AC1903 | 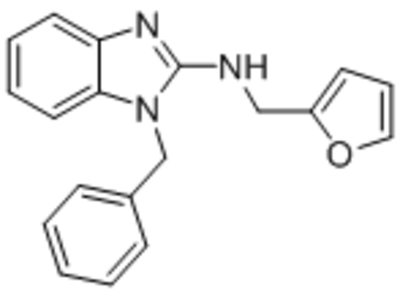 |
TRPC5 | IC50, 14.7 μM | Zhou et al., 2017 |
| Clemizole | 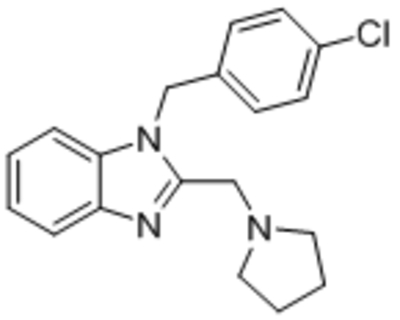 |
TRPC4β TRPC5 |
IC50, 6.4 μM IC50, 1.0–1.3 μM |
Richter et al., 2014a |
| GFB-887 | Not disclosed | TRPC5 | IC50, 11 nM | Mundel et al., 2019 |
| A54 | 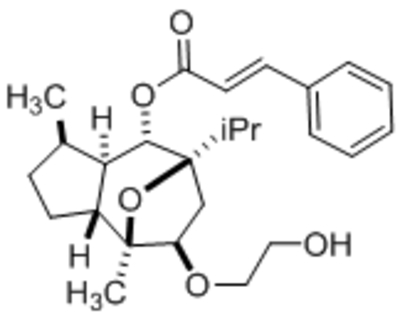 |
A498 cells TRPC1–C4 |
IC50, 62 nM | Rubaiy et al., 2018b |
| [6]-shogaol | 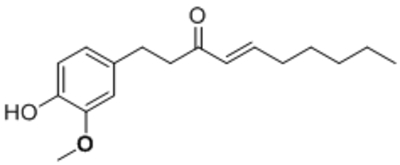 |
TRPC5 | IC50, 18.3 μM | Kim et al., 2016 |
| Galangin | 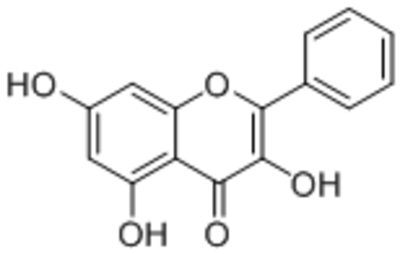 |
TRPC5 | IC50, 0.45 μM | Naylor et al., 2016 |
| Kaempferol | 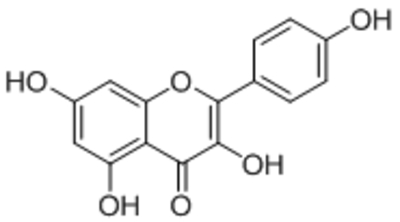 |
TRPC5 | IC50, 3.9 μM | Naylor et al., 2016 |
| Quercetin | 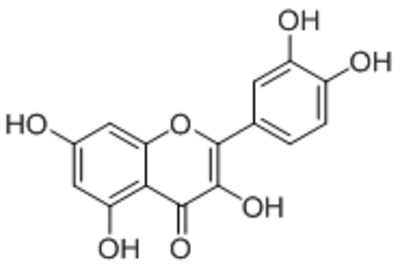 |
TRPC5 | IC50, 6.5 μM | Naylor et al., 2016 |
| AM12 | 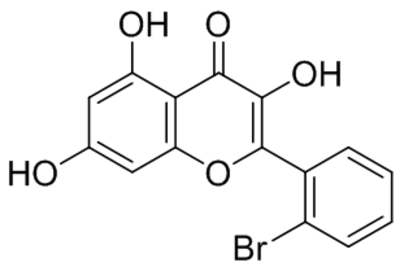 |
TRPC5 TRPC4 |
IC50, 0.28 μM | Naylor et al., 2016 |
| Chlorogenic acid (CGA) | 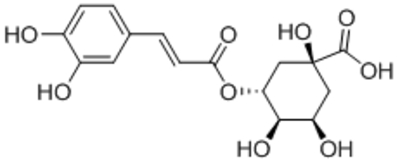 |
Suppressed TRPC1 expression | 300 μM | Jung et al., 2017 |
| Spironolactone | 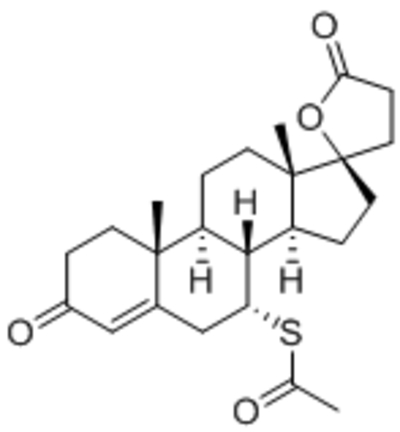 |
Suppressed TRPC1, C6 expression | 3 mg/kg body weight/day (in vivo) | Li et al., 2017 |
| Topotecan (TPT), | 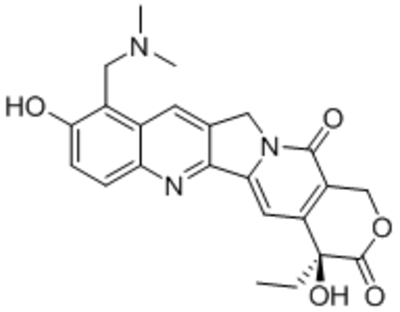 |
Suppressed expression of TRPC1, C4 | IC50, 1–10 nM | Jiang et al., 2018 |
| Rosiglitazone (RSG) | 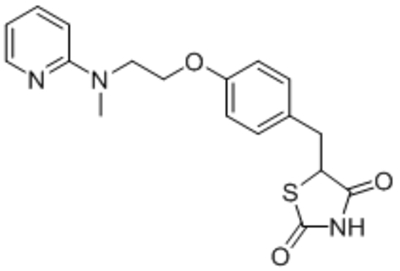 |
Reduced TRPC1 activation | IC50, 10 μM | Wei et al., 2017 |
| Apigenin | 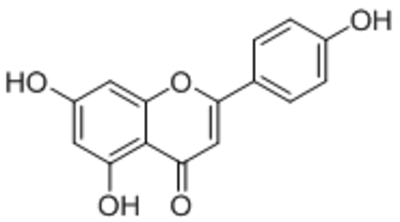 |
TRPC5 | IC50 >>10 μM | Naylor et al., 2016 |
Compounds with selectivity information and likelihood of direct interaction with the channel are highlighted in bold letters and shaded rows.
3.2.1. Modulators for cardiovascular disorders
In cardiovascular system, TRPC channels are directly or indirectly regulated by several endogenous factors involved in the pathogenesis of various cardiovascular disorders. Recent studies show that brain-derived neurotrophic factor (BDNF) protects against myocardial infarction through TRPC3/6 channels (Hang et al., 2015); prolonged activation of the exchange protein directly activated by cAMP (EPAC) causes upregulation in the expression of TRPC3 and TRPC4 proteins and enhanced store-operated Ca2+ entry in adult rat ventricular cardiomyocytes, which amounts to a proarrhythmic effect (Dominguez-Rodriguez et al., 2015); Transforming growth factor beta 1 (TGFβ1) induces upregulation of TRPC6 in vascular smooth muscle cells and in turn stress fiber formation that may underlie pathogenesis of vascular fibrosis (Park et al., 2017); lysophosphatidylcholine (LPC) activates TRPC6 channels in bovine aortic endothelial cells to inhibit endothelial cell migration and thereby delaying the healing of arterial injuries (Chaudhuri et al., 2008; Chaudhuri, Rosenbaum, Birnbaumer, & Graham, 2017). Other factors, such as atrial natriuretic peptide (ANP), endoglin, MicroRNAs, nitric oxide (NO) and protein kinase G, have also been shown to participate in various pathophysiological mechanisms by modulating TRPC channel function and/or expression [Chen et al., 2013; Feng, Xu, & Wang, 2018; Morine et al., 2016; Zhang et al., 2014].
In addition, several marketed drugs have recently been found to affect cardiovascular diseases by regulating function and/or expression of TRPC channels. For instance, spironolactone, a mineralocorticoid receptor inhibitor, was reported to attenuate coronary TRPC expression under long-term usage in the model of metabolic syndrome, which may help reduce coronary pathology (Li et al., 2017). Salvianolic acid B was found to attenuate doxorubicin-induced ER stress by inhibiting TRPC3/6-mediated Ca2+ overload in cardiomyocytes (Chen et al., 2017). Similarly, chlorogenic acid was shown to protect endothelial cells against LPC injury by attenuating TRPC1 expression and thereby inhibiting atherosclerosis (Jung, Im, Song, & Bae, 2017). Moreover, losartan effectively prevented the downregulation in endothelial cells and upregulation in smooth muscle cells of TRPC1 and TRPC6 in thoracic aortas of sinoaortic denervation rats and thereby the vasomotor function impairment (Liang, Zhong, Miao, Wu, & Liu, 2018).
In view of the crucial role of TRPC channels in cardiovascular pathogenesis, development of new TRPC modulators is required. In many of the early studies, SKF-96365 was used as a general TRPC channel antagonist, and therefore was also widely used in the study of cardiovascular disorders (Liu et al., 2016; Sabourin, Bartoli, Antigny, Gomez, & Benitah, 2016). Subsequently, Pyr3, a pyrazole compound that potently and selectively antagonizes TRPC3 (IC50 = 0.7 μM), was suggested to be of pharmaceutical potential in treating TRPC3-related diseases, like cardiac hypertrophy, and preventing stent-induced arterial remodeling (Kiyonaka et al., 2009; Koenig et al., 2013). However, because Pyr3 inhibits TRPC3 and Orai channels with equal potency, structural analogs had been developed, with Pyr10 showing about 18-fold selectivity for TRPC3 than Orai1 (Schleifer et al., 2012). Pyr10 exhibited an IC50 of 0.72 μM at Ca2+ entry mediated by TRPC3 while at 10 μM, it only inhibited TRPC4, C5, and C6 by less than 50% (Schleifer et al., 2012). Moreover, the combined TRPC3 and TRPC6 blockade by selective TRPC3/6 antagonists (GSK2332255B and GSK2833503A, IC50, 3–21 nM against TRPC3 and TRPC6) was shown to inhibit pathological cardiac hypertrophy (Seo et al., 2014). GSK2332255B and GSK2833503A display more than 100-fold selectivity over other calcium-permeable channels and 100-fold greater potency at TRPC3 compared with Pyr3. Moreover, they dose-dependently block cell hypertrophy signaling triggered by angiotensin II (Ang II) or endothelin-1 in neonatal and adult cardiac myocytes (Seo et al., 2014). Notably, the latest study has described a novel TRPC6-specific inhibitor (BI 749327) with IC50 of 13 nM and efficient oral bioavailability, which is able to protect cardiac function and reduce chamber dilation and fibrosis under abnormal hemodynamic stress. BI 749327 is 85-fold more selective for TRPC6 than TRPC3 (IC50 = 1.1 μM) and 42-fold over TRPC7 (IC50 = 0.55 μM). BI 749327 inhibited NFAT activation in HEK293T cells expressing wild-type or gain-of-function TRPC6 mutants, suppressed Ang II-induced expression of prohypertrophic genes in isolated myocytes, and improved heart function with reduced pathology in mice subjected to sustained pressure overload (Lin et al., 2019).
3.2.2. Modulators for pulmonary arterial hypertension (PAH)
Pulmonary circulation plays an essential role in gas exchange that supports all physiological activities. Dysfunction of pulmonary circulation often causes several progressive diseases such as pulmonary arterial hypertension (PAH) among others (Lambert et al., 2018). Ca2+ plays a key role in pulmonary circulation and TRPC channels contribute to the generation of Ca2+ signals in multiple lung tissues. Numerous studies have demonstrated the potential of modulating TRPC channels in the treatment of PAH. Several endogenous and exogenous factors have been found to regulate TRPC channels in pulmonary arterial smooth muscle cells (PASMCs). Lipopolysaccharide (LPS) and bone morphogenetic protein 4 (BMP4), for example, induce PASMC proliferation through upregulation of expression of TRPC1, TRPC6, and perhaps also TRPC4 (Boucherat & Bonnet, 2015; Jiang et al., 2016; Wang et al., 2015; Zhang et al., 2014). On the other hand, Guanabenz (100 μM) has been shown to elicit Ca2+ influx in human airway epithelial cells independently of the α2-adrenoceptors, the drug’s known clinic target. Based on the effects of isoform specific TRPC siRNAs, it was suggested that guanabenz may directly activate TRPC6, but not TRPC1. Subsequently, the [Ca2+]c increase evoked by guanabenz causes activation of Ca2+-activated Cl− channels in these cells (Bertrand et al., 2015).
Because of the prominent expression of TRPC6 in lung tissues and its involvement in lung function and diseases, including hypoxic vasoconstriction, lung ischemia-reperfusion edema (LIRE) and idiopathic PAH (IPAH) (Weissmann et al., 2006; Weissmann et al., 2012; Yu et al., 2004), it would be highly desirable to verify the therapeutic value of inhibiting TRPC6 channels in lung diseases using pharmacological tools. A number of compounds, such as SKF-96365, econazole, W7, compound 8009–5364, norgestimate, and sildenafil, have been shown to inhibit TRPC6 through either direct or indirect mechanisms (Bon & Beech, 2013; Harteneck & Gollasch, 2011; Lu et al., 2010; Miehe et al., 2012; Urban, Hill, Wang, Kuebler, & Schaefer, 2012; Wang et al., 2013). However, limitations exist due to their low potency and poor selectivity. In addition, a series of anilino-thiazoles have been shown to inhibit TRPC3/6 channels with high potency (Washburn et al., 2013). Although their low oral bioavailability makes them poor candidates of chronic pharmacological studies, some of the analogs should still be excellent research tools for studying TRPC3 and C6 channels.
Recently, SAR7334 was identified as a potent TRPC6 inhibitor (IC50 = 7.9 nM) from a series of aminoindanol derivatives. The compound exhibits reasonable selectivity for TRPC6 over TRPC3/7 channels (36–29 fold, IC50 = 282 nM for C3 and 226 nM for C7) and a good oral pharmacokinetic profile (Maier et al., 2015). In isolated perfused mouse lungs, SAR7334 blocked acute hypoxic pulmonary vasoconstriction in a TRPC6-dependent fashion. Furthermore, a bicyclo[4.3.0]nonane derivative, DS88790512, has been reported to inhibit TRPC6 with an IC50 of 11 nM. This compound only weakly inhibited hERG and hNaV1.5 channels at high micromolar concentrations and it displayed good oral bioavailability (Motoyama et al., 2018). However, the selectivity among TRPC channels is not known and no information is available about its therapeutic potentials. Larixyl N-methylcarbamate, SH045, derived from an abundant natural product, (+)-larixol, has also been shown to block TRPC6 channels expressed in cell lines at low nanomolar concentrations (IC50 = 5.8–62 nM depending on the assays used). The compound exhibited some selectivity over TRPC7 (~3.5 fold) and more so over TRPC3 (~13 fold). In rat PASMCs, SH045 inhibited OAG-induced [Ca2+]c increase with an IC50 of 340 nM, and in explanted mouse lungs, the treatment of SH045 (5 μM) attenuated lung ischemia-reperfusion edema (LIRE) (Hafner et al., 2018). Likewise, larixyl acetate, which inhibited heterologously expressed TRPC6 with IC50 values of 0.1–0.6 μM and suppressed DAG-evoked native TRPC6-like Ca2+ signals in rat PASMCs, also prevented acute hypoxia-induced vasoconstriction in isolated mouse lungs when applied at 5 μM (Urban et al., 2016). Larixyl acetate is one of the main ingredients of larch resin. The compound exhibited 12- and 5-fold selectivity for TRPC6 over TRPC3 and C7, respectively.
