Abstract
Combined radiation-wound injury (CRWI) is characterized by blood vessel damage and pro-inflammatory cytokine deficiency. Studies have identified that the direct application of leptin plays a significant role in angiogenesis and inflammation. We established a sustained and stable leptin expression system to study the mechanism. A lentivirus method was employed to explore the angiogenic potential and peripheral inflammation of irradiated human umbilical vein endothelial cells (HUVECs). Leptin was transfected into human placenta-derived mesenchymal stem cells (HPMSCs) with lentiviral vectors. HUVECs were irradiated by X-ray at a single dose of 20 Gy. Transwell migration assay was performed to assess the migration of irradiated HUVECs. Based on the Transwell systems, co-culture systems of HPMSCs and irradiated HUVECs were established. Cell proliferation was measured by cell counting kit-8 (CCK-8) assay. The secretion of pro-inflammatory cytokines (human granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin (IL)-1α, IL-6, and IL-8) was detected by enzyme-linked immunosorbent assay (ELISA). The expression of pro-angiogenic factors (vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF)) mRNA was detected by real-time quantitative polymerase chain reaction (RT-qPCR) assay. Relevant molecules of the nuclear factor-κB (NF-κB) and Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathways were detected by western blot assay. Results showed that leptin-modified HPMSCs (HPMSCs/leptin) exhibited better cell proliferation, migration, and angiogenic potential (expressed more VEGF and bFGF). In both the single HPMSCs/leptin and the co-culture systems of HPMSCs/leptin and irradiated HUVECs, the increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) was associated with the interaction of the NF-κB and JAK/STAT signaling pathways. We conclude that HPMSCs/leptin could promote angiogenic potential and peripheral inflammation of HUVECs after X-ray radiation.
Keywords: Leptin, Angiogenesis, Pro-inflammatory cytokines, X-ray radiation, Human placenta-derived mesenchymal stem cells (HPMSCs), Human umbilical vein endothelial cells (HUVECs)
1. Introduction
Combined radiation-wound injury (CRWI) is a refractory wound, which occurs in most malignant tumor patients with postoperative or preoperative radiation therapy. Compared with normal wound healing, the CRWI is refractory to healing mainly due to but not limited: (1) blood vessel damage brings about tissue anoxia and ischemic injury (van den Brenk et al., 1974a, 1974b; Hao et al., 2009); (2) inhibition of the hematopoietic function leads to a decrease in the total number of inflammatory cells and the cells recruited to the wound site (Hao et al., 2009). Thus, angiogenesis and pro-inflammation are the critical points of CRWI healing.
Leptin, a 16-kDa adipose tissue-specific cytokine, is mainly secreted from white adipose tissue (Adya et al., 2015; Park and Ahima, 2015). Leptin possesses multiple established physiological roles in the stable control of body weight, lipid metabolism, hematopoiesis, and body heat production (Frühbeck, 2006). Importantly, the widespread expression of leptin receptors in peripheral tissues contributes to the high functional polymorphism of this hormone, including metabolism, bone remodeling, wound healing, and reproduction (Lancha et al., 2012). Apart from the metabolic role, leptin is also considered as an angiogenic molecule (Sierra-Honigmann et al., 1998; Cao et al., 2001; Park et al., 2001; Kurtovic et al., 2015; Manjunathan and Ragunathan, 2015), which has been widely studied in wound healing (Liapaki et al., 2008; Umeki et al., 2014; Tadokoro et al., 2015).
Recently, it was reported that leptin plays a pro-inflammatory role in regulating immune responses and facilitating numerous autoimmune diseases (Abella et al., 2017). In addition, leptin promotes the stimulation and proliferation of various immune cells to generate and secrete inflammatory cytokines (Pérez-Pérez et al., 2017).
However, the effective concentration of local direct application of leptin often varies with individual differences and experimental conditions. Thus, it is worth considering the establishment of a sustained and stable leptin expression system.
Human placenta-derived mesenchymal stem cells (HPMSCs) belong to extra-fetal tissue-derived stem cells (Duscher et al., 2016) and can be isolated from discarded placental tissue (Alviano et al., 2007). The HPMSCs have plenty of inherent advantages: no invasive procedures, easier to access, fewer ethical restrictions, considerable multipotency, low immunogenicity, inability to form invasive colonies, and immunomodulatory effects (Liu et al., 2009; Lee et al., 2012; Sabapathy et al., 2012; Duscher et al., 2016). These advantages suggest that HPMSCs may be ideal leptin carriers and could be employed to establish a sustained and stable gene expression system.
To this end, in this study, we transfected leptin gene into HPMSCs by lentiviral vectors and investigated the effects of leptin-modified HPMSCs (HPMSCs/leptin) on angiogenesis and pro-inflammation of irradiated human umbilical vein endothelial cells (HUVECs). We speculated that the leptin expression system may be a positive regulator of angiogenesis and pro-inflammation during the healing of CRWI.
2. Materials and methods
2.1. Cells, agents, and antibodies
The human placenta and written informed consent for this study were provided by the Second Hospital of Jilin University (Changchun, China). The inclusion criteria of human placenta included full-term eutocous placenta without fetal malformation and being obtained from healthy lying-in women without infectious disease. HPMSCs were extracted, cultured, and identified in the Laboratory of the School of Stomatology, Jilin University. Construction of leptin was via a gene synthesis method, which was supported by Synbio Technologies (Suzhou, China). Recombinant lentiviral vectors pEB-copGFP (T2A) PURO-leptin (L.v.-pEB-copGFP (T2A) PURO-leptin) and pEB-copGFP (T2A) PURO (L.v.-pEB-copGFP (T2A) PURO) were constructed by Guangzhou Fit-gene Biotech Co., Ltd. (China). Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG), DMEM, penicillin-streptomycin, trypsin, and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA), and glutamine was obtained from Thermo Fisher Scientific (NJ, USA). HiFiScript gDNA Removal cDNA Synthesis Kit and UltraSYBR Mixture (High ROX) were obtained from Cowin Biosciences (Beijing, China). Transwell systems with 8.0 and 0.4 μm polyethylene terephthalate (PET) membranes were purchased from Corning (NY, USA). Anti-bodies against leptin, nuclear factor-κB (NF-κB) p65, inhibitor of NF-κB (IκBα), signal transducer and activator of transcription 3 (STAT3), β-Actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and enhanced chemiluminescence (ECL) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (H+L) and cell counting kit-8 (CCK-8) were purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits of human granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin (IL)-1α, IL-6, and IL-8 were purchased from Neobioscience Technology Co., Ltd. (Shenzhen, China).