In addition, several marketed drugs have been shown to be potential inhibitors of TRPC6 and beneficial for PAH therapy. Sodium tanshinone IIA sulfonate (STS), a medicine with high efficiency on chronic hypoxic pulmonary hypertension, was recently recognized to have inhibitory effect on TRPC6 channels, and shown to suppress hypoxia-induced enhancement of store-operated Ca2+ entry in PASMCs (Wang et al., 2013; Jiang et al., 2016). Moreover, Topotecan (TPT), a topoisomerase inhibitor that is used to treat several cancers, was found to ameliorate the hypoxia-induced PAH by suppressing the upregulation in the expression of hypoxia-inducible factor 1α (HIF-1α) as well as TRPC1, C4, and C6 (Jiang et al., 2018). This effect required low concentrations of TPT (1–10 nM) and extended treatment time (≥24 h), which inhibited the proliferation of PASMCs.
3.2.3. Modulators for neurological disorders
Neurons exhibit the highest expression of various TRPC subtypes, as compared to other cell types, and this is even more true during the early developmental stage (Li et al., 1999). Essentially, any factor that triggers PLC activation can also activate the TRPC channels in neurons, meaning that many of the neurotransmitters and neuromodulators that signal through Gq/11 or tyrosine kinases, or even Gi/o proteins if TRPC4/5 channels are involved (Jeon et al., 2012; Jeon et al., 2016; Thakur et al., 2016), may serve as endogenous triggers for TRPC channel activation, through which they may be linked to membrane excitability and Ca2+ signaling. For example, insulin and leptin can stimulate anorexigenic proopiomelanocortin neurons to participate in energy homeostasis through activation of TRPC channels (Qiu et al., 2014; Qiu, Wagner, Ronnekleiv, & Kelly, 2018). Several endogenous factors implicated in pathogenesis of neurological diseases may act through TRPC channels, including S1P, nerve growth factor, glutamate, serotonin and dopaminergic neurotoxins (Lepannetier et al., 2018; Shimizu et al., 2018; Shirakawa et al., 2017; Sukumaran, Sun, Antonson, & Singh, 2018; Yamamoto, Hatano, Sugai, & Kato, 2014).
A number of small molecular probes of TRPC channels have been found to be effective in animal models of neurological disorders. The first selective small molecular probe for TRPC4/5 channels, ML204, was identified out of a high-throughput screen effort. ML204 blocked TRPC4 and C5 channels in both fluorescent Ca2+ and electrophysiological assays. It showed an IC50 of 0.96 μM for TRPC4β channels in the Ca2+ assay when the channel was activated via stimulation of μOR by the μ agonist, DAMGO. The IC50 was 2.6 μM in the electrophysiological assay (Miller et al., 2011). ML204 has been a valuable probe since its discovery. In a rat visceral pain model induced by colonic exposure to mustard oil, oral feeding or intraperitoneal (i. p.) administration of ML204 led to dose-dependent relief in pain, without any adverse cardiovascular or other side effects (Westlund et al., 2014). In addition, microinjection of ML204 into amygdala of neuropathic pain rats suppressed mechanical hypersensitivity of the injured limb, as well as affective-like pain behavior, in a dose-dependent manner, without obvious side effects (Wei, Sagalajev, Yüzer, Koivisto, & Pertovaara, 2015). Furthermore, ML204 blocked Ca2+ entry and cytotoxicity induced by maitotoxin in human neuronal stem cells (Boente-Juncal, Vale, Alfonso, & Botana, 2018), although maitotoxin has been reported to evoke non-selective cation currents in mammalian cells and Xenopus oocytes in a manner that depends on the expression of TRPC1, but not TRPC4 (Brereton, Chen, Rychkov, Harland, & Barritt, 2001; Flores et al., 2017).
The same high throughput screen as above also identified M084, a 2-aminobenzimidazole derivative, as another TRPC4/5 inhibitor. Although not as potent as ML204, M084 has better pharmacokinetic properties and provides an alternative structural scaffold for further development of more potent TRPC4/5-selective antagonists. M084 has similar potency at TRPC4 and TRPC5 channels with IC50 values of 10.3 ± 0.5 and 8.2 ± 0.7 μM, respectively, when the channels were stimulated via activation of μOR. M084 shows a weak inhibitory effect on TRPC3 with IC50 of ~50 μM (Zhu et al., 2015). Since TRPC4 and TRPC5 had been implicated in fear and anxiety-like behaviors (Riccio et al., 2009; Riccio et al., 2014), inhibiting these channels with the TRPC4/5 blocker might be antidepressant. Indeed, a single i. p. administration of M084 in mice resulted in rapid antidepressant and anxiolytic-like effects, which are accompanied with increases in BDNF and phosphorylation of AKT and ERK in prefrontal cortex (Yang et al., 2015). More recently, a more potent TRPC4/5 antagonist, HC-070, which is structurally unrelated to M084, was also shown to have anxiolytic and antidepressant effects in mice upon oral dosing (Just et al., 2018). HC-070 selectively inhibits TRPC4 and C5 channels with IC50 values of 46.0 ± 3.9 nM and 9.3 ± 0.9 nM, respectively, in the Ca2+ influx assay, and weakly inhibits TRPC3 (IC50 ~1 μM). It shows good pharmacokinetics and sufficient penetration to brain. In electrophysiological recordings, HC-070 appears to show even higher potency than in the Ca2+ influx assay, yielding IC50 values close to or below 1 nM depending on the method of stimulation and the subtype and species of the channel examined. Some of these tests also included TRPC1 for probing the TRPC4–C1 or TRPC5–C1 heteromeric channels (Just et al., 2018). HC-070 is a structural analog of HC-608, also known as Pico145 and so named because it inhibits TRPC4/5 homomers or TRPC4/5 heteromers in complex with TRPC1 at picomolar concentrations (Rubaiy et al., 2017). In Ca2+ influx assay performed in parallel with HC-070, HC-608 exhibited IC50 values of 32.5 ± 1.8 nM and 6.2 ± 0.5 nM, respectively, on TRPC4 and TRPC5 homomeric channels (Just et al., 2018). In the study that described Pico145, the compound inhibited homomeric TRPC4 or TRPC5, heteromeric TRPC4–C1 or TRPC5–C1, and endogenous TRPC4/5-like channels in cancer cell lines with IC50 values that ranged from 9 to 1300 pM, depending on the stimulation method, channel type and the functional assay (Ca2+ versus patch clamping) used. Interestingly, Pico145 appears to be a competitive antagonist when the TRPC4 and TRPC5 channels are activated by (−)-englerin A, showing higher IC50 values as the concentration of (−)-englerin A increases (Rubaiy et al., 2017).
In an effort to determine the molecular target(s) of ginger extract, an analeptic in herbal medicine, for its antioxidant effects, TRPC5 was found to be inhibited by the extract and one of its pungent constituents, [6]-shogaol, which exhibited an IC50 of ~18.3 μM in the electrophysiological assay. Since TRPC5 channels are known to be activated by reactive oxygen species (ROS) and nitric oxide (NO) in central nervous system (CNS) neurons, the antioxidant effects of the ginger extract could be in part mediated by TRPC5 and this property may be applicable to the treatment of neurological diseases (Kim, Hong, Lee, Nam, & Kim, 2016). More interestingly, galangin, a flavonol from galangal which is closely related to ginger, was shown to inhibit TRPC5 with an IC50 of 0.45 μM. Other structurally similar natural flavonols either inhibited (kaempferol and quercetin, IC50 of 3.9 μM and 6.5 μM, respectively), or weakly stimulated (apigenin), or had no effect (myricetin, apigenin and luteolin) on TRPC5. A synthetic derivative of galangin, AM12, inhibited TRPC5 with IC50 of 0.28 μM (Naylor et al., 2016). AM12 can also inhibit TRPC4, but is very weak at the TRPC5–C1 heteromeric channels. Interestingly, AM12 only inhibited TRPC5 when the channel was activated by Gd3+ or (−)-englerin A. With the stimulation by S1P or LPC, AM12 acted more like allosteric activator and enhanced the TRPC5 current (Naylor et al., 2016).
TRPC6 has been shown to play roles in both neural development and neurodegeneration. A number of small molecular probes have shown their effects on brain function through acting at TRPC6 channels. Differing from TRPC4/5, the neuroprotective effect has often been associated with enhancing, rather than inhibiting, TRPC6 function. Hyperforin, an active component from Hypericum species including the medicinal plant, Hypericum perforatum (HPer), also known as St. John’s wort, induces Ca2+ influx to neurons through TRPC6 channels, Hyperforin had been known to have an antidepressive effect with few side-effects through blocking serotonin and norepinephrine uptake via an indirect mechanism (Galeotti, 2017). It was proposed that the Na+ currents generated by hyperforin activation of TRPC6 may dissipate the Na+ gradients across the membrane and thereby suppress the neuronal amine uptake. Interestingly, despite the high homology, TRPC3 is not activated by hyperforin (Leuner et al., 2007). On the other hand, the roles of hyperforin and HPer on neurons may not always be dependent on TRPC6. It has been shown that hyperforin induces TRPC6-independent H+ currents in several cell types and in artificial lipid bilayers not containing any proteins (Sell, Belkacemi, Flockerzi, & Beck, 2014). This latter effect suggests that hyperforin may act as a protonphore, which acidifies the cytoplasm in a voltage-dependent manner and in turn impairs neurotransmitter uptake through the PM Na+/H+ exchangers. In DRG neurons from rats that suffered spinal cord injury, hyperforin and HPer exerted protection through inhibition of TRPM2 and TRPV1 channels (Özdemir, Nazıroğlu, Şenol, & Ghazizadeh, 2016; Uslusoy, Nazıroğlu, & Çiğ, 2017). Despite these findings, hyperforin treatment alleviated the negative impacts of chronic unpredictable stress to rats on cognitive function, long-term potentiation of dentate gyrus, and dendrite morphology, including the length of dendrites, density of spines, and number of excitatory synapses; the same treatment of hyperforin also restored the expression of TRPC6 to normal in hippocampus, which were markedly reduced in rats subject to chronic stress (Liu, Liu, Qin, Zhu, & Yang, 2015). In rats subjected to middle cerebral artery occlusion to cause transient focal cerebral ischemia, intracerebroventricular injection of hyperforin at the onset of injury also mitigated brain damage and the neurological deficits, which were accompanied with the restoration of TRPC6 expression in hippocampus at 24 hr, but not 6 or 12 hr, after the ischemia reperfusion (Lin et al., 2013). Thus, the effect of hyperforin on TRPC6 may include both activation and expression.
Similarly, calycosin, as an antioxidant medicinal plant component, also exerts neuroprotective effects against cerebral ischemia by upregulating the expression of TRPC6 (Guo et al., 2017). For both hyperforin and calycosin, the neuroprotective actions include an inhibition of calpain-mediated degradation of TRPC6 and the restoration of CREB phosphorylation downstream of TRPC6-dependent activation of MAP kinase and CaMKIV pathways (Liu et al., 2015; Guo, Ma, et al., 2017). Moreover, hyperforin was reported to activate transcription factor AP-1 in HEK293 cells that stably expressed mouse TRPC6, but not the wild type HEK293 cells, an activity that was mimicked by another TRPC6 agonist, OAG (Thiel & Rossler, 2017). The TRPC6-dependent transcriptional regulation also includes c-Jun, c-Fos, with contributions from CREB and MAP kinases.
Furthermore, tetrahydrohyperforin (IDN5706), a derivative of hyperforin, was shown to be neuroprotective in the mouse model of Alzheimer’s disease and able to overcome the inhibitory effect of Aβ oligomers on field excitatory postsynaptic potential in hippocampal slices (Montecinos-Oliva, Schüller, Parodi, Melo, & Inestrosa, 2014). Tetrahydrohyperforin is thought to act through TRPC3/6/7 and [Ca2+]c elevation to exert its neuroprotective function as the compound shares the same structural moiety with hyperforin, Hyp9 (2,4-diacylphloroglucinol) (Leuner et al., 2010), and DAG (Sawamura et al., 2016), with a potential pharmacophore of two hydrogen bond acceptors, one hydrogen bond donor, and an extended lipophilic contact surface.
Additionally, a group of piperazine-derived compounds, represented by PPZ1 and PPZ2, were reported to activate TRPC3/6/7 channels and trigger Ca2+ signaling, which in turn resulted in neurotrophic effects such as promotion of neurite outgrowth and neuronal survival under serum-deprived conditions (Sawamura et al., 2016). PPZ1 activated TRPC3, C6, and C7 channels with EC50 values of 57.0, 67.3, and 45.9 μM, respectively; PPZ2 had EC50 values of 10.2, 8.4, and 2.9 μM, respectively. However, despite the apparent low potency, the piperazine compounds conferred neuroprotection at submicromolar concentrations of 3–300 nM. Higher concentrations of PPZ2 was found to be toxic to neurons. The neuroprotective effect of the piperazine compounds also required MAP kinases, CaMKs, and CREB.
Other TRPC3/6/7 activators include a small 1,3-dihydro-2H-benzo [d]imidazol-2-one-based potent agonist (GSK1702934A), which has been briefly described in the abstract form by GlaxoSmithKline-US as a tool to directly activate TRPC3/6 channels independently of PLC signaling. GSK1702934A induces currents in HEK293 cells expressing human TRPC3 and C6 with EC50 values of 80 and 440 nM (patchclamp), respectively (Xu et al., 2013). The compound has been successfully used by others to activate TRPC3, C6, and C7 channels (Ding et al., 2018; Doleschal et al., 2015). An improved method in the synthesis of GSK1702934A and its structural analogs has been described (de la Cruz et al., 2017). Moreover, an azobenzene photoswitch moiety has been added to the main scaffold of GSK1702934A to create a light-sensitive TRPC agonist, OptoBI-1, which allows for photoactivation of TRPC3/6/7, but not TRPC4/5, channels. Interestingly, light treatment of hippocampal neurons exposed to OptoBI-1 suppressed action potential firing elicited by current injection (Tiapko et al., 2019). Furthermore, the latest study has reported a positive allosteric TRPC6 modulator, C20, that selectively exaggerate TRPC6-dependent signals. Unlike OAG and many other TRPC3/6/7 drugs, C20 acts at TRPC6 without affecting the closely related TRPC3 and C7. Detailed analysis revealed that C20 rather functions as an enhancer of TRPC6 activation than a direct activator by itself, showing its role as an allosteric regulator of TRPC6 that enables low basal concentration of DAG to elicit TRPC6 channel activation (Hafner, Urban, & Schaefer, 2019). Moreover, recent studies have reported that mycotoxins, such as zeranol and aflatoxin B1, induce COX-2 expression by mediating TRPC3 activation in the placental cells JEG-3. This poses a potential threat to pregnant women because TRPC3 is commonly expressed in the reproductive tissues in human and plays a critical role in the female reproductive processes (Zhu, Tan, & Leung, 2016; Zhu, Yao, & Leung, 2016). However, at this point, no information is available about how the above drugs affect neurons and neurological functions.
Indeed, it is not always beneficial to the neurological function to activate TRPC6 channels. For example, TRPC6 activation is involved in endothelial dysfunction in concussions, or mild, traumatic brain injury. With the treatment of larixyl acetate, which inhibits TRPC6 as described above, the damage on aortic endothelial cells was alleviated (Chen et al., 2019).
3.2.4. Modulators for kidney diseases
Growing evidence has implicated the role of TRPC channels in the kidney and the development of kidney diseases. Most prominently, mutations in TRPC6 are linked to familial focal segmental glomerulosclerosis (FSGS) (Reiser et al., 2005; Winn et al., 2005). While many of these mutations exhibit gain-of-function in channel activity, some of them also display loss-of-function phenotypes (Riehle et al., 2016). Thus, either too high or too low a TRPC6 channel activity seems to be detrimental to kidney function. The TRPC6 mutations mainly affect the filtering function of podocytes, where the slit diaphragm formed between feet or processes of the podocytes blocks the passage of large molecules, such as proteins, to the proximal tubule, but allows small molecules like glucose, ions, and water to go through. As a key component of the slit diaphragm, TRPC6 is critical for glomerular development, allowing the terminally differentiated podocytes to properly interface with glomerular capillaries (Liu et al., 2015). The mutations in TRPC6 disrupt the slit diaphragm formation, causing proteins to leak into the urine, i. e. proteinuria. Therefore, manipulation of the TRPC6 function should bring benefit to FSGS.
Intriguingly, TRPC5 has also been implicated in the pathogenesis of progressive kidney diseases, including FSGS (Schaldecker et al., 2013; Zhou et al., 2017). It was suggested that TRPC5 and TRPC6 may antagonize each other on regulating actin cytoskeleton, and thereby the contractility of podocytes, via Rac1 and RhoA, respectively (Greka & Mundel, 2011). The balance between podocyte TRPC5 and C6 activities then may be the most critical for glomerular filtration barrier function. However, the role of TRPC5 in renal function was recently challenged by another research group who examined TRPC5 gain-in-function mutant mice but found no increase in proteinuria even under conditions of kidney injury (Wang et al., 2018).
Based on the premise for TRPC5 to be involved in progressive kidney disease, a specific TRPC5 inhibitor, AC1903, (IC50 = 14.7 μM, by patch clamp recording) was developed. AC1903 shares the benzimidazole backbone with M084 (Zhu et al., 2015) and clemizole (Richter, Schaefer, & Hill, 2014a). It exerted a very weak effect on TRPC4 and no effect on TRPC6. In the rat model of FSGS, chronic administration of AC1903 attenuated the severe proteinuria and protected podocytes from damage. In the hypertensive proteinuric kidney disease model, AC1903 also showed therapeutic benefit (Zhou et al., 2017). Another TRPC5 inhibitor, GFB-887, developed by a biotechnology company, Goldfinch Bio, was also claimed to suppress pathogenic podocyte motility and proteinuria in both a hypertension-induced FSGS rat model without altering blood pressure and a non-hypertensive FSGS model by puromycin aminonucleoside nephrosis. These results demonstrate a direct glomerular protection in vivo by GFB-887 and the drug’s potential in treating chronic kidney disease. GFB-887 is described as a potent, selective and orally bioavailable small molecule TRPC5 antagonist; however, its structure has not been disclosed (Mundel et al., 2019).
For TRPC6, 20-hydroxyeicosatetraenoic acid (20-HETE), a known agonist of TRPC6 channels (Basora, Boulay, Bilodeau, Rousseau, & Payet, 2003) and an agent that causes podocyte apoptosis (Eid et al., 2009), has been shown to activate TRPC6-like channels and increase TRPC6 surface abundance in cultured podocytes when applied exogenously. 20-HETE is a metabolite of arachidonic acid and known to regulate glomerular function during tubuloglomerular feedback. This could represent an endogenous cue of regulating renal function through, at least in part, TRPC6 in podocytes. However, in the cultured podocytes, 20-HETE does not appear to activate TRPC6 directly; the activation required ROS production and G proteins (Roshanravan, Kim, & Dryer, 2016). Consistent with the detrimental effect of TRPC6 overactivation on kidney, (+)-conocarpan, a benzofuran neolignane found in many medicinal plants, was shown to induce apoptosis of human proximal tubular epithelial (HK-2) cells through TRPC6, with accompanied [Ca2+]c increase and caspase-3 activation. In whole-cell recordings, (+)-conocarpan evoked currents in TRPC6-expressing HEK293 cells with an EC50 of 6.01 μM (Yang et al., 2019). Because TRPC6 function is linked to NFAT activation through calcineurin, the calcineurin inhibitor, FK506, has been tested for its ability to ameliorate podocyte injury in the rat model of type 2 diabetic nephropathy. With the long-term (12 weeks) administration, FK506 was found to mitigate many of the hallmarks of nephropathy and this was accompanied with attenuation of the upregulation of TRPC6 and NFAT expression found in untreated diabetic animals (Ma, Liu, Jiang, Yu, & Song, 2015). Additionally, an antidiabetic drug, rosiglitazone (RSG), has been shown to inhibit the proliferation of rat glomerular mesangial cells (HBZY-1) induced by Ang II. Here, the drug was thought to activate PPAR-γ, which upregulates RGS4 expression and in turn reduces Gq signaling. The decreased Gq activity then results in lower activation of TRPC1 and TRPC6 by Ang II (Wei et al., 2017). Also acting through PPAR-γ, the protein kinase G activator, sildenafil, was shown to prevent proteinuria through suppressing the increase in the podocyte expression of TRPC6 in rats with nephropathy induced by adriamycin and mice suffering from renal injury due to hyperglycemia. The PPAR-γ activator, pioglitazone, had similar protective effects. In this case, PPAR-γ was thought to act at the TRPC6 promoter to suppress its expression (Sonneveld et al., 2017). However, in contrast to the above scenarios, downregulation of TRPC6 has been implicated in kidney injury associated with iatrogenic hyperinsulinemia due to long-term use of insulin to treat diabetes. The damage was found to be alleviated by astragaloside IV, a type of saponin from Astragalus membranaceus (Fisch) Bunge, which also ameliorated the downregulation of TRPC6 expression seen in the iatrogenic hyperinsulinemia model (He et al., 2018).
Some of the direct antagonists of TRPC6, or of TRPC3/6/7, have also been tested for their effect on kidney or renal cells. For example, BI 749327 was shown to dose dependently reduce renal fibrosis and changes in the gene expression pattern found in the mouse model of unilateral ureteral obstruction (Lin et al., 2019). SAR7334 not only diminished TGFβ1-induced fibrogenesis of HK-2 cells (Zhou et al., 2018), but also protected renal proximal tubular cells from apoptotic death induced by oxidative stress (Hou et al., 2018). In a model of glomerular permeability response to the drop of oncotic pressure, SAR7334 also attenuated the inhibitory action of Ang II on glomeruli volume increase (Ilatovskaya, Palygin, Levchenko, Endres, & Staruschenko, 2017). Additionally, two novel TRPC6 inhibitors isolated from Ribes manshuricum, Ribemansides A and B, which inhibited TRPC6 activity with IC50 values of 24.5 and 25.6 μM, respectively, were also shown to suppress TGF-β1-induced fibrogenesis in the HK-2 cells in a similar fashion as SAR7334 (Zhou et al., 2018). Recently, BTDM was reported to be a potent inhibitor of TRPC3 (IC50 = 11 nM) and TRPC6 (IC50 = 10 nM), as well as some of the gain-of-function TRPC6 mutants found in FSGS; the compound directly binds to TRPC6 (Tang et al., 2018). However, the effects of this drug on kidney cells and kidney disease models have yet to be reported.
Collectively speaking, small molecular probes that inhibit TRPC5 or TRPC6 either directly or indirectly through downregulation of the channel’s expression, function or its downstream signaling pathways have shown promising protective effects in kidney diseases. Further investigation into the roles of these channels in different renal cell types and pathogenesis of various renal diseases, as well as the effects of TRPC5 and C6 modulators on kidney dysfunction, will bring new insights into therapeutic development. In this context, the recently reported series of pyrazolopyrimidines, developed based on a lead compound identified using a cell-based high throughput screening assay against TRPC6, are worth noting. One of the modified analogs, 4n, designed based on the lead compound, exhibits the most efficacious activation on TRPC3 (EC50 = 19 nM), followed by TRPC7 (EC50 = 90 nM) and TRPC6 (EC50 = 1.39 μM) (Qu et al., 2017). Further modifications of this series also gave rise to a potent partial agonist, 14a, which essentially suppresses TRPC6 activity with an IC50 of 1 μM (Ding et al., 2018). Given that the agonists from this series activated native TRPC3/6 channels in rat glomerular mesangial cells (Qu et al., 2017), it is possible that this series of compounds may lead to more effective and specific TRPC modulators and contribute to the development of new treatments for renal diseases.
3.2.5. Modulators for cancers
TRPC channels have been implicated in the pathogenesis of various cancers [He & Ma, 2016; Gaunt et al., 2016; Jardin & Rosado, 2016; Li & Ding, 2017; Zhan & Shi, 2017]. These Ca2+-permeable nonselective cation channels likely participate in tumor development to different extents by affecting cell metabolism, controlling cell proliferation, promoting angiogenesis, and supporting cell migration and invasion during tumor progression. One of the common effects that had been frequently seen for TRPC channels is the role on epithelial mesenchymal transition (EMT) (Ge et al., 2018; Xu et al., 2018; Xu, Liu, Li, Ma, & Zhang, 2017), but the underlying mechanism remains to be elucidated. In addition, as described earlier, TRPC5 has been implicated in chemoresistance (Ma et al., 2012; Wang et al., 2015)
Although much evidence about the involvement of individual TRPC subtypes in various cancers have been obtained by the examination of mRNA and protein expression and gene silencing with siRNA, pharmacological evidence is also accumulating. Indeed, some TRPCs have been shown to be expressed at high levels in certain cancers and at particular stages of cancer progression, making them potential diagnostic markers (Jiang et al., 2013; Zeng, Yuan, Yang, Atkin, & Xu, 2013). Among these is TRPC4 in many renal cell carcinomas (Akbulut et al., 2015). Interestingly, a sesquiterpene agent, (−)-englerin A, a natural product obtained from Phyllanthus engleri and known to be highly toxic to renal cell carcinoma, has been found to be a highly selective and nanomolar-potent TRPC4 and C5 activator, with EC50 values of 11.2 nM and 7.6 nM, respectively (Akbulut et al., 2015). Although it was originally thought that (−)-englerin A inhibits proliferation of tumor cells that express high levels of TRPC4 or TRPC5 by promoting Ca2+ entry and causing intracellular Ca2+ overload, a more recently study suggests that (−)-englerin A may confer cancer cell cytotoxicity through sustained Na+ entry via activation of heteromeric TRPC1–C4 channels (Ludlow et al., 2017). (−)-Englerin A has been found to be extremely toxic in vivo due to its stability in the plasma (Carson et al., 2015), or it is rapidly metabolized to an inactive product, (−)-englerin B (Minard et al., 2018). Either will preclude its clinical use. Therefore, chemical synthesis of (−)-englerin A and its analogs has been undertaken in order to improve its bioavailability and better understand the mechanism of its action (Radtke et al., 2011). Similar to the situation for the pyrazolopyrimidine compounds described above, one of the analogs, A54, was found to reverse the action of the original compound. Instead of activation, A54 inhibited (−)-englerin A-evoked [Ca2+]c increase in A498 renal cell carcinoma with an IC50 of 62 nM in a competitive manner. It also strongly blocked (−)-englerin A-evoked currents of TRPC1–C4 heteromeric channels and TRPC5 homomeric channels, and weakly inhibited the activation of TRPC4 homomers. On the other hand, A54 potentiated the activation of TRPC4 by S1P and that of TRPC5 by Gd3+. These results indicate that minor modifications of the side chain in the compound can enable drastic changes in the outcome of the ligand binding (Rubaiy et al., 2018).
Similar to (−)-englerin A, another natural product, Tonantzitlolone (TZL), from Euphorbiaceae, also exerts cytotoxicity towards certain types of cancers including renal cell carcinoma lines. Although chemically distinct, TZL was shown to also activate TRPC4, C5, C1–C4 and C1–C5 heteromeric channels with EC50 values of 123 nM, 83 nM, 140 nM, and 61 nM, respectively. Moreover, the effects of TZL were reversible on wash-out and effectively inhibited by the TRPC1/4/5 inhibitor, Pico145. Therefore, TZL offers another option of achieving selective cytotoxicity against certain types of cancer cells through activating TRPC1/4/5 (Rubaiy et al., 2018).
To more specifically target TRPC5, riluzole has been identified as a selective TRPC5 agonist from a library of approved drugs and natural compounds. It was reported to activate TRPC5 with an EC50 of 9.2 ± 0.5 μM, independently of G protein signaling and PLC activity. Moreover, riluzole is able to activate both heterologously expressed TRPC5 in HEK293 cells and endogenous channels in U-87 glioblastoma cells (Richter, Schaefer, & Hill, 2014b). However, riluzole is also known to inhibit voltage-gated Na+ and Ca2+ channels and thereby attenuate neurotransmitter release (Bellingham, 2011). To validate the specificity of riluzole activation of TRPC5 in U-87 cells, the potent inhibitor of TRPC5, clemizole hydrochloride, was used. Clemizole inhibited TRPC5 with IC50 of about 1.0–1.3 μM, which is about 6-fold selective than TRPC4β (IC50 = 6.4 μM) and 9- to 10-fold selective than TRPC3 (IC50 = 9.1 μM) and TRPC6 (IC50 = 11.3 μM) (Richter et al., 2014a).
Additional TRPC5 activators include a benzothiadiazine derivative (BTD, EC50 = 1.4 μM) and methylprednisolone (EC50, 12 μM). Prednisolone also acts as a weak activator of TRPC5 with an EC50 of 64 ± 15 μM (Beckmann et al., 2017). In addition, a structural analog of HC-070 and Pico145, AM237, was shown to specifically activate TRPC5 with EC50 values of 15–20 nM (Minard et al., 2019). The lack of stimulatory effect of these compounds on TRPC4 and TRPC1–C4 heteromers could make them good probes for distinguishing TRPC4 and TRPC5 channels in native systems. However, their effects on cancer have not been reported. In addition, not only did AM237 show a decline in stimulating TRPC5 when used at high concentrations, but it also inhibited (−)-englerin A-induced activation of TRPC5, as well as TRPC4 and TRPC1–C4, channels, implicating complex gating effects (Minard et al., 2019).
Functional TRPC6 channels are found to be overexpressed in prostate, stomach, breast cancers, as well as neuroglia, as compared to the low or undetectable levels in the corresponding normal tissues. Recent studies have demonstrated [Ca2+]c rise mediated via activation of α1-adrenoceptors in normal mesangial cells and prostate cancer cells, which elicits a proliferative signal, implicating the control in cell proliferation (Santoni et al., 2015). In prostate cancer mouse model of knock-In mouse adenocarcinoma prostate (KIMAP), dietary supplement of high Ca2+ accelerated the progression of prostate tumors in a manner that involves increasing the expression of calcium sensing receptor and TRPC6, an effect that was attenuated through dietary supplement of Vitamin D3. This effect of Ca2+ and its inhibition by Vitamin D3 were also reproduced in cultured prostate cancer cell lines (Bernichtein et al., 2017). Additionally, (−)-oleocanthal (OLCT), a phenolic compound from olive oil, was reported to inhibit the proliferation and migration of breast cancer cells, including triple negative breast cancer cells, via decreasing the expression of TRPC6 without having effects on non-tumoral breast cells (Diez-Bello et al., 2019). Lovastatin, through inhibition of cholesterol synthesis, also suppressed the expression of TRPC6 and thereby the proliferation of human B lymphoma Daudi cell. Here, the effect of cholesterol on TRPC6 expression required ROS production, as reducing ROS with TEMPOL or apocynin had similar effects as lovastatin (Song et al., 2014).
Direct TRPC3/6/7 antagonists have also shown effectiveness in suppressing cancer. Although not specific, SKF-96365, has been frequently shown to exert cytotoxic potential in several types of human cancers. For instance, SKF-96365 was reported to induce cell cycle arrest in G2/M phase and inhibit the growth of gastric cancer cells through blocking TRPC6 (Cai et al., 2009); it was also shown to suppress the growth of glioblastoma cells by promoting the reverse mode of Na+/Ca2+ exchangers and thereby increasing [Ca2+]c (Song, Chen, & Yu, 2014); in B lymphoma Daudi cells; SKF-96365 inhibited cell proliferation of human hepatocellular carcinoma cell lines, HepG2 and Huh7, suppressed TGFβ-induced [Ca2+]c elevation, cell migration and invasion, and the EMT (Xu et al., 2018). Moreover, the novel pyrazolo[1,5-a] pyrimidine TRPC6 antagonists, compound, 14a, displayed a potent inhibitory effect on the growth of gastric cancer cells in culture and suppressed xenograft tumor formation in nude mice with excellent bioavailability. In the in vitro cytotoxicity assay, 14a concentration-dependently inhibited the proliferation of gastric cancer cells, AGS and MKN45, with IC50 values of 17.1 and 18.5 μM, respectively, while exhibiting no cytotoxicity at HK-2 cells at a high concentration of 100 μM, indicating an excellent therapeutic potential against human gastric cancer (Ding et al., 2018).
At the time of this writing, the effects of many of the new TRPC modulators described above for other systems have not been described. Given the critical involvement of individual TRPC subtypes in different aspects of various cancers, it is anticipated that many of the novel TRPC probes will find their use in cancer therapies. Furthermore, microRNA (miRNA), endogenous single-stranded, noncoding RNA molecules, that modulate gene transcription and expression, have also been shown to affect TRPC channels. For example, miR-200b-3p inhibits TRPC6 in podocytes (Yin, Zhang, Li, Li, & Yang, 2018), miR-26a-5p suppresses the expression of TRPC3, which promotes the growth and invasion of melanoma (Gao, Zeng, Liu, Gao, & Liu, 2019). All these could potentially be targeted for therapeutic benefit.