2.2. Experimental groups
HPMSCs were randomly divided into three groups, including HPMSCs (non-transfected), HPMSCs/NC (negative control, transfected with L.v.-pEB-copGFP (T2A) PURO), and HPMSCs/leptin (transfected with L.v.-pEB-copGFP (T2A) PURO-leptin). These groups were cultured under the same condition.
2.3. Transfection of HPMSCs with lentiviral vectors
We cultured HPMSCs in DMEM-LG supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2-containing humidified atmosphere. Cells were passaged and plated in a six-well plate, at a density of 5×106 cells/well. When it reached approximately 40%–50% confluence, the medium was changed to antibiotic-free DMEM-LG containing 10% FBS and cells were cultured at 37 °C in 5% CO2 for 1 h. Respectively, 1 mL L.v.-pEB-copGFP (T2A) PURO-leptin supernatant, L.v.-pEB-copGFP (T2A) PURO supernatant, and phosphate-buffered saline (PBS) were added to the planned three groups and each group was incubated at the same condition for 6 h. The infected cells were transferred to DMEM-LG supplemented with 10% FBS and 1% penicillin-streptomycin, and cultured at 37 °C in 5% CO2 and 95% relative humidity. After 48 h, the HPMSCs that successfully expressed L.v.-pEB-copGFP (T2A) PURO (HPMSCs/NC and HPMSCs/leptin) were screened from selection medium I (DMEM-LG containing 10% FBS, 1% penicillin-streptomycin, and 1 μg/mL puromycin), and then the screened cells were expanded in selection medium II (DMEM-LG containing 10% FBS, 1% penicillin-streptomycin, and 0.5 μg/mL puromycin). Finally, these cells were cryopreserved for the subsequent assays. The expression of copGFP was detected by an inverted fluorescence microscope (MF51, MSHOT, China). The expression of leptin mRNA was determined by real-time quantitative polymerase chain reaction (RT-qPCR) assay. GAPDH was selected as an internal control. Primers for leptin and GAPDH are shown in Table 1. The expression of leptin protein was determined by western blot assay. β-Actin was selected as an internal control. Primary antibody against leptin (1:200 dilution) and secondary antibody (HRP-labeled goat anti-rabbit IgG (H+L), 1:8000 dilution) were used.
Table 1.
Primers of each gene of RT-qPCR assay
| Gene | Forward primer sequence (5'→3') | Reverse primer sequence (5'→3') |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Leptin | CATTTCACACACGCAGTCAGT | CTGGAAGGCATACTGGTGAGG |
| VEGF | TCACCAAGGCCAGCACATAG | TTTCTCCGCTCTGAGCAAGG |
| bFGF | CAAAAACGGGGGCTTCTTCC | GTTGTAGCTTGATGTGAGGGTC |
RT-qPCR: real-time quantitative polymerase chain reaction; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor
2.4. Cell proliferation of HPMSCs, HPMSCs/NC, and HPMSCs/leptin
Anabiotic cells of the three groups were cultured in DMEM-LG supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in 5% CO2. When approximately 80% confluence was reached, cells were trypsinized, centrifuged, and resuspended. After the cell density was adjusted to 5×104 cells/mL, the cells were seeded in a 96-well plate, with approximately 5×103 cells in 100 μL cell suspension per well. After cells adhered, 10 μL CCK-8 solution (at a 1:10 ratio) was added per well and incubated at 37 °C in 5% for 4 h. The optical density at 450 nm (OD450) was measured by a microplate reader (318CT, Peiou, China).
2.5. RT-qPCR assay for VEGF and bFGF in HPMSCs, HPMSCs/NC, and HPMSCs/leptin
We isolated the total RNA from the three groups by TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. The concentration of RNA was measured by an SMA4000 ultramicrospectrophotometer (Metrinton, China). The integrity of RNA was analyzed by 1.0% (0.01 g/mL) agarose gel electrophoresis. DNA was removed, and cDNA was synthesized by reverse transcription using HiFiScript gDNA Removal cDNA Synthesis Kit. Primers for vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and GAPDH are shown in Table 1. GAPDH was selected as an internal control. The relative expression of these target genes was calculated by fold change=2−ΔΔ C T, ΔC T=C T ( leptin )–C T ( GAPDH ).
2.6. ELISA for pro-inflammatory cytokines of HPMSCs, HPMSCs/NC, and HPMSCs/leptin
Human GM-CSF, IL-1α, IL-6, and IL-8 secretion levels in the medium of HPMSCs, HPMSCs/NC, HPMSCs/leptin were detected using the appropriate ELISA kits, and the OD450 value was measured by a microplate reader (ELX800, BioTek, USA). The NC consisted of DMEM-LG containing 10% FBS and 1% penicillin-streptomycin only. All ELISAs were carried out according to the manufacturer’s instructions.
2.7. Detection of NF-κB p65, IκBα, and STAT3 proteins in HPMSCs/NC and HPMSCs/leptin by western blot assay
Anabiotic cells of each group were harvested and lysed. Total protein was extracted and quantified according to the bicinchoninic acid (BCA) method. Upon denaturation by heating at 100 °C for 5 min, the proteins were separated using 12% sodium dodecyl sulfate-polyarylamide gel electrophoresis (SDS-PAGE) and electrically transferred onto a nitrocellulose membrane with 300 mA constant current for 90 min. The membrane was blocked in 5% skimmed milk Tris-buffered saline and Tween 20 (TBST) solution at room temperature (RT) for 1 h. The blot was washed with TBST three times for 5 min each and incubated with a primary antibody against NF-κB p65 (1:500 dilution), IκBα (1:500 dilution), and STAT3 (1:500 dilution) at RT overnight. The blot was washed with TBST and incubated with a secondary antibody (HRP-labeled goat anti-rabbit IgG (H+L), 1:8000 dilution) at RT for 2 h. After being washed, the protein bands were visualized using a chemiluminescence reagent.