4. Conclusion
The pivotal roles of TRPC channels in physiology and disease are only beginning to be more fully understood. TRPC channels are critically involved in normal physiological and pathophysiological responses in many systems including vasculature, nervous system, kidney, inflammation and immunity. These channels play important roles in various cellular functions through regulation of membrane potential and calcium signaling and they are under tight regulation by multiple mechanisms to allow them to function as sensors to different environmental cues. The recent development of a large collection of novel TRPC small molecular probes complements the traditional genetic approaches, which not only provides powerful tools for further understanding the physiological significance of TRPC channels and the mechanisms of their regulation, but also offers potential new strategies to devise clinic therapies to treat TRPC-associated diseases. Certainly, considerable work is still needed to more fully understand the structural, functional, and mechanistic aspects of TRPC channel biology. An improved understanding of the underlying mechanisms of TRPC regulation may assist in the identification of more selective pharmacological agonists and antagonists of TRPCs or interdependent channels and promote exciting opportunities to develop new therapies that prevent or treat TRPCs-related diseases. With the advancement of the cryo-EM technology and the vastly improved capability to obtain high-resolution structural information of these channels, it is expected that more efficient and selective tool compounds and highly effective drugs targeting TRPCs will be discovered at an accelerated rate.
Table 2.
Small molecule TRPC3, C6, C7 antagonists.
| Namea | Structure | Target | Concentration or IC50 | Reference |
|---|---|---|---|---|
|
GSK2332255B (GSK255B) |
 |
TRPC3 TRPC6 |
IC50, 5 nM IC50, 4 nM |
Seo et al., 2014 |
|
GSK2833503A (GSK503A) |
 |
TRPC3 TRPC6 |
IC50, 21 nM IC50, 3 nM |
Seo et al., 2014 |
| BI 749327 | 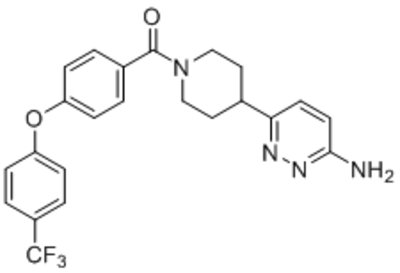 |
TRPC3 TRPC6 TRPC7 |
IC50, 1,100 nM IC50, 13 nM IC50, 550 nM |
Lin et al., 2019 |
| Anilino-thiazoles |  |
TRPC3 TRPC6 |
100 nM 16 nM |
Washburn et al., 2013 |
| SAR7334 |  |
TRPC3 TRPC6 TRPC7 |
IC50, 282 nM IC50, 7.9 nM IC50, 226 nM |
Maier, et al., 2015 |
| BTDM |  |
TRPC3 TRPC6 |
IC50, 11 nM IC50, 10 nM |
Tang, et al., 2018 |
| Larixyl acetate | 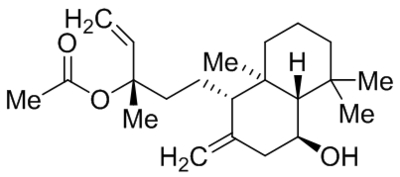 |
TRPC3 TRPC6 TRPC7 |
12 fold > C6 IC50, ~0.1 μM 5 fold > C6 |
Urban et al., 2016 |
| SH045 | 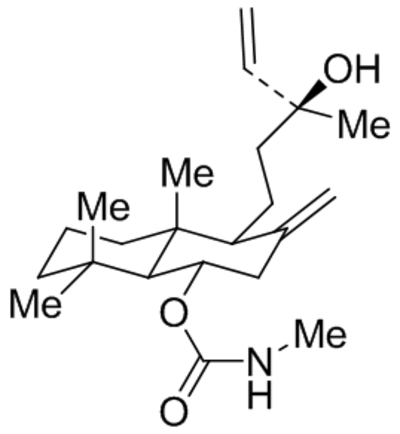 |
TRPC3 TRPC6 TRPC7 |
13 fold > C6 IC50, ~5.8 nM 3.5 fold > C6 |
Hafner et al., 2018 |
| Pyrazolopyrimidines 14a | 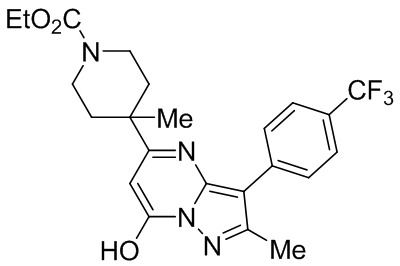 |
TRPC6 | IC50, 1 μM | Ding et al., 2018 |
| Pyr10 | 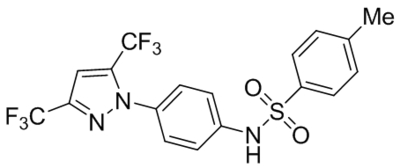 |
TRPC3 TRPC6 |
IC50, 0.72 μM IC50 ≥ 10 μM |
Schleifer et al., 2012 |
| Pyr3 | 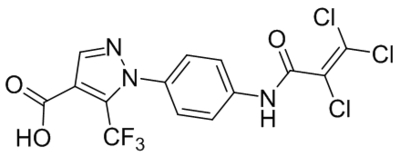 |
TRPC3 | IC50, 0.7 μM | Kiyonaka et al., 2009; Koenig et al., 2013 |
| DS88790512 | 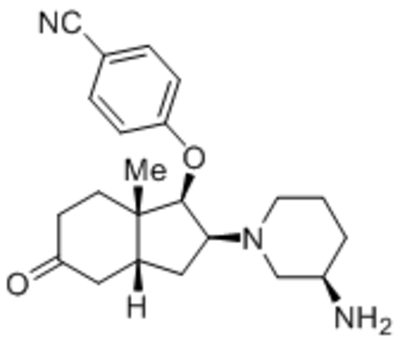 |
TRPC6 | IC50, 11 nM | Motoyama, et al., 2018 |
| Salvianolic acid B |  |
TRPC3 TRPC6 |
27.8 μM | Chen et al., 2017b |
| Sodium tanshinone IIA sulfonate (STS) |  |
Attenuated TRPC6, C1 upregulation | 12.5 μM |
Wang et al., 2013a Jiang et al., 2016a. |
| Ribemansides A | 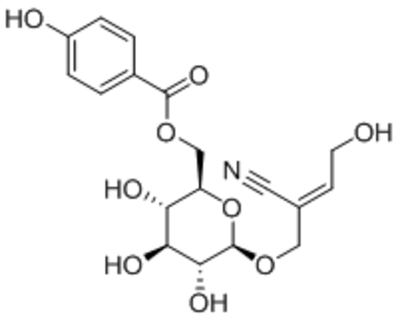 |
TRPC6 | IC50, 24.5 μM | Zhou et al., 2018 |
| Ribemansides B | 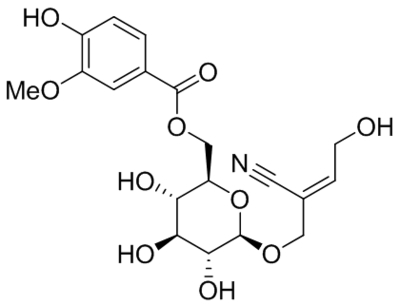 |
TRPC6 | IC50, 25.6 μM | Zhou et al., 2018 |
| Topotecan (TPT), |  |
Suppressed expression of TRPC6 | 1–10 nM | Jiang et al., 2018 |
| FK506 |  |
Attenuated TRPC6 upregulation | 6.2 μM | Ma et al., 2015 |
| Rosiglitazone (RSG) | 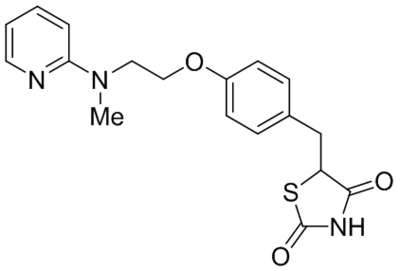 |
Reduced TRPC6, C1 activation | 10 μM | Wei et al., 2017 |
| Sildenafil | 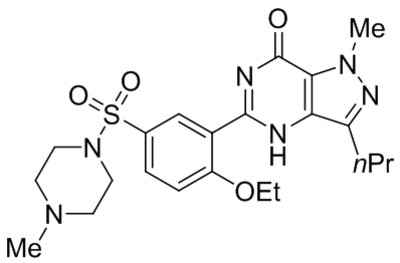 |
Suppressed TRPC6 expression | 300 nM | Lu et al., 2010 |
| (‑)‑Oleocanthal (OLCT) | 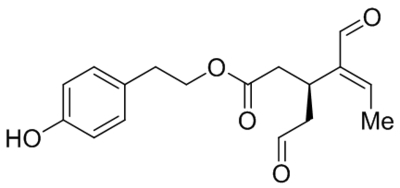 |
Suppressed TRPC6 expression | 10–20 μM | Diez-Bello, et al., 2019 |
| Pioglitazone | 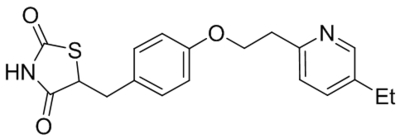 |
Suppressed TRPC6 expression | 12 mg/kg body weight (in vivo), 1 μM (in vitro) | Sonneveld et al., 2017 |
Compounds with selectivity information and likelihood of direct interaction with the channel are highlighted in bold letters and shaded rows.
Table 3.
Small molecule TRPC1, C4, C5 agonists.
| Namea | Structure | Target | Concentration or EC50 | Reference |
|---|---|---|---|---|
| (−)-Englerin A | 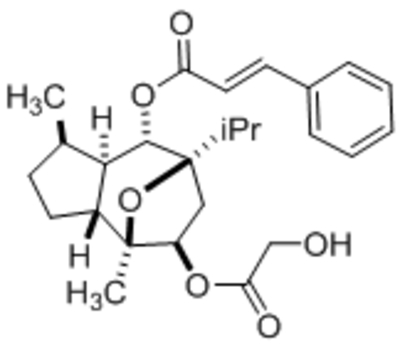 |
TRPC4 TRPC5 |
EC50, 11.2 nM EC50, 7.6 nM |
Akbulut et al., 2015 |
| Tonantzitlolone (TZL), | 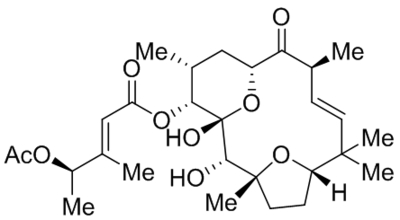 |
TRPC4 TRPC5 TRPC1–C4 TRPC1–C5 |
EC50, 123 nM EC50, 83 nM EC50, 140 nM EC50, 61 nM |
Rubaiy et al., 2018a |
| AM237 | 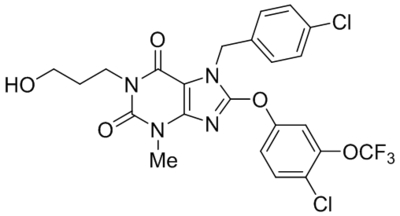 |
TRPC5 | EC50, 15–20 nM | Minard et al., 2019. |
| Benzothiadiazine derivative (BTD) | 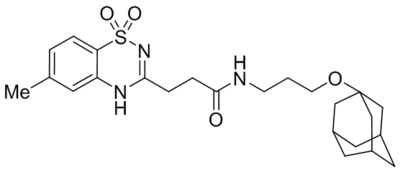 |
TRPC5 | EC50, 1.4 μM | Beckmann et al., 2017 |
| Riluzole | 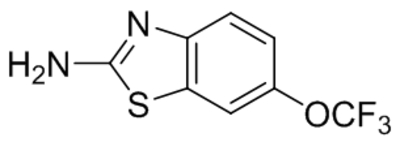 |
TRPC5 | EC50, 9.2 μM, | Richter et al., 2014b |
| Maitotoxin | 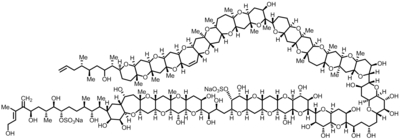 |
TRPC1 | EC50, 10 pM | Brereton et al., 2001; Flores, et al., 2017 |
| Methyl-prednisolone |  |
TRPC5 | EC50, 12 μM | Beckmann et al., 2017 |
| Prednisolone |  |
TRPC5 | EC50, 64 μM | Beckmann et al., 2017 |
Compounds with selectivity information and likelihood of direct interaction with the channel are highlighted in bold letters and shaded rows.
Acknowledgments
We thank members of the Hong lab, Wang lab, and Zhu lab for their constant support. The research in the labs of H Wang is supported by grants on Key Research Project of Shandong Province (2017GSF18177), The Science and Technology Support Program for Youth Innovation in Universities of Shandong (2019KJM009), Taishan Scholar Project and Key Research Project of Yantai (2019XDHZ102), of X. Hong by grants from NSFC (81773674, 81573383, 21473041), the Applied Basic Research Program of WMBST (2019020701011429), and Tibet Autonomous Region Science and Technology Plan Project Key Project (XZ201901-GB-11), Project First-Class Disciplines Development Supported by Chengdu University of Traditional Chinese Medicine (CZYJC1903), and of M. X. Zhu by grants from National Institutes of Health (R01 NS092377, R01 NS102452).
Footnotes
Declaration of Competing Interest
The authors claim no conflict of interest.