2.8. Cell culture and treatment
HUVECs were cultured in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin at 37 °C in 5% CO2 atmosphere. When approximately 90% confluence was reached, HUVECs were irradiated by an X-ray irradiator (MultiRad 225, Faxitron, USA) at a single dose of 20 Gy.
2.9. Transwell migration assay
Transwell migration assay was conducted using a Transwell system with an 8.0 μm PET membrane. A suspension of approximately 1.0×105 irradiated HUVECs in 100 μL serum-free DMEM was added to the upper chamber, while a suspension of approximately 1.0×104 HPMSCs/leptin or HPMSCs/NC in DMEM-LG was added to the lower chamber. The non-migrating cells on the upper surface of the PET membrane were wiped off by sterile cotton swabs after 48-h incubation. Cells which had migrated to the lower surface of the PET membrane were fixed with 4% paraformaldehyde for 20 min and stained with crystal violet for 10 min. Lastly, images were randomly captured in the objective field by an inverted fluorescence microscope (MF51, Mshot, China).
2.10. Construction of co-culture systems of irradiated HUVECs and HPMSCs/NC or HPMSCs/leptin
The mesenchymal stem cells (HPMSCs/NC, HPMSCs/leptin) and irradiated HUVECs were co-cultured using a Transwell system with 0.4 μm PET membrane. A 400-μL suspension containing approximately 1.0×105 irradiated HUVECs was added to the lower chamber. Then, a 200-μL suspension containing approximately 1.0×104 HPMSCs/NC or HPMSCs/leptin was added to the upper chamber. The co-culture systems of irradiated HUVECs and HPMSCs/NC or HPMSCs/leptin were incubated at 37 °C in 5% CO2.
2.11. Cell proliferation of the irradiated HUVECs co-cultured with HPMSCs/NC or HPMSCs/leptin
Co-cultured with HPMSCs/NC or HPMSCs/leptin for 1, 2, 3, and 4 d, the irradiated HUVECs were collected and seeded in 96-well plates at a density of 2×103 cells/well and incubated at 37 °C in 5% CO2. After 24 h, these cells were treated with 10 μL CCK-8 solution for another 2 h. The plates were subsequently subjected to a microplate reader (ELX800, BioTek, USA) for measuring absorbance at 450 nm.
2.12. ELISA for the irradiated HUVEC-derived pro-inflammatory cytokines in co-culture systems
Human GM-CSF, IL-1α, IL-6, and IL-8 secretion levels in the medium of co-culture systems were detected using the appropriate ELISA kits, and the OD450 value was measured by a microplate reader (ELX800, BioTek, USA). The NC for ELISA consisted of DMEM containing 10% FBS and 1% penicillin-streptomycin only. All ELISAs were carried out according to the manufacturer’s instructions.
2.13. Western blot assay for NF-κB p65, IκBα, and STAT3 proteins of the irradiated HUVECs
After being co-cultured with HPMSCs/NC or HPMSCs/leptin for 72 h, the irradiated HUVECs were collected, and the expression levels of NF-κB p65, IκBα, and STAT3 proteins were detected by western blotting as described in Section 2.7.
2.14. Statistical analysis
All statistical data were analyzed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Data are presented as mean±standard deviation (SD). Comparisons among three groups were made using a one-way analysis of variance (ANOVA) complemented with the Bonferroni correction. Comparisons between the two groups were measured using a two-tailed unpaired Student’s t-test. At an α level of 0.05, P<0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Transfection efficiency of HPMSCs with lentiviral vectors
The structure of L.v.-pEB-copGFP (T2A) PURO-leptin is shown in Fig. 1a. After transfection of the leptin gene with lentiviral vectors, the copGFP fluorescence of HPMSCs/NC and HPMSCs/leptin was observed under an inverted fluorescence microscope (Fig. 1b). The expression of leptin mRNA (Fig. 1c) and leptin protein (Fig. 1d) in HPMSCs/leptin was higher than that in the other two groups.
Fig. 1.
Transfection of HPMSCs with lentiviral vectors
(a) Structure of L.v.-pEB-copGFP (T2A) PURO-leptin. (b) Fluorescence microscope analysis of transfection (scale bar=200 μm). RT-qPCR assay (c) and western blot assay (d) of leptin expression. Lane 1: HPMSCs/leptin; Lane 2: HPMSCs/NC; Lane 3: HPMSCs; LTR: long terminal repeat; L.v.: lentiviral vector; RT-qPCR: real-time quantitative polymerase chain reaction; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control. Data are expressed as mean±standard deviation (SD), n=3. *** P<0.001
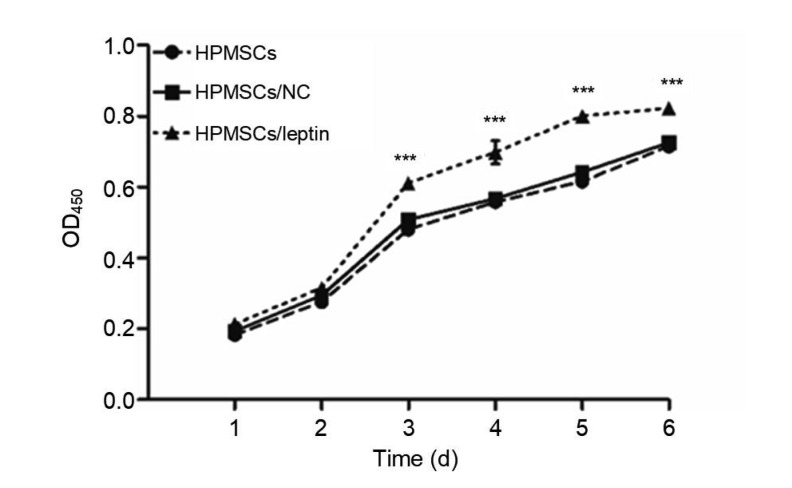
3.2. Better cell proliferation in HPMSCs/leptin
The CCK-8 assay (Fig. 2) showed that HPMSCs/leptin had a better cell proliferation than HPMSCs/NC or HPMSCs, which suggested that leptin could promote cell proliferation of HPMSCs.