References
- Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, Bruns A, … Waldmann H (2015). (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angewandte Chemie (International Ed. in English) 54, 3787–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarran L, Dionisio N, Lopez E, Salido GM, Redondo PC, & Rosado JA (2014). STIM1 regulates TRPC6 heteromultimerization and subcellular location. The Biochemical Journal 463, 373–381. [DOI] [PubMed] [Google Scholar]
- Albert AP, & Large WA (2003). Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. The Journal of Physiology 552, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Saleh SN, & Large WA (2008). Inhibition of native TRPC6 channel activity by phosphatidylinositol 4,5-bisphosphate in mesenteric artery myocytes. The Journal of Physiology 586, 3087–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Carbajo L, Kecskes M, Jacobs G, Pironet A, Syam N, Talavera K, & Vennekens R (2017). Muscling in on TRP channels in vascular smooth muscle cells and cardiomyocytes. Cell Calcium 66, 48–61. [DOI] [PubMed] [Google Scholar]
- Al-Shawaf E, Naylor J, Taylor H, Riches K, Milligan CJ, O’Regan D, … Beech DJ (2010). Short-term stimulation of calcium-permeable transient receptor potential canonical 5-containing channels by oxidized phospholipids. Arteriosclerosis, Thrombosis, and Vascular Biology 30, 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Shawaf E, Tumova S, Naylor J, Majeed Y, Li J, & Beech DJ (2011). GVI phospholipase A2 role in the stimulatory effect of sphingosine-1-phosphate on TRPC5 cationic channels. Cell Calcium 50, 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS (2016). Calcium signalling in salivary gland physiology and dysfunction. The Journal of Physiology 594, 2813–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampem PT, Smedlund K, & Vazquez G (2016). Pharmacological evidence for a role of the transient receptor potential canonical 3 (TRPC3) channel in endoplasmic reticulum stress-induced apoptosis of human coronary artery endothelial cells. Vascular Pharmacology 76, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antigny F, Sabourin J, Sauc S, Bernheim L, Koenig S, & Frieden M (2017). TRPC1 and TRPC4 channels functionally interact with STIM1L to promote myogenesis and maintain fast repetitive Ca2+ release in human myotubes. Biochimica et Biophysica Acta, Molecular Cell Research 1864, 806–813. [DOI] [PubMed] [Google Scholar]
- Asanov A, Sampieri A, Moreno C, Pacheco J, Salgado A, Sherry R, & Vaca L (2015). Combined single channel and single molecule detection identifies subunit composition of STIM1-activated transient receptor potential canonical (TRPC) channels. Cell Calcium 57, 1–13. [DOI] [PubMed] [Google Scholar]
- Autzen HE, Myasnikov AG, Campbell MG, Asarnow D, Julius D, & Cheng Y (2017). Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Medina J, Mayoral-Gonzalez I, Dominguez-Rodriguez A, Gallardo-Castillo I, Ribas J, Ordoñez A, & Smani T (2018). The complex role of store operated calcium entry pathways and related proteins in the function of cardiac, skeletal and vascular smooth muscle cells. Frontiers in Physiology 9, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumaya CM, Sierra-Valdez F, Cordero-Morales JF, & Nakagawa T (2018). Cryo-EM structure of the cytoplasmic domain of murine transient receptor potential cation channel subfamily C member 6 (TRPC6). The Journal of Biological Chemistry 293, 10381–10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, & Payet MD (2003). 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. The Journal of Biological Chemistry 278, 31709–31716. [DOI] [PubMed] [Google Scholar]
- Bavencoffe A, Zhu MX, & Tian JB (2017). New aspects of the contribution of ER to SOCE regulation: TRPC proteins as a link between plasma membrane ion transport and intracellular Ca2+ stores. Advances in Experimental Medicine and Biology 993, 239–255. [DOI] [PubMed] [Google Scholar]
- Becker EB, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, … Davies KE (2009). A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proceedings of the National Academy of Sciences of the United States of America 106, 6706–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann H, Richter J, Hill K, Urban N, Lemoine H, & Schaefer M (2017). A benzothiadiazine derivative and methylprednisolone are novel and selective activators of transient receptor potential canonical 5 (TRPC5) channels. Cell Calcium 66, 10–18. [DOI] [PubMed] [Google Scholar]
- Beech DJ (2013). Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circulation Journal 77, 570–579. [DOI] [PubMed] [Google Scholar]
- Bellingham MC (2011). A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neuroscience & Therapeutics 17, 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernichtein S, Pigat N, Barry Delongchamps N, Boutillon F, Verkarre V, Camparo P, … Goffin V (2017). Vitamin D3 prevents calcium-induced progression of early-stage prostate tumors by counteracting TRPC6 and calcium sensing receptor upregulation. Cancer Research 77, 355–365. [DOI] [PubMed] [Google Scholar]
- Berridge MJ (1995). Capacitative calcium entry. The Biochemical Journal 312, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Dannhoffer L, Antigny F, Vachel L, Jayle C, Vandebrouck C, … Norez C (2015). A functional tandem between transient receptor potential canonical channels 6 and calcium-dependent chloride channels in human epithelial cells. European Journal of Pharmacology 765, 337–345. [DOI] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, & Clapham DE (2004). Rapid vesicular translocation and insertion of TRP channels. Nature Cell Biology 6, 709–720. [DOI] [PubMed] [Google Scholar]
- Blair NT, Kaczmarek JS, & Clapham DE (2009). Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. The Journal of General Physiology 133, 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boente-Juncal A, Vale C, Alfonso A, & Botana LM (2018). Synergistic effect of transient receptor potential antagonist and amiloride against maitotoxin induced calcium increase and cytotoxicity in human neuronal stem cells. ACS Chemical Neuroscience 9, 2667–2678. [DOI] [PubMed] [Google Scholar]
- Bon RS, & Beech DJ (2013). In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels - mirage or pot of gold? British Journal of Pharmacology 170, 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O, & Bonnet S (2015). NOGGIN: A new therapeutic target for PH? Focus on “Noggin inhibits hypoxia-induced proliferation by targeting store-operated calcium entry and transient receptor potential cation channels”. American Journal of Physiology. Cell Physiology 308, C867–C868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Brown D, Qin N, Jiang M, Dietrich A, Zhu MX, … Birnbaumer L (1999). Modulation of Ca2+ entry by TRP-binding polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R). Evidence for roles of TRP and IP3R in store-depletion-activated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America 96, 14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, & Birnbaumer L (1997). Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. The Journal of Biological Chemistry 272, 29672–29680. [DOI] [PubMed] [Google Scholar]
- Boustany CE, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, & Capiod T (2010). Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 47, 2068–2077. [DOI] [PubMed] [Google Scholar]
- Brereton HM, Chen J, Rychkov G, Harland ML, & Barritt GJ (2001). Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochimica et Biophysica Acta 1540, 107–126. [DOI] [PubMed] [Google Scholar]
- Bröker-Lai J, Kollewe A, Schindeldecker B, Pohle J, Nguyen Chi V, Mathar I, … Freichel M (2017). Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. The EMBO Journal 36, 2770–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, … Wang Y (2009). Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. International Journal of Cancer 125, 2281–2287. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Locknar SA, & Parsons RL (2002). TRPC6 immunoreactivity is colocalized with neuronal nitric oxide synthase in extrinsic fibers innervating guinea pig intrinsic cardiac ganglia. The Journal of Comparative Neurology 450, 283–291. [DOI] [PubMed] [Google Scholar]
- Carson C, Raman P, Tullai J, Xu L, Henault M, Thomas E, … Solomon JM (2015). Englerin A agonizes the TRPC4/C5 cation channels to inhibit tumor cell line proliferation. PLoS One 10, e0127498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Berwick ZC, Bartlett PJ, Kumar S, Thomas AP, Sturek M, … Obukhov AG (2011). Bromoenol lactone inhibits voltage-gated Ca2+ and transient receptor potential canonical channels. The Journal of Pharmacology and Experimental Therapeutics 339, 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, & Graham LM (2008). Elucidation of a TRPC6–TRPC5 channel cascade that restricts endothelial cell movement. Molecular Biology of the Cell 19, 3203–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Rosenbaum MA, Birnbaumer L, & Graham LM (2017). Integration of TRPC6 and NADPH oxidase activation in lysophosphatidylcholine-induced TRPC5 externalization. American Journal of Physiology. Cell Physiology 313, C541–C555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Sun GB, Ye JX, Wang J, Zhang MD, & Sun XB (2017). Salvianolic acid B attenuates doxorubicin-induced ER stress by inhibiting TRPC3 and TRPC6 mediated Ca2+ overload in rat cardiomyocytes. Toxicology Letters 276, 21–30. [DOI] [PubMed] [Google Scholar]
- Chen W, Oberwinkler H, Werner F, Gassner B, Nakagawa H, Feil R, … Kuhn M (2013). Atrial natriuretic peptide-mediated inhibition of microcirculatory endothelial Ca2+ and permeability response to histamine involves cGMP-dependent protein kinase I and TRPC6 channels. Arteriosclerosis, Thrombosis, and Vascular Biology 33, 2121–2129. [DOI] [PubMed] [Google Scholar]
- Chen X, Li W, Riley AM, Soliman M, Chakraborty S, Stamatkin CW, & Obukhov AG (2017). Molecular determinants of the sensitivity to Gq/11-phospholipase C-dependent gating, Gd3+ potentiation, and Ca2+ permeability in the transient receptor potential canonical type 5 (TRPC5) channel. The Journal of Biological Chemistry 292, 898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Taylor-Nguyen NN, Riley AM, Herring BP, White FA, & Obukhov AG (2019). The TRPC6 inhibitor, larixyl acetate, is effective in protecting against traumatic brain injury-induced systemic endothelial dysfunction. Journal of Neuroinflammation 16, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Liu X, Ong HL, & Ambudkar IS (2008). Functional requirement for Orai1 in store operated TRPC1-STIM1 channels. The Journal of Biological Chemistry 283, 12935–12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KT, Ong HL, Liu X, & Ambudkar IS (2013). Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Current Topics in Membranes 71, 149–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Tong Q, Cheung JY, Wozney J, Conrad K, Mazack V, … Miller BA (2004). Interaction of TRPC2 and TRPC6 in erythropoietin modulation of calcium influx. The Journal of Biological Chemistry 279, 10514–10522. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Wu S, Chen H, Alexeyev M, St Croix CM, Pitt BR, … Stevens T (2012). Orai1 determines calcium selectivity of an endogenous TRPC heterotetramer channel. Circulation Research 110, 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillot M, Giacco V, & Hamilton NB (2019). The role of TRP channels in white matter function and ischaemia. Neuroscience Letters 690, 202–209. [DOI] [PubMed] [Google Scholar]
- Corteling RL, Li S, Giddings J, Westwick J, Poll C, & Hall IP (2004). Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. American Journal of Respiratory Cell and Molecular Biology 30, 145–154. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, & Manning A (1969). Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287. [DOI] [PubMed] [Google Scholar]
- de la Cruz GG, Svobodova B, Lichtenegger M, Tiapko O, Groschner K, & Glasnov T (2017). Intensified microwave-assisted N-acylation procedure - synthesis and activity evaluation of TRPC3 channel agonists with a 1,3-dihydro-2H-benzo[d]imidazol-2-one core. Synlett. 28, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, & Trebak M (2015). Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Science Signaling 8, ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Steinritz D, & Gudermann T (2017). Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium 67, 123–137. [DOI] [PubMed] [Google Scholar]
- Diez-Bello R, Jardin I, Lopez JJ, El Haouari M, Ortega-Vidal J, Altarejos J, … Rosado JA (2019). (−)Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochimica et Biophysica Acta, Molecular Cell Research, 474–485. [DOI] [PubMed] [Google Scholar]
- Ding M, Wang H, Qu C, Xu F, Zhu Y, Lv G, … Hong X (2018). Pyrazolo[1,5-a]pyrimidine TRPC6 antagonists for the treatment of gastric cancer. Cancer Letters 432, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doleschal B, Primessnig U, Wölkart G, Wolf S, Schernthaner M, Lichtenegger M, … Groschner K (2015). TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovascular Research 106, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Ruiz-Hurtado G, Sabourin J, Gomez AM, Alvarez JL, & Benitah JP (2015). Proarrhythmic effect of sustained EPAC activation on TRPC3/4 in rat ventricular cardiomyocytes. Journal of Molecular and Cellular Cardiology 87, 74–78. [DOI] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, & Xu H (2008). The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, … Xu H (2010). PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nature Communications 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Li J, Chen G-L, Ge Y, Liu J, Xie K, … Zhang J (2019). Cryo-EM structure of TRPC5 at 2.8-Å resolution reveals unique and conserved structural elements essential for channel function. Science Advances 5 eaaw7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Li J, Zeng B, Chen GL, Peng X, Zhang Y, … Zhang J (2018). Structure of the mouse TRPC4 ion channel. Nature Communications 9, 3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, & Brayden JE (2015). Transient receptor potential channels in the vasculature. Physiological Reviews 95, 645–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, & Abboud HE (2009). Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 58, 1201–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser R, Montani G, Tirindelli R, & Paysan J (2005). Phosphatidyl-inositide signalling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. The European Journal of Neuroscience 21, 2692–2700. [DOI] [PubMed] [Google Scholar]
- Falcon D, Galeano-Otero I, Calderon-Sanchez E, Del Toro R, Martin-Bornez M, Rosado JA, … Smani T (2019). TRP channels: Current perspectives in the adverse cardiac remodeling. Frontiers in Physiology 10, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Choi W, Sun W, Du J, & Lu W (2018). Structure of the human lipid-gated cation channel TRPC3. Elife 7 [pii: e36852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasolato C, Hoth M, Matthews G, & Penner R (1993). Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proceedings of the National Academy of Sciences of the United States of America 90, 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Xu D, & Wang L (2018). miR-26a inhibits atherosclerosis progression by targeting TRPC3. Cell & Bioscience 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, He Z, Li H, & Wang Y (2015). Ca2+ signaling initiated by canonical transient receptor potential channels in dendritic development. Neuroscience Bulletin 31, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, … Rao A (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Chrétien C, Leloup C, & Pénicaud L (2017). Recent advances in the cellular and molecular mechanisms of hypothalamic neuronal glucose detection. Frontiers in Physiology 8, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores PL, Rodriguez E, Zapata E, Carbo R, Farias JM, & Martinez M (2017). Maitotoxin is a potential selective activator of the endogenous transient receptor potential canonical Type 1 channel in Xenopus laevis oocytes. Marine Drugs 15 pii: RE198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Kyriaki S, Ozkan ED, Phillips CW, & Cooper DC (2007). Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One 2, e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, & Flockerzi V (2005). Functional role of TRPC proteins in native systems: Implications from knockout and knock-down studies. The Journal of Physiology 567, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gao Z, Shen B, & Zhu MX (2015). Canonical transient receptor potential 4 and its small molecule modulators. Science China. Life Sciences 58, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N (2017). Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. Journal of Ethnopharmacology 200, 136–146. [DOI] [PubMed] [Google Scholar]
- Gao J, Zeng K, Liu Y, Gao L, & Liu L (2019). LncRNA SNHG5 promotes growth and invasion in melanoma by regulating the miR-26a-5p/TRPC3 pathway. OncoTargets and Therapy 12, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt HJ, Vasudev NS, & Beech DJ (2016). Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. European Biophysics Journal 45, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Wei L, Zhang M, Hu B, Wang K, Li Y, … Li Y (2018). TRPC1/3/6 inhibition attenuates the TGF-beta1-induced epithelial-mesenchymal transition in gastric cancer via the Ras/Raf1/ERK signaling pathway. Cell Biology International 42, 975–984. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, & Schilling WP (2002). Selective association of TRPC channel subunits in rat brain synaptosomes. The Journal of Biological Chemistry 277, 48303–48310. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins WG, Zuo CD, Estacion M, & Schilling WP (2006). Identification and localization of TRPC channels in the rat kidney. American Journal of Physiology. Renal Physiology 290, F1241–F1252. [DOI] [PubMed] [Google Scholar]
- Goel M, Zuo CD, Sinkins WG, & Schilling WP (2007). TRPC3 channels colocalize with Na+/Ca2+ exchanger and Na+ pump in axial component of transverse-axial tubular system of rat ventricle. American Journal of Physiology. Heart and Circulatory Physiology 292, H874–H883. [DOI] [PubMed] [Google Scholar]
- Götz V, Qiao S, Beck A, & Boehm U (2017). Transient receptor potential (TRP) channel function in the reproductive axis. Cell Calcium 67, 138–147. [DOI] [PubMed] [Google Scholar]
- Grayson TH, Murphy TV, & Sandow SL (2017). Transient receptor potential canonical type 3 channels: Interactions, role and relevance - A vascular focus. Pharmacology & Therapeutics 174, 79–96. [DOI] [PubMed] [Google Scholar]
- Greka A, & Mundel P (2011). Balancing calcium signals through TRPC5 and TRPC6 in podocytes. Journal of the American Society of Nephrology 22, 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greka A, Navarro B, Oancea E, Duggan A, & Clapham DE (2003). TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nature Neuroscience 6, 837–845. [DOI] [PubMed] [Google Scholar]
- Griesi-Oliveira K, Suzuki AM, & Muotri AR (2017). TRPC channels and mental disorders. Advances in Experimental Medicine and Biology 976, 137–148. [DOI] [PubMed] [Google Scholar]
- Groschner K, & Tiapko O (2018). Revelation of an enigmatic signaling machinery-First insights into the mammalian TRPC architecture. Cell Calcium 74, 144–146. [DOI] [PubMed] [Google Scholar]