Fig. 2.
Assessment of cell proliferation of HPMSCs, HPMSCs/NC, and HPMSCs/leptin by CCK-8 assay
HPMSCs/leptin exhibited better cell proliferation, compared with HPMSCs and HPMSCs/NC. Data are expressed as mean±standard deviation (SD), n=4. *** P<0.001 compared with other groups. HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control; CCK-8: cell counting kit-8; OD450: optical density at 450 nm
3.3. Increased expression of VEGF and bFGF in HPMSCs/leptin
The RT-qPCR showed that the relative expression of VEGF and bFGF in HPMSCs/leptin was higher than that in HPMSCs/NC and HPMSCs (Fig. 3). This confirmed that leptin could increase the expression of VEGF and bFGF, and further, promote cell proliferation and migration.
Fig. 3.
Quantification of VEGF and bFGF expression by RT-qPCR assay
Leptin increased the expression of VEGF and bFGF in HPMSCs/leptin. Data are expressed as mean±standard deviation (SD), n=3. *** P<0.001. VEGF: vascular endothelial growth factor; bFGF: basic fibroblast growth factor; RT-qPCR: real-time quantitative polymerase chain reaction; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control
3.4. Upregulated production of pro-inflammatory cytokines in HPMSCs/leptin
ELISA was used to quantify the concentration of secreted pro-inflammatory cytokines (human GM-CSF, IL-1α, IL-6, and IL-8) in HPMSCs/leptin, HPMSCs/NC, and HPMSCs (Fig. 4). The levels of IL-6, GM-CSF, and IL-1α secreted by HPMSCs/leptin in culture supernatant were higher than the average levels of the other two groups. However, the concentration of IL-8 did not reveal a significant difference among groups. Taken together, our current work showed that leptin promoted HPMSCs/leptin to secrete the human GM-CSF, IL-1α, and IL-6.
Fig. 4.
Upregulated production of pro-inflammatory cytokines in HPMSCs/leptin
Enzyme-linked immunosorbent assay (ELISA) was used to quantify the concentrations of secreted pro-inflammatory cytokines (human granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin (IL)-1α, IL-6, and IL-8) in HPMSCs/leptin, HPMSCs/NC, and HPMSCs. Leptin increased the human GM-CSF, IL-1α, and IL-6 secreted by HPMSCs. Data are expressed as mean±standard deviation (SD), n=3. One way analysis of variance (ANOVA) with Bonferroni correction was performed among three groups to compare the quantity of the concentrations of secreted pro-inflammatory cytokines. ** P<0.01, *** P<0.001. HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control
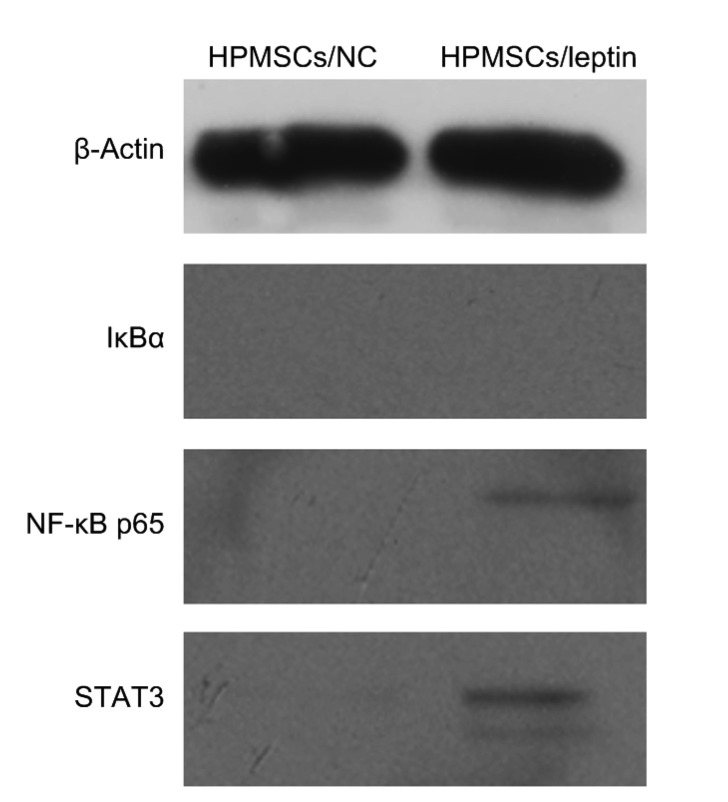
3.5. Promoted expression of NF-κB p65 and STAT3 proteins in HPMSCs/leptin
Cells of each group were collected and lysed. The total proteins were collected and then separated by 12% (0.12 g/mL) SDS-PAGE. Results revealed that the expression of NF-κB p65 and STAT3 proteins was observed in the HPMSCs/leptin, but not in the HPMSCs/NC (Fig. 5). IκBα was not observed in any group. These results suggested that leptin could facilitate the expression of NF-κB p65 and STAT3 proteins in HPMSCs/leptin.
Fig. 5.