- Gross SA, Guzmán GA, Wissenbach U, Philipp SE, Zhu MX, Bruns D, & Cavalié A (2009). TRPC5 is a Ca2+-activated channel functionally coupled to Ca2+-selective ion channels. The Journal of Biological Chemistry 284, 34423–34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Ma Y, Ma S, Mu F, Deng J, Duan J, … Wen A (2017). The Role of TRPC6 in the neuroprotection of calycosin against cerebral ischemic injury. Scientific Reports 7, 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, She J, Zeng W, Chen Q, Bai XC, & Jiang Y (2017). Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner S, Burg F, Kannler M, Urban N, Mayer P, Dietrich A, … Schaefer M (2018). A (+)-Larixol congener with high affinity and subtype selectivity toward TRPC6. ChemMedChem. 13, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Hafner S, Urban N, & Schaefer M (2019). Discovery and characterization of a positive allosteric modulator of transient receptor potential canonical 6 (TRPC6) channels. Cell Calcium 78, 26–34. [DOI] [PubMed] [Google Scholar]
- Han L, & Li J (2018). Canonical transient receptor potential 3 channels in atrial fibrillation. European Journal of Pharmacology 837, 1–7. [DOI] [PubMed] [Google Scholar]
- Hang P, Zhao J, Cai B, Tian S, Huang W, Guo J, … Du Z (2015). Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. International Journal of Biological Sciences 11, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, & Minke B (1992). The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651. [DOI] [PubMed] [Google Scholar]
- Hardie RC, & Minke B (1993). Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: Implications for phosphoinositide-mediated Ca2+ mobilization. Trends in Neurosciences 16, 371–376. [DOI] [PubMed] [Google Scholar]
- Harteneck C, & Gollasch M (2011). Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Current Pharmaceutical Biotechnology 12, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, … Konnerth A (2008). TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59, 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DX, & Ma X (2016). Transient receptor potential channel C5 in cancer chemoresistance. Acta Pharmacologica Sinica 37, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He KQ, Li WZ, Chai XQ, Yin YY, Jiang Y, & Li WP (2018). Astragaloside IV prevents kidney injury caused by iatrogenic hyperinsulinemia in a streptozotocininduced diabetic rat model. International Journal of Molecular Medicine 41, 1078–1088. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, & Schultz G (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, & Gudermann T (2002). Subunit composition of mammalian transient receptor potential channels in living cells. Proceedings of the National Academy of Sciences of the United States of America 99, 7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, & Penner R (1992). Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hou X, Xiao H, Zhang Y, Zeng X, Huang M, Chen X, … Liao Y (2018). Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death & Disease 9, 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, & Schilling WP (1994). Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochemical and Biophysical Research Communications 201, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, & Worley PF (2006). STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nature Cell Biology 8, 1003–1010. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, & Hardie RC (2010). Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Current Biology 20, 189–197. [DOI] [PubMed] [Google Scholar]
- Huang Y, Winkler PA, Sun W, Lü W, & Du J (2018). Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature 562, 145–149. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Zhu X, Boulay G, Birnbaumer L, & Stefani E (1998). Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Letters 422, 333–338. [DOI] [PubMed] [Google Scholar]
- Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, & Staruschenko A (2017). The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Scientific Reports 22;7(1), 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Varnell AL, Ostertag EM, Klipec WD, & Cooper DC (2012). TRPC4 ion channel protein is selectively expressed in a subpopulation of dopamine neurons in the ventral tegmental area. Nature Precedings arXiv:1204.0142 [q-NC]. doi: 10.1038/npre.2011.6577.1. [DOI] [Google Scholar]
- Imai Y, Itsuki K, Okamura Y, Inoue R, & Mori MX (2012). A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. The Journal of Physiology 590, 1101–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsuki K, Imai Y, Hase H, Okamura Y, Inoue R, & Mori MX (2014). PLC-mediated PI (4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels. The Journal of General Physiology 143, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin I, & Rosado JA (2016). STIM and calcium channel complexes in cancer. Biochimica et Biophysica Acta 1863, 1418–1426. [DOI] [PubMed] [Google Scholar]
- Jeon JP, Hong C, Park EJ, Jeon JH, Cho NH, Kim IG, … So I (2012). Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. The Journal of Biological Chemistry 287, 17029–17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JP, Lee KP, Park EJ, Sung TS, Kim BJ, Jeon JH, & So I (2008). The specific activation of TRPC4 by Gi protein subtype. Biochemical and Biophysical Research Communications 377, 538–543. [DOI] [PubMed] [Google Scholar]
- Jeon JP, Thakur DP, Tian JB, So I, & Zhu MX (2016). Regulator of G-protein signalling and GoLoco proteins suppress TRPC4 channel function via acting at Galphai/o. The Biochemical Journal 473, 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Rodriguez M, Williams AG, & Yuan JP (2017). Homer binds to Orai1 and TRPC channels in the neointima and regulates vascular smooth muscle cell migration and proliferation. Scientific Reports 7, 5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HN, Zeng B, Chen GL, Lai B, Lu SH, & Qu JM (2016). Lipopolysaccharide potentiates endothelin-1-induced proliferation of pulmonary arterial smooth muscle cells by upregulating TRPC channels. Biomedicine & Pharmacotherapy 82, 20–27. [DOI] [PubMed] [Google Scholar]
- Jiang HN, Zeng B, Zhang Y, Daskoulidou N, Fan H, Qu JM, & Xu SZ (2013). Involvement of TRPC channels in lung cancer cell differentiation and the correlation analysis in human non-small cell lung cancer. PLoS One 8, e67637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lu W, Yang K, Hadadi C, Fu X, Chen Y, … Sun D (2016). Sodium tanshinone IIA sulfonate inhibits hypoxia-induced enhancement of SOCE in pulmonary arterial smooth muscle cells via the PKG-PPAR-gamma signaling axis. American Journal of Physiology. Cell Physiology 311, C136–C149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhou Y, Peng G, Liu N, Tian H, Pan D, … Dai A (2018). Topotecan prevents hypoxia-induced pulmonary arterial hypertension and inhibits hypoxia-inducible factor-1alpha and TRPC channels. The International Journal of Biochemistry & Cell Biology 104, 161–170. [DOI] [PubMed] [Google Scholar]
- Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, Huynh W, … Cheng Y (2017). Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Im SS, Song DK, & Bae JH (2017). Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Reports 50, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, & Plant TD (2003). Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. The Journal of Biological Chemistry 278, 3562–3571. [DOI] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lémos JR, & Florman HM (2001). Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biology 3, 499–502. [DOI] [PubMed] [Google Scholar]
- Just S, Chenard BL, Ceci A, Strassmaier T, Chong JA, Blair NT, … Moran MM (2018). Treatment with HC-070, a potent inhibitor of TRPC4 and TRPC5, leads to anxiolytic and antidepressant effects in mice. PLoS One 13, e0191225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki H, Kinoshita M, Akaike A, Satoh M, Mori Y, & Kaneko S (2001). Activation of inositol 1,4,5-trisphosphate receptor is essential for the opening of mouse TRP5 channels. Molecular Pharmacology 60, 989–998. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, & Ronnekleiv OK (2018). TRPCing around the hypothalamus. Frontiers in Neuroendocrinology 51, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Kim MT, Jeon JH, Kim SJ, & So I (2008). Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biological & Pharmaceutical Bulletin 31, 1733–1738. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwak M, Jeon JP, Myeong J, Wie J, Hong C, … So I (2014). Isoform- and receptor-specific channel property of canonical transient receptor potential (TRPC) 1/4 channels. Pflügers Archiv 466, 491–504. [DOI] [PubMed] [Google Scholar]
- Kim YS, Hong CS, Lee SW, Nam JH, & Kim BJ (2016). Effects of ginger and its pungent constituents on transient receptor potential channels. International Journal of Molecular Medicine 38, 1905–1914. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Mignery GA, Zhu MX, & Muallem S (1999). The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Molecular Cell 4, 423–429. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Kuo TH, Mozhayeva G, Pessah I, Mignery G, … Muallem S (1998). Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 396, 478–482. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, … Mori Y (2009). Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proceedings of the National Academy of Sciences of the United States of America 106, 5400–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Schernthaner M, Maechler H, Kappe CO, Glasnov TN, Hoefler G, … Groschner K (2013). A TRPC3 blocker, ethyl-1-(4-(2,3,3-trichloroacrylamide) phenyl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate (Pyr3), prevents stent-induced arterial remodeling. The Journal of Pharmacology and Experimental Therapeutics 344, 33–40. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mishra AK, Swain DK, Singh V, Yadav S, & Saxena A (2018). Role of transient receptor potential channels in regulating spermatozoa functions: A mini-review. Veterinary World 11, 1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, & Montell C (2007). Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Molecular Cell 25, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Capuano V, Olschewski A, Sabourin J, Nagaraj C, Girerd B, … Antigny F (2018). Ion channels in pulmonary hypertension: A therapeutic interest? International Journal of Molecular Sciences 19, pii: E3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OC, Shen B, Wong CO, Tjong YW, Lo CY, Wang HC, … Yao X (2016). TRPC5 channels participate in pressure-sensing in aortic baroreceptors. Nature Communications 7, 11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, … Yuan JP (2014). Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). The Journal of Biological Chemistry 289, 6372–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier L, Trebak M, & Putney JW Jr. (2008). Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium 43, 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, … Boulay G (2006). Identification of two domains involved in the assembly of transient receptor potential canonical channels. The Journal of Biological Chemistry 281, 30356–30364. [DOI] [PubMed] [Google Scholar]
- Lepannetier S, Gualdani R, Tempesta S, Schakman O, Seghers F, Kreis A, … Gailly P (2018). Activation of TRPC1 channel by metabotropic glutamate receptor mGluR5 modulates synaptic plasticity and spatial working memory. Frontiers in Cellular Neuroscience 12, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, … Müller WE (2010). Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators–identification of a novel pharmacophore. Molecular Pharmacology 77, 368–377. [DOI] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Müller M, Essin K, Henke B, Gollasch M, … Müller WE (2007). Hyperforin—a key constituent of St. John’s wort specifically activates TRPC6 channels. The FASEB Journal 21, 4101–4111. [DOI] [PubMed] [Google Scholar]
- Lewis RS, & Cahalan MD (1989). Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regulation 1, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, & Axel R (2002). Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America 99, 6376–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HS, Xu XZ, & Montell C (1999). Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24, 261–273. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang X, Song X, Liu R, Zhang J, & Li Z (2019). The structure of TRPC ion channels. Cell Calcium 80, 25–28. [DOI] [PubMed] [Google Scholar]
- Li S, & Ding X (2017). TRPC channels and glioma. Advances in Experimental Medicine and Biology 976, 157–165. [DOI] [PubMed] [Google Scholar]
- Li W, Chen X, Riley AM, Hiett SC, Temm CJ, Beli E, … Obukhov AG (2017). Long-term spironolactone treatment reduces coronary TRPC expression, vasoconstriction, and atherosclerosis in metabolic syndrome pigs. Basic Research in Cardiology 112, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Zhang Y, Zhuo D, Lo CY, Yu L, Lau CW, … Yao X (2019). Endothelial cell transient receptor potential channel C5 (TRPC5) is essential for endothelium-dependent contraction in mouse carotid arteries. Biochemical Pharmacology 159, 11–24. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhong W, Miao F, Wu H, & Liu Y (2018). Effects of losartan on vasomotor function and canonical transient receptor potential channels in the aortas of sinoaortic denervation rats. Clinical and Experimental Hypertension 40, 39–48. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, & Birnbaumer L (2008). Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/ICRAC channels. Proceedings of the National Academy of Sciences of the United States of America 105, 2895–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, & Birnbaumer L (2007). Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proceedings of the National Academy of Sciences of the United States of America 104, 4682–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, & Birnbaumer L (2009). A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America 106, 3202–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenegger M, Tiapko O, Svobodova B, Stockner T, Glasnov TN, Schreibmayer W, … Groschner K (2018). An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nature Chemical Biology 14, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Corey DP, & Dulac C (1999). TRP2: A candidate transduction channel for mammalian pheromone sensory signaling. Proceedings of the National Academy of Sciences of the United States of America 96, 5791–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BL, Matera D, Doerner JF, Zheng N, del Camino D, Mishra S, … Blair NT (2019). In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proceedings of the National Academy of Sciences of the United States of America 116, 10156–10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhang J-C, Fu J, Chen F, Wang J, Wu Z-L, & Yuan S-Y (2013). Hyperforin attenuates brain damage induced by transient middle cerebral artery occlusion (MCAO) in rats via inhibition of TRPC6 channels degradation. Journal of Cerebral Blood Flow and Metabolism 33, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, & Groschner K (2000). Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. The Journal of Biological Chemistry 275, 27799–27805. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr., & Meyer T (2005). STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Current Biology 15, 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, & Montell C (2015). Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochemical and Biophysical Research Communications 460, 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang L, Chen KH, Sun HY, Jin MW, Xiao GS, … Li GR (2016). SKF-96365 blocks human ether-a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Pharmacological Research 104, 61–69. [DOI] [PubMed] [Google Scholar]
- Liu W, Wen W, Wei Z, Yu J, Ye F, Liu CH, … Zhang M (2011). The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell 145, 1088–1101. [DOI] [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Singh BB, Groschner K, & Ambudkar IS (2005). Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. The Journal of Biological Chemistry 280, 21600–21606. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O’Connell B, … Ambudkar IS (2000). Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. The Journal of Biological Chemistry 275, 3403–3411. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu C, Qin X, Zhu M, & Yang Z (2015). The change of spatial cognition ability in depression rat model and the possible association with down-regulated protein expression of TRPC6. Behavioural Brain Research 294, 186–193. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang J, Zhang X, Xu P, Zhang T, & Yang Z (2015). Developmental changes in the expression and function of TRPC6 channels related the F-actin organization during differentiation in podocytes. Cell Calcium 58, 541–548. [DOI] [PubMed] [Google Scholar]
- Lopez J, Salido GM, Pariente JA, & Rosado JA (2006). Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. The Journal of Biological Chemistry 281, 28254–28264. [DOI] [PubMed] [Google Scholar]
- Lu R, He Q, & Wang J (2017). TRPC channels and Alzheimer’s disease. Advances in Experimental Medicine and Biology 976, 73–83. [DOI] [PubMed] [Google Scholar]
- Lu W, Ran P, Zhang D, Peng G, Li B, Zhong N, & Wang J (2010). Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. American Journal of Physiology. Cell Physiology 298, C114–C123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P, Ukhanov K, Leinders-Zufall T, & Zufall F (2003). A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: Mechanism of pheromone transduction. Neuron 40, 551–561. [DOI] [PubMed] [Google Scholar]
- Ludlow MJ, Gaunt HJ, Rubaiy HN, Musialowski KE, Blythe NM, Vasudev NS, … Beech DJ (2017). (−)-Englerin A-evoked cytotoxicity Is mediated by Na+ Influx and counteracted by Na+/K+-ATPase. The Journal of Biological Chemistry 292, 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Liu L, Jiang W, Yu Y, & Song H (2015). FK506 ameliorates podocyte injury in type 2 diabetic nephropathy by down-regulating TRPC6 and NFAT expression. International Journal of Clinical and Experimental Pathology 8, 14063–14074. [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY, … Yao X (2012). Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proceedings of the National Academy of Sciences of the United States of America 109, 16282–16287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Chen Z, Hua D, He D, Wang L, Zhang P, … Yao X (2014). Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proceedings of the National Academy of Sciences of the United States of America 111, 6389–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, … Yao X (2010). Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arteriosclerosis, Thrombosis, and Vascular Biology 30, 851–858. [DOI] [PubMed] [Google Scholar]
- Maier T, Follmann M, Hessler G, Kleemann HW, Hachtel S, Fuchs B, … Strübing C (2015). Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. British Journal of Pharmacology 172, 3650–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malczyk M, Erb A, Veith C, Ghofrani HA, Schermuly RT, Gudermann T, … Sydykov A (2017). The role of transient receptor potential channel 6 channels in the pulmonary vasculature. Frontiers in Immunology 8, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos Y, Schnitzler M, Gudermann T, & Storch U (2018). Emerging roles of diacylglycerol-sensitive TRPC4/5 channels. Cells 7 [pii: E218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum E, Parker PJ, & Carozzi A (1991). The PtdIns-PLC superfamily and signal transduction. Biochimica et Biophysica Acta 1092, 49–71. [DOI] [PubMed] [Google Scholar]