Western blot analyses of IκBα, NF-κB p65, and STAT3 proteins in HPMSCs/NC and HPMSCs/leptin
NF-κB: nuclear factor-κB; IκBα: inhibitor of NF-κB; STAT3: signal transducer and activator of transcription 3; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control
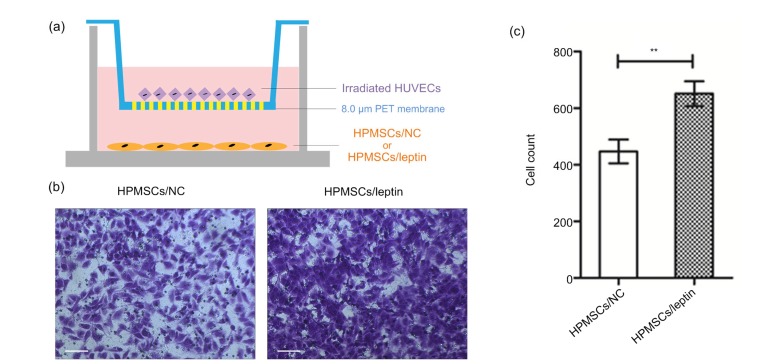
3.6. Promoted migration of the irradiated HUVECs in HPMSCs/leptin
Transwell migration assay was employed to assess the migration of irradiated HUVECs (Fig. 6a). Results showed that the cell migration of irradiated HUVECs was significantly stimulative in the Transwell system of irradiated HUVECs and HPMSCs/leptin (Figs. 6b and 6c), which confirmed that HPMSCs/leptin could promote the migration of irradiated HUVECs.
Fig. 6.
Transwell migration assay
(a) Transwell migration system was employed to investigate the migration of irradiated HUVECs induced by HPMSCs/NC or HPMSCs/leptin. (b, c) Compared with HPMSCs/NC, HPMSCs/leptin promoted the migration of irradiated HUVECs (scale bar=100 μm). Data are expressed as mean±standard deviation (SD), n=3. ** P<0.01. HUVECs: human umbilical vein endothelial cells; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control; PET: polyethylene terephthalate
3.7. Promoted cell proliferation of the irradiated HUVECs in HPMSCs/leptin
Based on the Transwell system, co-culture systems of HPMSCs/NC or HPMSCs/leptin and irradiated HUVECs were constructed (Fig. 7a). CCK-8 assay showed that, compared with those co-cultured with HPMSCs/NC, the irradiated HUVECs possessed better cell proliferation co-cultured with HPMSCs/leptin (Fig. 7b), which suggested that HPMSCs/leptin could promote cell proliferation of irradiated HUVECs.
Fig. 7.
CCK-8 analysis of cell proliferation of the irradiated HUVECs in co-culture systems
(a) Co-culture systems of irradiated HUVECs and HPMSCs/NC or HPMSCs/leptin. (b) HPMSCs/leptin promoted cell proliferation of irradiated HUVECs in the co-culture system of HPMSCs/leptin and irradiated HUVECs. Data are expressed as mean±standard deviation (SD), n=4. *** P<0.001 vs. HPMSCs/NC. CCK-8: cell counting kit-8; HUVECs: human umbilical vein endothelial cells; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control; PET: polyethylene terephthalate; OD450: optical density at 450 nm
3.8. Upregulated secretion of pro-inflammatory cytokines in co-culture systems of HPMSCs/leptin and irradiated HUVECs
ELISA was used to quantify the concentrations of secreted pro-inflammatory cytokines (human GM-CSF, IL-1α, IL-6, and IL-8) in co-culture systems (Fig. 8). The levels of IL-6, GM-CSF, and IL-1α secreted by the co-culture system of HPMSCs/leptin and irradiated HUVECs in culture supernatant were higher than the average levels of the co-culture system of HPMSCs/NC and irradiated HUVECs. However, the concentration of IL-8 did not reveal a significant difference between groups. Taken together, HPMSCs/leptin increased the production of the pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in the co-culture system of HPMSCs/leptin and irradiated HUVECs.
Fig. 8.
ELISA for the pro-inflammatory cytokines of the irradiated HUVECs in the co-culture system
The concentrations of pro-inflammatory cytokines (human granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin (IL)-1α, IL-6, and IL-8) in the co-culture system of HPMSCs/leptin or HPMSCs/NC and irradiated HUVECs were calculated. HPMSCs/leptin upregulated the production of human GM-CSF, IL-1α, and IL-6 in the co-culture system of HPMSCs/leptin and irradiated HUVECs. Data are expressed as mean±standard deviation (SD), n=3. Two-tailed unpaired Student’s t-test was performed between two groups. * P<0.05, *** P<0.001. ELISA: enzyme-linked immunosorbent assay; HUVECs: human umbilical vein endothelial cells; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control
3.9. Promoted expression of STAT3 protein in the irradiated HUVECs co-cultured with HPMSCs/NC
The irradiated HUVECs of each co-culture system were collected and lysed. The total proteins of irradiated HUVECs were collected and then separated by 12% (0.12 g/mL) SDS-PAGE. As shown in Fig. 9, this revealed that the expression of STAT3 protein was observed in the irradiated HUVECs that were co-cultured with the HPMSCs/leptin, but not in the irradiated HUVECs that were co-cultured with the HPMSCs/NC. The irradiated HUVECs of each co-culture system expressed NF-κB p65 protein, and there was no significant difference between the two co-culture systems. IκBα was not observed in any group. These results suggested that leptin could promote the expression of STAT3 protein in irradiated HUVECs.
Fig. 9.
Western blot analyses of IκBα, NF-κB p65, and STAT3 proteins in the co-culture system of HPMSCs/leptin or HPMSCs/NC and irradiated HUVECs
NF-κB: nuclear factor-κB; IκBα: inhibitor of NF-κB; STAT3: signal transducer and activator of transcription 3; HPMSCs: human placenta-derived mesenchymal stem cells; NC: negative control; HUVECs: human umbilical vein endothelial cells
4. Discussion
CRWI is characterized by blood vessel damage and pro-inflammatory cytokine deficiency. Studies have identified that the direct application of leptin plays a significant role in angiogenesis (Umeki et al., 2014; Kurtovic et al., 2015; Manjunathan and Ragunathan, 2015; Tadokoro et al., 2015; Nwadozi et al., 2019) and inflammation (Sun et al., 2018; Yu et al., 2019). However, the effective concentration of local direct application of leptin often varies with individual differences and experimental conditions. Therefore, it is worth considering establishing a sustained and stable leptin expression system to explore the mechanism. In our examination, we successfully transfected leptin into HPMSCs using the lentiviral vectors method, and obtained HPMSCs/leptin. Compared with our previous work, we improved the transfection efficiency of HPMSCs/leptin (Jin et al., 2014). HPMSCs/leptin exhibited better cell proliferation, migration, and angiogenic potential. In addition, increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6), both in the single HPMSCs/leptin and in the co-culture system of HPMSCs/leptin and irradiated HUVECs, was associated with the interaction of the NF-κB signaling pathway and the Janus kinase (JAK)/STAT signaling pathway. Our study indicated that HPMSCs/leptin could promote angiogenic potential and peripheral inflammation of HUVECs following X-ray radiation.
Different from the methods of most related works (Umeki et al., 2014; Kurtovic et al., 2015; Manjunathan and Ragunathan, 2015; Tadokoro et al., 2015; Sun et al., 2018; Nwadozi et al., 2019; Yu et al., 2019), in which leptin protein was directly added to medium, our study established a gene expression system that allowed HPMSC expression of leptin in a sustained and stable manner. By observing the copGFP fluorescence and detecting the expression of leptin mRNA and leptin protein in the HPMSCs/leptin, our work confirmed the success of constructing a leptin expression system. HPMSCs/leptin exhibited better cell proliferation than non-leptin-modified HPMSCs. This is consistent with our previous work (Jin et al., 2014). Many previous studies have proved that leptin stimulates VEGF and bFGF expression in angiogenesis (Park et al., 2001; Manjunathan and Ragunathan, 2015). In our study, the expression of VEGF and bFGF in HPMSCs/leptin was significantly higher than that in non-leptin-modified HPMSCs. It is confirmed that leptin could promote HPMSCs to express more VEGF and bFGF, and further promote angiogenic potential.
Our findings confirm that leptin increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in the single HPMSCs/leptin. It is generally known that leptin is a pro-inflammatory factor (Abella et al., 2017; Pérez-Pérez et al., 2017; Yu et al., 2019), which is able to promote the secretion levels of numerous pro-inflammatory cytokines in a variety of tissues (Nwadozi et al., 2019; Yu et al., 2019). Our work found that leptin could upregulate the production of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in HPMSCs/leptin. This suggests that HPMSCs/leptin could increase the secretion levels of pro-inflammatory cytokines of itself; nevertheless, up to now, there has been no study examining this phenomenon.
Our findings suggest that increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in the single HPMSCs/leptin is associated with the interaction of the NF-κB signaling pathway and the JAK/STAT signaling pathway. It has been reported that leptin induces IL-6 expression in ligamentum flavum cells through NF-κB (Sun et al., 2018). In addition, IL-6/gp130 is related to the activation of the JAK/STAT signaling pathway in the inflammatory processes of vascular diseases (Grote et al., 2005). Thus, leptin upregulates pro-inflammatory cytokines and is implicated in the NF-κB signaling pathway, even the JAK/STAT signaling pathway. NF-κB p65 is an essential member of NF-κB heterodimeric protein, which induces the transcription of pro-inflammatory cytokines (Sun et al., 2018) and is mainly involved in inflammatory responses (Baldwin, 1996). In the HPMSCs/leptin, we observed that leptin promotes the expression of NF-κB p65 and upregulates the production of the pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6). IκBα is an inhibitory protein that maintains balance by inhibiting the inflammatory response caused by signal transduction through non-covalent interactions with NF-κB dimers in unstimulated cells. The degradation of IκBα allows the activation of NF-κB complex to translocate into the nucleus and induce gene expression (Baeuerle, 1998). In our present study, we observed no IκBα expression in HPMSCs/leptin or HPMSCs/NC. The former may activate NF-κB complex via leptin stimulation, while the latter may be determined by the property of HPMSCs. In addition, leptin binds to the leptin receptor long isoform and transmits extracellular signals through the JAK2/STAT3 signaling pathway. STAT3 is a kind of STATs, which can be induced by leptin (Sierra-Honigmann et al., 1998) to form active STAT3 dimers. These translocate into the nucleus to regulate gene expression and alter target cell proliferation and differentiation (Grote et al., 2005; Abella et al., 2017). Moreover, the function of activated STAT3 can be altered by association with NF-κB (Walker and Smith, 2005). In HPMSCs/leptin, to some extent, the result that leptin facilitates the expression of NF-κB p65 and STAT3 could be attributed to the interaction between the NF-κB signaling pathway and the JAK/STAT signaling pathway.
In HPMSCs/leptin, to some extent, our findings provide evidence for the angiogenic potential of leptin in irradiated HUVECs. Surgery and radiation are necessary for most malignant tumor patients; however, to no small extent, postoperative or preoperative radiation therapy usually results in refractory wounds (Powers et al., 1967). This wound is characterized by tissue anoxia and ischemic injury caused by blood vessel damage (van den Brenk et al., 1974a, 1974b; Hao et al., 2009). This is called CRWI. It has been proved that a low dose (0.3 Gy) of ionizing radiation promotes the angiogenic potential of adipocyte conditioned medium from mature adipocytes, which differentiated from irradiated pre-adipocytes (Marques et al., 2019). Moreover, under low (0.2 or 1.0 Gy) doses of radiation, the irradiated HUVECs induce the expression of pro-angiogenic microRNA and promote the formation of capillary-like tubes (Vincenti et al., 2011). High (20.0 Gy) doses of radiation can induce the production of angiostatic chemokine and apoptosis in human endothelial cells (Chang et al., 2009). Given this fact, we placed HUVECs under an X-ray irradiator at a single dose of 20.0 Gy (van den Brenk et al., 1974a; Mustoe et al., 1989). We designed Transwell migration systems and co-culture systems to test and verify whether HPMSCs/leptin is capable of promoting cell migration and proliferation when cells are irradiated. In the Transwell migration systems, the irradiated HUVECs were placed in the upper chamber; meanwhile, the HPMSCs/leptin or the HPMSCs/NC were placed in the lower chamber. We found that HPMSCs/leptin significantly promoted the migration of irradiated HUVECs compared to the HPMSCs/NC. In the co-culture systems, the HPMSCs/leptin or the HPMSCs/NC were placed in the upper chamber, and the irradiated HUVECs were placed in the lower chamber. In this design, we co-cultured HPMSCs/leptin with irradiated HUVECs to investigate whether the HPMSCs/leptin have a positive effect on the cell proliferation of irradiated HUVECs. We found that HPMSCs/leptin promoted the cell proliferation of irradiated HUVECs. It has been reported that leptin alone and in synergism with VEGF or bFGF promotes the cell proliferation of bovine capillary endothelial (BCE) (Cao et al., 2001). Also, leptin plays a pro-angiogenic role in skeletal myocytes by promoting the production of VEGF-A (Nwadozi et al., 2019). VEGF and bFGF are well-known pro-angiogenic factors and have a synergistic pro-angiogenic effect on cell proliferation and migration of endothelial cells (Cao et al., 2001; van Hove and Benoit, 2015; Bai et al., 2018; Shentu et al., 2018). These facts suggest that leptin could promote cell migration and proliferation of irradiated HUVECs via improving the expression of VEGF and bFGF, and further to promote the angiogenesis potential of irradiated HUVECs.