- Miehe S, Bieberstein A, Arnould I, Ihdene O, Rütten H, & Strübing C (2010). The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5. The Journal of Biological Chemistry 285, 12426–12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miehe S, Crause P, Schmidt T, Löhn M, Kleemann HW, Licher T, … Strübing C (2012). Inhibition of diacylglycerol–sensitive TRPC channels by synthetic and natural steroids. PLoS One 7, e35393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, … Zhu MX (2011). Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. The Journal of Biological Chemistry 286, 33436–33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard A, Bauer CC, Chuntharpursat-Bon E, Pickles IB, Wright DJ, Ludlow MJ, … Bon RS (2019). Potent, selective, and subunit-dependent activation of TRPC5 channels by a xanthine derivative. British Journal of Pharmacology 176, 3924–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard A, Bauer CC, Wright DJ, Rubaiy HN, Muraki K, Beech DJ, & Bon RS (2018). Remarkable progress with small-molecule modulation of TRPC1/4/5 channels: Implications for understanding the channels in health and disease. Cells 7 pii: E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F, Lucariello A, & Guerra G (2018). TRPC3-mediated Ca2+ signals as a promising strategy to boost therapeutic angiogenesis in failing hearts: The role of autologous endothelial colony forming cells. Journal of Cellular Physiology 233, 3901–3917. [DOI] [PubMed] [Google Scholar]
- Montecinos-Oliva C, Schüller A, Parodi J, Melo F, & Inestrosa NC (2014). Effects of tetrahydrohyperforin in mouse hippocampal slices: Neuroprotection, long-term potentiation and TRPC channels. Current Medicinal Chemistry 21, 3494–3506. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, … Zhu MX (2002). A unified nomenclature for the superfamily of TRP cation channels. Molecular Cell 9, 229–231. [DOI] [PubMed] [Google Scholar]
- Montell C, & Rubin GM (1989). Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323. [DOI] [PubMed] [Google Scholar]
- Morine KJ, Paruchuri V, Qiao X, Aronovitz M, Huggins GS, DeNofrio D, … Kapur NK (2016). Endoglin selectively modulates transient receptor potential channel expression in left and right heart failure. Cardiovascular Pathology 25, 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama K, Nagata T, Kobayashi J, Nakamura A, Miyoshi N, Kazui M, … Sakakura T (2018). Discovery of a bicyclo[4.3.0]nonane derivative DS88790512 as a potent, selective, and orally bioavailable blocker of transient receptor potential canonical 6 (TRPC6). Bioorganic & Medicinal Chemistry Letters 28, 2222–2227. [DOI] [PubMed] [Google Scholar]
- Mundel P, Westerling-Bui A, Ledeboer M, Coeffet-Le Gal M, Pan-Zhou XR, Yu M, … Reilly J (2019). GFB-887, a small molecule inhibitor of TRPC5, protects against podocyte injury and attenuates proteinuria in models of FSGS. Kidney International Reports 4, S237. [Google Scholar]
- Myeong J, Ko J, Hong C, Yang D, Lee KP, Jeon JH, & So I (2016). The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochemical and Biophysical Research Communications 474, 476–481. [DOI] [PubMed] [Google Scholar]
- Myeong J, Kwak M, Hong C, Jeon JH, & So I (2014). Identification of a membrane-targeting domain of the transient receptor potential canonical (TRPC)4 channel unrelated to its formation of a tetrameric structure. The Journal of Biological Chemistry 289, 34990–35002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Minard A, Gaunt HJ, Amer MS, Wilson LA, Migliore M, … Bon RS (2016). Natural and synthetic flavonoid modulation of TRPC5 channels. British Journal of Pharmacology 173, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaga-Tomita T, Oda S, Nishiyama K, Tanaka T, Nishimura A, & Nishida M (2019). TRPC channels in exercise-mimetic therapy. Pflügers Archiv 471, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaga-Tomita T, Oda S, Shimauchi T, Nishimura A, Mangmool S, & Nishida M (2017). TRPC3 channels in cardiac fibrosis. Frontiers in Cardiovascular Medicine 4, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukhov AG, & Nowycky MC (2004). TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50). Journal of Cellular Physiology 201, 227–235. [DOI] [PubMed] [Google Scholar]
- Odell AF, Van Helden DF, & Scott JL (2008). The spectrin cytoskeleton influences the surface expression and activation of human transient receptor potential channel 4 channels. The Journal of Biological Chemistry 283, 4395–4407. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, … Mori Y (1999). Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. The Journal of Biological Chemistry 274, 27359–27370. [DOI] [PubMed] [Google Scholar]
- Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, … Mori Y (1998). Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. The Journal of Biological Chemistry 273, 10279–10287. [DOI] [PubMed] [Google Scholar]
- Ong HL, & Ambudkar IS (2017). STIM-TRP pathways and microdomain organization: Contribution of TRPC1 in store-operated Ca2+ entry: Impact on Ca2+ signaling and cell function. Advances in Experimental Medicine and Biology 993, 159–188. [DOI] [PubMed] [Google Scholar]
- Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, … Ambudkar IS (2007). Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. The Journal of Biological Chemistry 282, 9105–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HL, Jang SI, & Ambudkar IS (2012). Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca2+]i signals determine specificity of Ca2+-dependent gene expression. PLoS One 7, e47146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, & Vaca L (2005). Calmodulin and calcium interplay in the modulation of TRPC5 channel activity. Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. The Journal of Biological Chemistry 280, 30788–30796. [DOI] [PubMed] [Google Scholar]
- Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, … Zholos AV (2008). Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. The Journal of Biological Chemistry 283, 10026–10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir ÜS, Nazıroğlu M, Şenol N, & Ghazizadeh V (2016). Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: Involvement of TRPM2 and TRPV1 channels. Molecular Neurobiology 53, 3540–3551. [DOI] [PubMed] [Google Scholar]
- Park S, Lee S, Park EJ, Kang M, So I, Jeon JH, & Chun JN (2017). TGFbeta1 induces stress fiber formation through upregulation of TRPC6 in vascular smooth muscle cells. Biochemical and Biophysical Research Communications 483, 129–134. [DOI] [PubMed] [Google Scholar]
- Pchitskaya E, Popugaeva E, & Bezprozvanny I (2018). Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, Shwe U, Abramowitz J, Wu H, Rhee S, Howell M, … Zheng F (2013). Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Molecular Pharmacology 83, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Cavalié A, Freichel M, Wissenbach U, Zimmer S, Trost C, … Flockerzi V (1996). A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. The EMBO Journal 15, 6166–6171. [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Hambrecht J, Braslavski L, Schroth G, Freichel M, Murakami M, … Flockerzi V (1998). A novel capacitative calcium entry channel expressed in excitable cells. The EMBO Journal 17, 4274–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Bull A, & Kelly LE (1992). Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 8, 631–642. [DOI] [PubMed] [Google Scholar]
- Polat OK, Uno M, Maruyama T, Tran HN, Imamura K, Wong CF, … Mori MX (2019). Contribution of coiled-coil assembly to Ca2+/calmodulin-dependent inactivation of trpc6 channel and its impacts on FSGS-associated phenotypes. Journal of the American Society of Nephrology 30, 1587–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, … Groschner K (2006). TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. The Journal of Biological Chemistry 281, 13588–13595. [DOI] [PubMed] [Google Scholar]
- Poteser M, Schleifer H, Lichtenegger M, Schernthaner M, Stockner T, Kappe CO, … Groschner K (2011). PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proceedings of the National Academy of Sciences of the United States of America 108, 10556–10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucovský V, Zholos AV, & Bolton TB (1998). Muscarinic cation current and suppression of Ca2+ current in guinea pig ileal smooth muscle cells. European Journal of Pharmacology 346, 323–330. [DOI] [PubMed] [Google Scholar]
- Putney JW Jr. (1986). A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Qiu J, Wagner EJ, Ronnekleiv OK, & Kelly MJ (2018). Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels. Journal of Neuroendocrinology 30 10.1111/jne.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, … Kelly MJ (2014). Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism 19, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Ding M, Zhu Y, Lu Y, Du J, Miller M, … Hong X (2017). Pyrazolopyrimidines as potent stimulators for transient receptor potential canonical 3/6/7 channels. Journal of Medicinal Chemistry 60, 4680–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke L, Willot M, Sun H, Ziegler S, Sauerland S, Strohmann C, … Christmann M (2011). Total synthesis and biological evaluation of (−)-englerin A and B: Synthesis of analogues with improved activity profile. Angewandte Chemie (International Ed. in English) 50, 3998–4002. [DOI] [PubMed] [Google Scholar]
- Ramirez GA, Coletto LA, Sciorati C, Bozzolo EP, Manunta P, Rovere-Querini P, & Manfredi AA (2018). Ion channels and transporters in inflammation: Special focus on TRP channels and TRPC6. Cells 7 pii: E70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, … Pollak MR (2005). TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nature Genetics 37, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, & Bae YS (1997). Regulation of phosphoinositide-specific phospholipase C isozymes. The Journal of Biological Chemistry 272, 15045–15048. [DOI] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, … Clapham DE (2009). Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137, 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, … Pangalos MN (2002). mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Research. Molecular Brain Research 109, 95–104. [DOI] [PubMed] [Google Scholar]
- Riccio A, Yan L, Tsvetkov E, Gapon S, Gui LY, Smith KS, … Clapham DE (2014). Decreased anxiety-like behavior and Gαq/11-dependent responses in the amygdala of mice lacking TRPC4 channels. The Journal of Neuroscience 34, 3653–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, & Hill K (2014a). Clemizole hydrochloride is a novel and potent inhibitor of transient receptor potential channel TRPC5. Molecular Pharmacology 86, 514–521. [DOI] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, & Hill K (2014b). Riluzole activates TRPC5 channels independently of PLC activity. British Journal of Pharmacology 171, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle M, Buscher AK, Gohlke BO, Kassmann M, Kolatsi-Joannou M, Brasen JH, … Harteneck C (2016). TRPC6 G757D loss-of-function mutation associates with FSGS. Journal of the American Society of Nephrology 27, 2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, … Stauderman KA (2005). STIM1, an essential and conserved component of store-operated Ca2+ channel function. The Journal of Cell Biology 169, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanravan H, Kim EY, & Dryer SE (2016). 20-Hydroxyeicosatetraenoic acid (20-HETE) modulates canonical transient receptor potential-6 (TRPC6) Channels in podocytes. Frontiers in Physiology 7, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaiy HN, Ludlow MJ, Henrot M, Gaunt HJ, Miteva K, Cheung SY, … Beech DJ (2017). Picomolar, selective, and subtype-specific small-molecule inhibition of TRPC1/4/5 channels. The Journal of Biological Chemistry 292, 8158–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaiy HN, Ludlow MJ, Siems K, Norman K, Foster R, Wolf D, … Beech DJ (2018). Tonantzitlolone is a nanomolar potency activator of transient receptor potential canonical 1/4/5 channels. British Journal of Pharmacology 175, 3361–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubaiy HN, Seitz T, Hahn S, Choidas A, Habenberger P, Klebl B, … Beech DJ (2018). Identification of an (−)-englerin A analogue, which antagonizes (−)-englerin A at TRPC1/4/5 channels. British Journal of Pharmacology 175, 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin J, Bartoli F, Antigny F, Gomez AM, & Benitah JP (2016). Transient receptor potential canonical (TRPC)/Orai1-dependent store-operated Ca2+ channels: New targets of aldosterone in cardiomyocytes. The Journal of Biological Chemistry 291, 13394–13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G, Morelli MB, Amantini C, Santoni M, Nabissi M, Cardinali C, … Quaglia W (2015). Cooperative interaction between the alpha1-adrenoceptors (α1-AR) and transient receptor potential (TRP) triggers a proliferative cell signal in prostate cancer cell lines. Journal of Genetic Syndromes and Gene Therapy 6, 275. [Google Scholar]
- Sauc S, & Frieden M (2017). Neurological and motor disorders: TRPC in the skeletal muscle. Advances in Experimental Medicine and Biology 993, 557–575. [DOI] [PubMed] [Google Scholar]
- Saul S, Stanisz H, Backes CS, Schwarz EC, & Hoth M (2014). How ORAI and TRP channels interfere with each other: Interaction models and examples from the immune system and the skin. European Journal of Pharmacology 739, 49–59. [DOI] [PubMed] [Google Scholar]
- Sawamura S, Hatano M, Takada Y, Hino K, Kawamura T, Tanikawa J, … Mori Y (2016). Screening of transient receptor potential canonical channel activators identifies novel neurotrophic piperazine compounds. Molecular Pharmacology 89, 348–363. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Plant TD, Obukhov AG, Hofmann T, Gudermann T, & Schultz G (2000). Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. The Journal of Biological Chemistry 275, 17517–17526. [DOI] [PubMed] [Google Scholar]
- Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, … Hopkins CR (2013). Inhibition of the TRPC5 ion channel protects the kidney filter. The Journal of Clinical Investigation 123, 5298–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindl R, Fritsch R, Jardin I, Frischauf I, Kahr H, Muik M, … Romanin C (2012). Canonical transient receptor potential (TRPC) 1 acts as a negative regulator for vanilloid TRPV6-mediated Ca2+ influx. The Journal of Biological Chemistry 287, 35612–35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, … Groschner K (2012). Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca2+ entry pathways. British Journal of Pharmacology 167, 1712–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff J (2017). TRPC6 and kidney disease: Sclerosing more than just glomeruli? Kidney International 91, 773–775. [DOI] [PubMed] [Google Scholar]
- Secondo A, Bagetta G, & Amantea D (2018). On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Frontiers in Molecular Neuroscience 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell TS, Belkacemi T, Flockerzi V, & Beck A (2014). Protonophore properties of hyperforin are essential for its pharmacological activity. Scientific Reports 4, 7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, Rainer PP, Hahn VS, Lee D, Jo S-H, Andersen A, … Lepore JJ (2014). Combined TRPC3 and TRPC6 blockade by selective small-molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America 111, 1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Geshi N, Takahashi S, Kiyonaka S, Ichikawa J, Hu Y, … Inoue R (2013). Molecular determinants for cardiovascular TRPC6 channel regulation by Ca2+/calmodulin-dependent kinase II. The Journal of Physiology 591, 2851–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Ju M, Abramowitz J, Large WA, Birnbaumer L, & Albert AP (2012). TRPC1 proteins confer PKC and phosphoinositol activation on native heteromeric TRPC1/C5 channels in vascular smooth muscle: Comparative study of wild-type and TRPC1−/− mice. The FASEB Journal 26, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Miralles F, Kinet JP, Birnbaumer L, Large WA, & Albert AP (2017). Evidence that Orai1 does not contribute to store-operated TRPC1 channels in vascular smooth muscle cells. Channels (Austin, Tex.) 11, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, & Inoue R (2004). Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. The Journal of Physiology 561, 415–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Wada N, Shimizu T, Suzuki T, Takaoka EI, Kanai AJ, … Yoshimura N (2018). Effects of nerve growth factor neutralization on TRP channel expression in laser-captured bladder afferent neurons in mice with spinal cord injury. Neuroscience Letters 683, 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Katsumoto R, Iida S, Miyake T, Higuchi T, Nagashima T, … Kaneko S (2017). Sphingosine-1-phosphate induces Ca2+ signaling and CXCL1 release via TRPC6 channel in astrocytes. Glia 65, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Sierra-Valdez F, Azumaya CM, Romero LO, Nakagawa T, & Cordero-Morales JF (2018). Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating. The Journal of Biological Chemistry 293, 16102–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BB, Liu X, Tang J, Zhu MX, & Ambudkar IS (2002). Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Molecular Cell 9, 739–750. [DOI] [PubMed] [Google Scholar]
- Smith KA, Ayon RJ, Tang H, Makino A, & Yuan JX (2016). calcium-sensing receptor regulates cytosolic [Ca2+] and plays a major role in the development of pulmonary hypertension. Frontiers in Physiology 7, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Chen D, & Yu SP (2014). The TRPC channel blocker SKF 96365 inhibits glioblastoma cell growth by enhancing reverse mode of the Na+/Ca2+ exchanger and increasing intracellular Ca2+. British Journal of Pharmacology 171, 3432–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Liu BC, Lu XY, Yang LL, Zhai YJ, Eaton AF, … Shen BZ (2014). Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression. Biochimica et Biophysica Acta 1843, 894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld R, Hoenderop JG, Isidori AM, Henique C, Dijkman HB, Berden JH, … Nijenhuis T (2017). Sildenafil prevents podocyte injury via PPAR-γ-mediated TRPC6 inhibition. Journal of the American Society of Nephrology 28(5), 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sours-Brothers S, Ding M, Graham S, & Ma R (2009). Interaction between TRPC1/TRPC4 assembly and STIM1 contributes to store-operated Ca2+ entry in mesangial cells. Experimental Biology and Medicine (Maywood, N.J.) 234, 673–682. [DOI] [PubMed] [Google Scholar]
- de Souza LB, Ong HL, Liu X, & Ambudkar IS (2015). Fast endocytic recycling determines TRPC1-STIM1 clustering in ER-PM junctions and plasma membrane function of the channel. Biochimica et Biophysica Acta 1853, 2709–2721. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, & Gwack Y (2012). Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1). Proceedings of the National Academy of Sciences of the United States of America 109, 8682–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamboulian S, Moutin MJ, Treves S, Pochon N, Grunwald D, Zorzato F, … Arnoult C (2005). Junctate, an inositol 1,4,5-triphosphate receptor associated protein, is present in rodent sperm and binds TRPC2 and TRPC5 but not TRPC1 channels. Developmental Biology 286, 326–337. [DOI] [PubMed] [Google Scholar]