Our findings confirm that HPMSCs/leptin increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in the co-culture system of HPMSCs/leptin and irradiated HUVECs. A previous study has shown that JAK/STAT signaling pathway is involved in regulating the inflammatory processes of many cells in the vessel wall (Grote et al., 2005). In the co-culture systems, HPMSCs/leptin or HPMSCs/NC were placed in the upper chamber and irradiated HUVECs were placed in the lower chamber. In this experiment, we co-cultured HPMSCs/leptin with irradiated HUVECs to study the effects of HPMSCs/leptin on the secretion levels of pro-inflammatory cytokines in irradiated HUVECs. We found that in the co-culture system of HPMSCs/leptin and irradiated HUVECs significantly higher secretion levels of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) were detected, indicating that HPMSCs/leptin were capable of increasing the secretion levels of pro-inflammatory cytokines of irradiated HUVECs. This may help to maintain the normal progression of inflammatory response during CRWI healing. Nevertheless, so far, there has been no study examining this phenomenon.
Our findings suggest that increased secretion of pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) in the co-culture system of HPMSCs/leptin and irradiated HUVECs was associated with the interaction of the NF-κB signaling pathway and the JAK/STAT signaling pathway. It has been shown that exposure of cells to ionizing radiation stimulates the signaling pathway to activate transcription factor NF-κB (Li and Karin, 1998). In each co-culture system, the expression of NF-κB p65 and the degradation of IκBα were observed. However, only in the co-culture system of HPMSCs/leptin and irradiated HUVECs was the expression of STAT3 protein detected. Since the function of activated STAT3 can be altered by NF-κB (Walker and Smith, 2005), and the JAK/STAT signaling pathway is related to the inflammatory process of many cells in the vascular wall (Grote et al., 2005), the upregulation of pro-inflammatory cytokines secretion in the co-culture system of HPMSCs/leptin and irradiated HUVECs may be attributed to the interaction of the NF-κB signaling pathway and the JAK/STAT signaling pathway.
5. Conclusions
In conclusion, we have succeeded in obtaining the HPMSCs/leptin, a sustained and stable leptin expression system, by transfecting lentiviral vectors (L.v.-pEB-copGFP (T2A) PURO-leptin) into HPMSCs. HPMSCs/leptin exhibited better cell proliferation, migration, and angiogenic potential. In addition, in both the single HPMSCs/leptin and the co-culture system of HPMSCs/leptin and irradiated HUVECs, the increased secretion of the pro-inflammatory cytokines (human GM-CSF, IL-1α, and IL-6) was associated with the interaction of the NF-κB signaling pathway and the JAK/STAT signaling pathway.
Acknowledgments
We thank Ji-zhou ZHANG (Department of Biochemistry, Basic Medical School, Jilin University, Changchun, China) for guiding the writing of this manuscript.
Footnotes
Project supported by the Special Fund for Cooperation of Local Government and College (Schools and Institutes) in Changchun, Jilin Province (No. 17DY024), China
Contributors: Shu CHEN performed the experimental research. Qian WANG, Bing HAN, Jia WU, Ding-kun LIU, Jun-dong ZOU, and Mi WANG participated in carrying out the experiment, analyzing data, and discussing. Shu CHEN and Qian WANG draw charts and wrote the manuscript. Zhi-hui LIU designed, organized, and supervised the project. Zhi-hui LIU, Shu CHEN, and Qian WANG proofread the manuscript. All authors have read, revised, and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Shu CHEN, Qian WANG, Bing HAN, Jia WU, Ding-kun LIU, Jun-dong ZOU, Mi WANG, and Zhi-hui LIU declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Abella V, Scotece M, Conde J, et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 2.Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res, 2015:648239. 2015 doi: 10.1155/2015/648239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol, 7:11. 2007 doi: 10.1186/1471-213x-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeuerle PA. IκB-NF-κB structures: at the interface of inflammation control. Cell. 1998;95(6):729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Bai LJ, Zhou J, et al. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018;323:19–32. doi: 10.1016/j.cellimm.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Cao RH, Brakenhielm E, Wahlestedt C, et al. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA. 2001;98(11):6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Lerman OZ, Thanik VD, et al. Dose-dependent effect of radiation on angiogenic and angiostatic CXC chemokine expression in human endothelial cells. Cytokine. 2009;48(3):295–302. doi: 10.1016/j.cyto.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Duscher D, Barrera J, Wong VW, et al. Stem cells in wound healing: the future of regenerative medicine? A mini-review. Gerontology. 2016;62(2):216–225. doi: 10.1159/000381877. [DOI] [PubMed] [Google Scholar]
- 10.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(1):7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grote K, Luchtefeld M, Schieffer B. JANUS under stress–role of JAK/STAT signaling pathway in vascular diseases. Vascul Pharmacol. 2005;43(5):357–363. doi: 10.1016/j.vph.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Hao L, Wang J, Zou Z, et al. Transplantation of BMSCs expressing hPDGF-A/hBD2 promotes wound healing in rats with combined radiation-wound injury. Gene Ther. 2009;16(1):34–42. doi: 10.1038/gt.2008.133. [DOI] [PubMed] [Google Scholar]
- 13.Jin JL, Wang BW, Zhu ZW, et al. Construction of a recombinant eukaryotic expression vector containing a leptin gene and its expression in HPMSCs. Cytotechnology. 2014;66(3):471–479. doi: 10.1007/s10616-013-9599-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtovic S, Ng TT, Gupta A, et al. Leptin enhances endothelial cell differentiation and angiogenesis in murine embryonic stem cells. Microvasc Res. 2015;97:65–74. doi: 10.1016/j.mvr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Lancha A, Frühbeck G, Gómez-Ambrosi J. Peripheral signalling involved in energy homeostasis control. Nutr Res Rev. 2012;25(2):223–248. doi: 10.1017/s0954422412000145. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Jung J, Lee HJ, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13(2):219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Li NX, Karin M. Ionizing radiation and short wavelength UV activate NF-κB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95(22):13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liapaki I, Anagnostoulis S, Karayiannakis A, et al. Burn wound angiogenesis is increased by exogenously administered recombinant leptin in rats. Acta Cir Bras. 2008;23(2):118–124. doi: 10.1590/s0102-86502008000200002. [DOI] [PubMed] [Google Scholar]
- 19.Liu CL, Liu ZH, Wang BW, et al. Study on multi-directional differentiation potentiality of human placenat derived mesenchymal–like stem cells in vitro. Chin J Lab Diagn. 2009;13(8):1022–1024. doi: 10.3969/j.issn.1007-4287.2009.08.010. (in Chinese) [DOI] [Google Scholar]
- 20.Manjunathan R, Ragunathan M. In ovo administration of human recombinant leptin shows dose dependent angiogenic effect on chicken chorioallantoic membrane. Biol Res, 48:29. 2015 doi: 10.1186/s40659-015-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques FG, Poli E, Rino J, et al. Low doses of ionizing radiation enhance the angiogenic potential of adipocyte conditioned medium. Radiat Res. 2019;192(5):517–526. doi: 10.1667/rr15438.1. [DOI] [PubMed] [Google Scholar]
- 22.Mustoe TA, Purdy J, Gramates P, et al. Reversal of impaired wound healing in irradiated rats by platelet-derived growth factor-BB. Am J Surg. 1989;158(4):345–350. doi: 10.1016/0002-9610(89)90131-1. [DOI] [PubMed] [Google Scholar]
- 23.Nwadozi E, Ng A, Strömberg A, et al. Leptin is a physiological regulator of skeletal muscle angiogenesis and is locally produced by PDGFRα and PDGFRβ expressing perivascular cells. Angiogenesis. 2019;22(1):103–115. doi: 10.1007/s10456-018-9641-6. [DOI] [PubMed] [Google Scholar]
- 24.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64(1):24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HY, Kwon HM, Lim HJ, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro . Exp Mol Med. 2001;33(2):95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, et al. Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 2017;35:71–84. doi: 10.1016/j.cytogfr.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Powers WE, Ogura JH, Palmer LA. Radiation therapy and wound healing delay: animals and man. Radiology. 1967;89(1):112–115. doi: 10.1148/89.1.112. [DOI] [PubMed] [Google Scholar]
- 28.Sabapathy V, Ravi S, Srivastava V, et al. Long-term cultured human term placenta-derived mesenchymal stem cells of maternal origin displays plasticity. Stem Cells Int, 2012:174328. 2012 doi: 10.1155/2012/174328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shentu WH, Yan CH, Liu CM, et al. Use of cationic microbubbles targeted to P-selectin to improve ultrasound-mediated gene transfection of hVEGF165 to the ischemic myocardium. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(9):699–707. doi: 10.1631/jzus.B1700298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, Wang Z, Tian JW, et al. Leptin-induced inflammation by activating IL-6 expression contributes to the fibrosis and hypertrophy of ligamentum flavum in lumbar spinal canal stenosis. Biosci Rep. 2018;38(2):BSR20171214. doi: 10.1042/bsr20171214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tadokoro S, Ide S, Tokuyama R, et al. Leptin promotes wound healing in the skin. PLoS ONE. 2015;10(3):e0121242. doi: 10.1371/journal.pone.0121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umeki H, Tokuyama R, Ide S, et al. Leptin promotes wound healing in the oral mucosa. PLoS ONE. 2014;9(7):e101984. doi: 10.1371/journal.pone.0101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Brenk HAS, Orton C, Stone M, et al. Effects of X-radiation on growth and function of the repair blastema (granulation tissue). I. Wound contraction. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;25(1):1–19. doi: 10.1080/09553007414550011. [DOI] [PubMed] [Google Scholar]
- 35.van den Brenk HAS, Sharpington C, Orton C, et al. Effects of X-radiation on growth and function of the repair blastema (granulation tissue). II. Measurements of angiogenesis in the Selye pouch in the rat. Int J Radiat Biol Relat Stud Phys Chem Med. 1974;25(3):277–289. doi: 10.1080/09553007414550331. [DOI] [PubMed] [Google Scholar]
- 36.van Hove AH, Benoit DSW. Depot-based delivery systems for pro-angiogenic peptides: a review. Front Bioeng Biotechnol, 3:102. 2015 doi: 10.3389/fbioe.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincenti S, Brillante N, Lanza V, et al. HUVEC respond to radiation by inducing the expression of pro-angiogenic microRNAs. Radiat Res. 2011;175(5):535–546. doi: 10.1667/rr2200.1. [DOI] [PubMed] [Google Scholar]
- 38.Walker JG, Smith MD. The JAK-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32(9):1650–1653. [PubMed] [Google Scholar]
- 39.Yu YY, Yang J, Fu SS, et al. Leptin promotes monosodium urate crystal-induced inflammation in human and murine models of gout. J Immunol. 2019;202(9):2728–2736. doi: 10.4049/jimmunol.1801097. [DOI] [PubMed] [Google Scholar]