- Staruschenko A, Spires D, & Palygin O (2019). Role of TRPC6 in progression of diabetic kidney disease. Current Hypertension Reports 21, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch U, Forst AL, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, … Gudermann T (2017). Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proceedings of the National Academy of Sciences of the United States of America 114, E37–E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, & Koentges G (2002). Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500. [DOI] [PubMed] [Google Scholar]
- Stroh O, Freichel M, Kretz O, Birnbaumer L, Hartmann J, & Egger V (2012). NMDA receptor-dependent synaptic activation of TRPC channels in olfactory bulb granule cells. The Journal of Neuroscience 32, 5737–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing C, Krapivinsky G, Krapivinsky L, & Clapham DE (2001). TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29, 645–655. [DOI] [PubMed] [Google Scholar]
- Sukumaran P, Sun Y, Antonson N, & Singh BB (2018). Dopaminergic neurotoxins induce cell death by attenuating NF-kappaB-mediated regulation of TRPC1 expression and autophagy. The FASEB Journal 32, 1640–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran P, Sun Y, Schaar A, Selvaraj S, & Singh BB (2017). TRPC channels and Parkinson’s disease. Advances in Experimental Medicine and Biology 976, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sukumaran P, Bandyopadhyay BC, & Singh BB (2014). Physiological function and characterization of TRPCs in neurons. Cells 3, 455–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, … Tiruppathi C (2012). The Ca2+ sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca2+ entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Molecular Pharmacology 81, 510–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TS, Jeon JP, Kim BJ, Hong C, Kim SY, Kim J, … So I (2011). Molecular determinants of PKA-dependent inhibition of TRPC5 channel. American Journal of Physiology. Cell Physiology 301, C823–C832. [DOI] [PubMed] [Google Scholar]
- Tabata H, Tanaka S, Sugimoto Y, Kanki H, Kaneko S, & Ichikawa A (2002). Possible coupling of prostaglandin E receptor EP1 to TRP5 expressed in Xenopus laevis oocytes. Biochemical and Biophysical Research Communications 298, 398–402. [DOI] [PubMed] [Google Scholar]
- Tai Y, & Jia Y (2017). TRPC channels and neuron development, plasticity, and activities. Advances in Experimental Medicine and Biology 976, 95–110. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, & Zhu MX (2001). Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. The Journal of Biological Chemistry 276, 21303–21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Guo W, Zheng L, Wu JX, Liu M, Zhou X, … Chen L (2018). Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Research 28, 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, … Zhu MX (2000). Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. The Journal of Biological Chemistry 275, 37559–37564. [DOI] [PubMed] [Google Scholar]
- Thakur DP, Tian J, Jeon J, Xiong J, Huang Y, Flockerzi V, & Zhu MX (2016). Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proceedings of the National Academy of Sciences of the United States of America 113, 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, & Rossler OG (2017). Hyperforin activates gene transcription involving transient receptor potential C6 channels. Biochemical Pharmacology 129, 96–107. [DOI] [PubMed] [Google Scholar]
- Thyagarajan B, Poteser M, Romanin C, Kahr H, Zhu MX, & Groschner K (2001). Expression of Trp3 determines sensitivity of capacitative Ca2+ entry to nitric oxide and mitochondrial Ca2+ handling: Evidence for a role of Trp3 as a subunit of capacitative Ca2+ entry channels. The Journal of Biological Chemistry 276, 48149–48158. [DOI] [PubMed] [Google Scholar]
- Tian J, Thakur DP, Lu Y, Zhu Y, Freichel M, Flockerzi V, & Zhu MX (2014). Dual depolarization responses generated within the same lateral septal neurons by TRPC4-containing channels. Pflügers Archiv 466, 1301–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Thakur DP, & Zhu MX (2014). TRPC channels In Zheng J, & Trudeau MC (Eds.), Handbook of ion channels . Chapter 27 (pp. 411–426). CRC Press. [Google Scholar]
- Tiapko O, Bacsa B, de la Cruz GG, Glasnov T, & Groschner K (2016). Optopharmacological control of TRPC channels by coumarin-caged lipids is associated with a phototoxic membrane effect. Science China. Life Sciences 59, 802–810. [DOI] [PubMed] [Google Scholar]
- Tiapko O, Shrestha N, Lindinger S, Guedes de la Cruz G, Graziani A, Klec C, … Groschner K (2019). Lipid-independent control of endothelial and neuronal TRPC3 channels by light. Chemical Science 10, 2837–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, DeHaven WI, Wedel BJ, Bird GS, & Putney JW Jr. (2009). Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels. Pflügers Archiv 457, 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, Bird GSJ, & Putney JW Jr. (2003). The TRPC3/6/7 subfamily of cation channels. Cell Calcium 33, 451–461. [DOI] [PubMed] [Google Scholar]
- Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, … Zorzato F (2004). Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. The Journal of Cell Biology 166, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S, Vukcevic M, Griesser J, Armstrong CF, Zhu MX, & Zorzato F (2010). Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions. Journal of Cell Science 123, 4170–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost C, Bergs C, Himmerkus N, & Flockerzi V (2001). The transient receptor potential, TRP4, cation channel is a novel member of the family of calmodulin binding proteins. The Biochemical Journal 355, 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, & Sukhatme VP (1999). Specific association of the gene product of PKD2 with the TRPC1 channel. Proceedings of the National Academy of Sciences of the United States of America 96, 3934–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, … Flockerzi V (2009). Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N, Hill K, Wang L, Kuebler WM, & Schaefer M (2012). Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium 51, 194–206. [DOI] [PubMed] [Google Scholar]
- Urban N, Wang L, Kwiek S, Rademann J, Kuebler WM, & Schaefer M (2016). Identification and validation of larixyl acetate as a potent TRPC6 inhibitor. Molecular Pharmacology 89, 197–213. [DOI] [PubMed] [Google Scholar]
- Uslusoy F, Nazıroğlu M, & Çiğ B (2017). Inhibition of the TRPM2 and TRPV1 channels through Hypericum perforatum in sciatic nerve injury-induced rats demonstrates their key role in apoptosis and mitochondrial oxidative stress of sciatic nerve and dorsal root ganglion. Frontiers in Physiology 8, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L (2010). SOCIC: The store-operated calcium influx complex. Cell Calcium 47, 199–209. [DOI] [PubMed] [Google Scholar]
- Vaca L, & Kunze DL (1993). Depletion and refilling of intracellular Ca2+ stores induce oscillations of Ca2+ current. The American Journal of Physiology 264, H1319–H1322. [DOI] [PubMed] [Google Scholar]
- Vaca L, & Sampieri A (2002). Calmodulin modulates the delay period between release of calcium from internal stores and activation of calcium influx via endogenous TRP1 channels. The Journal of Biological Chemistry 277, 42178–42187. [DOI] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, & Schilling WP (1994). Activation of recombinant trp by thapsigargin in Sf9 insect cells. The American Journal of Physiology 267, C1501–C1505. [DOI] [PubMed] [Google Scholar]
- Vazquez G, Lievremont JP, St J Bird G, & Putney JW Jr. (2001). Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America 98, 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, … Kinet J-P (2006). CRACM1 Is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayagam D, Mager T, Apelbaum A, Bothe A, Merino F, Hofnagel O, … Raunser S (2018). Electron cryo-microscopy structure of the canonical TRPC4 ion channel Elife, 7, e36615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fu X, Yang K, Jiang Q, Chen Y, Jia J, … Lu W (2015). Hypoxia inducible factor-1-dependent up-regulation of BMP4 mediates hypoxia-induced increase of TRPC expression in PASMCs. Cardiovascular Research 107, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang Q, Wan L, Yang K, Zhang Y, Chen Y, … Lu W (2013). Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. American Journal of Respiratory Cell and Molecular Biology 48, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, … Lu W (2013). Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARgamma axis. American Journal of Respiratory Cell and Molecular Biology 49, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fu TM, Zhou Y, Xia S, Greka A, & Wu H (2018). Structures and gating mechanism of human TRPM2. Science, 362 pii: eaav4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Thakur DP, Tian J, Jeon J, & Zhu MX (2018). Mechanism of regulation of Gi/o-mediated TRPC4 activation by intracellular protons. Biophysical Journal 114, 397a [abstract]. [Google Scholar]
- Wang T, Chen Z, Zhu Y, Pan Q, Liu Y, Qi X, … Hua D (2015). Inhibition of transient receptor potential channel 5 reverses 5-Fluorouracil resistance in human colorectal cancer cells. The Journal of Biological Chemistry 290, 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dande RR, Yu H, Samelko B, Miller RE, Altintas MM, & Reiser J (2018). TRPC5 does not cause or aggravate glomerular disease. Journal of the American Society of Nephrology 29, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bu J, Shen H, Li H, Wang Z, & Chen G (2017). Targeting transient receptor potential canonical channels for diseases of the nervous system. Current Drug Targets 18, 1460–1465. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, & Robinson DW (2006). The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. The European Journal of Neuroscience 23, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn DG, Holt DA, Dodson J, Mcatee JJ, Terrell LR, Barton L, … Dan JG (2013). The discovery of potent blockers of the canonical transient receptor channels, TRPC3 and TRPC6, based on an anilino-thiazole pharmacophore. Bioorganic & Medicinal Chemistry Letters 23, 4979–4984. [DOI] [PubMed] [Google Scholar]
- Wei H, Sagalajev B, Yüzer MA, Koivisto A, & Pertovaara A (2015). Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neuroscience Letters 608, 12–17. [DOI] [PubMed] [Google Scholar]
- Wei L, Mao J, Lu J, Gao J, Zhu D, Tian L, … Fu R (2017). Rosiglitazone inhibits angiotensin II-induced proliferation of glomerular mesangial cells via the Galphaq/Plcbeta4/TRPC signaling pathway. Cellular Physiology and Biochemistry 44, 2228–2242. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, … Gudermann T (2006). Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proceedings of the National Academy of Sciences of the United States of America 103, 19093–19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, … Dietrich A (2012). Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nature Communications 3, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, & Montell C (1995). TRPC1, a human homolog of a Drosophila store-operated channel. Proceedings of the National Academy of Sciences of the United States of America 92, 9652–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlund KN, Zhang LP, Ma F, Nesemeier R, Ruiz JC, Ostertag EM, … Marcinkiewicz MM (2014). A rat knockout model implicates TRPC4 in visceral pain sensation. Neuroscience 262, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Merry Kay F, Tony C, Hawkins AF, … Burchette JL (2005). A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804. [DOI] [PubMed] [Google Scholar]
- Wong F, Schaefer EL, Roop BC, LaMendola JN, Johnson-Seaton D, & Shao D (1989). Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron 3, 81–94. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu HX, Shen K, Cao W, & Li XQ (2017). Canonical transient receptor potential channels and their link with cardio/cerebro-vascular diseases. Biomolecules & Therapeutics 25, 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu XC, Li L, Ma CN, & Zhang YJ (2017). Effects of TRPC1 on epithelial mesenchymal transition in human airway in chronic obstructive pulmonary disease. Medicine (Baltimore) 96, e8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y, Xie R, Liu J, Nie X, An J, … Tuo B (2018). The NCX1/TRPC6 complex mediates TGFβ-driven migration and invasion of human hepatocellular carcinoma cells. Cancer Research 78, 2564–2576. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, … Beech DJ (2006). A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circulation Research 98, 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, … Beech DJ (2008). TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Lozinskaya I, Costell M, Lin Z, Ball JA, Bernard R, … Schnackenberg CG (2013). Characterization of small molecule TRPC3 and TRPC6 agonist and antagonists. Biophysical Journal 104, 454a. [Google Scholar]
- Yamamoto R, Hatano N, Sugai T, & Kato N (2014). Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT2C receptor. Neuropharmacology 82, 49–58. [DOI] [PubMed] [Google Scholar]
- Yan HD, Okamoto H, Unno T, Tsytsyura YD, Prestwich SA, Komori S, … Bolton TB (2003). Effects of G-protein-specific antibodies and G beta gamma subunits on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. British Journal of Pharmacology 139, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Ma H, Wu Y, Zhou B, Zhang C, Chai C, & Cao Z (2019). Activation of TRPC6 channels contributes to (+)-conocarpan-induced apoptotic cell death in HK-2 cells. Food and Chemical Toxicology 129, 281–290. [DOI] [PubMed] [Google Scholar]
- Yang LP, Jiang FJ, Wu GS, Deng K, Wen M, Zhou X, … Luo HR (2015). Acute treatment with a novel TRPC4/C5 channel inhibitor produces antidepressant and anxiolytic-like effects in mice. PLoS One 10, e0136255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zhang X, Li K, Li Z, & Yang Z (2018). Effects of miR-200b-3p inhibition on the TRPC6 and BKCa channels of podocytes. Archives of Biochemistry and Biophysics 653, 80–89. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, & Lee SY (2017). Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, … Yuan JX (2004). Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proceedings of the National Academy of Sciences of the United States of America 101, 13861–13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, & Muallem S (2009). TRPC channels as STIM1-regulated SOCs. Channels (Austin, Tex.) 3, 221–225. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, & Muallem S (2007). STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nature Cell Biology 9, 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Xie J, Yu AS, Stock J, Du J, & Yue L (2015). Role of TRP channels in the cardiovascular system. American Journal of Physiology. Heart and Circulatory Physiology 308, H157–H182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B, Yuan C, Yang X, Atkin SL, & Xu SZ (2013). TRPC channels and their splice variants are essential for promoting human ovarian cancer cell proliferation and tumorigenesis. Current Cancer Drug Targets 13, 103–116. [PubMed] [Google Scholar]
- Zeng C, Tian F, & Xiao B (2016). TRPC channels: Prominent candidates of underlying mechanism in neuropsychiatric diseases. Molecular Neurobiology 53, 631–647. [DOI] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, & Muallem S (2008). STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Molecular Cell 32, 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, & Shi Y (2017). TRPC channels and cell proliferation. Advances in Experimental Medicine and Biology 976, 149–155. [DOI] [PubMed] [Google Scholar]
- Zhang E, & Liao P (2015). Brain transient receptor potential channels and stroke. Journal of Neuroscience Research 93, 1165–1183. [DOI] [PubMed] [Google Scholar]
- Zhang P, Ma Y, Wang Y, Ma X, Huang Y, Li RA, … Yao X (2014). Nitric oxide and protein kinase G act on TRPC1 to inhibit 11,12-EET-induced vascular relaxation. Cardiovascular Research 104, 138–146. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, … Cahalan MD (2006). Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proceedings of the National Academy of Sciences of the United States of America 103, 9357–9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, … Cahalan MD (2005). STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Yang K, Tian L, Fu X, Wang Y, … Wang J (2014). BMP4 increases the expression of TRPC and basal [Ca2+]i via the p38MAPK and ERK1/2 pathways independent of BMPRII in PASMCs. PLoS One 9, e112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, … Zhu MX (2001). Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proceedings of the National Academy of Sciences of the United States of America 98, 3168–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, & Phelan KD (2014). The role of canonical transient receptor potential channels in seizure and excitotoxicity. Cells 3, 288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, & Bolton TB (1997). Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. British Journal of Pharmacology 122, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang Y, Zhang C, Yang G, Zhang F, Yu B, … Cao Z (2018). Ribemansides A and B, TRPC6 inhibitors from Ribes manshuricum that suppress TGF-beta1-induced fibrogenesis in HK-2 Cells. Journal of Natural Products 81, 913–917. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, … Greka A (2017). A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358, 1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, & Greka A (2016). Calcium-permeable ion channels in the kidney. American Journal of Physiology. Renal Physiology 310, F1157–F1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX (2005). Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflügers Archiv 451, 105–115. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, & Birnbaumer L (1995). Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Letters 373, 193–198. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, & Birnbaumer L (1998). Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. The Journal of Biological Chemistry 273, 133–142. [DOI] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, & Birnbaumer L (1996). trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell 85, 661–671. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gao M, Zhou T, Xie M, Mao A, Feng L, … Ma X (2019). The TRPC5 channel regulates angiogenesis and promotes recovery from ischemic injury in mice. The Journal of Biological Chemistry 294, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Lu Y, Qu C, Miller M, Tian J, Thakur DP, … Luo HR (2015). Identification and optimization of 2-aminobenzimidazole derivatives as novel inhibitors of TRPC4 and TRPC5 channels. British Journal of Pharmacology 172, 3495–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tan YQ, & Leung LK (2016). Aflatoxin B1 disrupts transient receptor potential channel activity and increases COX-2 expression in JEG-3 placental cells. Chemico-Biological Interactions 260, 84–90. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yao X, & Leung LK (2016). Zeranol induces COX-2 expression through TRPC-3 activation in the placental cells JEG-3. Toxicology In Vitro 35, 17–23. [DOI] [PubMed] [Google Scholar]
- Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Lückhoff A, & Schultz G (1996). Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16, 1189–1196. [DOI] [PubMed] [Google Scholar]


