Abstract
Objective: Drug-resistance and metastasis are major reasons for the high mortality of ovarian cancer (OC) patients. Cyclooxygenase-2 (COX-2) plays a critical role in OC development. This study was designed to evaluate the effects of COX-2 on migration and cisplatin (cis-dichloro diammine platinum, CDDP) resistance of OC cells and explore its related mechanisms. Methods: Cell counting kit-8 (CCK-8) assay was used to detect the cytotoxicity effects of celecoxib (CXB) and CDDP on SKOV3 and ES2 cells. The effect of COX-2 on migration was evaluated via the healing test. Western blot and real-time quantitative polymerase chain reaction (qPCR) were used to analyze E-cadherin, vimentin, Snail, and Slug levels. Results: COX-2 promoted drug-resistance and cell migration. CXB inhibited these effects. The combination of CDDP and CXB increased tumor cell sensitivity, reduced the amount of CDDP required, and shortened treatment administration time. COX-2 upregulation increased the expression of Snail and Slug, resulting in E-cadherin expression downregulation and vimentin upregulation. Conclusions: COX-2 promotes cancer cell migration and CDDP resistance and may serve as a potential target for curing OC.
Keywords: Ovarian cancer (OC), Cyclooxygenase-2 (COX-2), Drug resistance, Migration, Epithelial-mesenchymal transition (EMT)
1. Introduction
Ovarian cancer (OC) mortality ranks first in gynecological malignancies. Cisplatin (cis-dichloro diammine platinum, CDDP) promotes DNA chain and interchain crosslinks, interferes with DNA replication, and promotes cell apoptosis. CDDP has been used as a chemotherapy drug since the end of the 1970s, and platinum has become the most important drug for the treatment of OC. Cytoreductive surgery combined with platinum-based combination chemotherapy has been the main treatment for an OC tumor, as it is difficult to completely remove the cancer with surgery. Heterologous antineoplastic agents, such as the combination chemotherapy consisting of paclitaxel and CDDP, have achieved a certain curative effect. The five-year survival rate of patients with OC is still 20% to 30%, while 70% of patients eventually relapse (Buys et al., 2011). The main cause of treatment failure is platinum resistance, which has become an increasingly important clinical issue affecting OC patient prognosis. OC drug resistance and metastasis are key issues affecting OC patient prognosis and are also hot issues in gynecological oncology research (JNCI, 2011). Although drug resistance mechanisms remain unclear, tumor microenvironmental change and abnormal tumor metabolism are thought to be chemoresistance-related factors (Koti et al., 2015; Li et al., 2018).
Recent studies have found that first-line platinum-and taxane-based chemotherapies may induce a protumorigenic microenvironment by triggering the release of proinflammatory mediators (Gartung et al., 2019) and that celecoxib (CXB), an anti-inflammatory drug, could inhibit the growth and proliferation of malignant tumors, promote tumor cells apoptosis, and enhance the sensitivity of tumor cells to anticancer drugs (Subbaramaiah et al., 2000; Kısmet et al., 2004).
Cyclooxygenase-2 (COX-2) expression is very low in normal tissues and is generally difficult to detect. In addition to participating in inflammatory reactions, COX-2 is also involved in the formation of tumors and promotes cancer development. High COX-2 expression can be detected in various solid tumors, such as gastric cancer, lung cancer, and breast cancer (Ferrandina et al., 2002). Studies have found almost no COX-2 expression in the normal ovarian epithelium, but high COX-2 expression can be detected in OC cells (Landen et al., 2003; Shigemasa et al., 2003; Li et al., 2004). Furthermore, COX-2 expression level is closely related to OC stage (Denkert et al., 2003, 2004) and prognosis (Ferrandina et al., 2004; Jemal et al., 2010; Chen et al., 2011, 2019). Li et al. (2004) found that high COX-2 expression was more likely in metastasis and new OC foci. In addition, COX-2 overexpression may cause resistance to OC chemotherapy (Harris et al., 2014). Thus, COX-2 may play an important role in the development and progression of OC.
However, research on the effect of COX-2 in OC resistance and the synergy of CXB and CDDP in preventing cancer and reducing drug resistance is poorly expounded. In this study, we evaluated the effects of COX-2 on the anticancer effect of CDDP and CXB in OC cells in order to provide some evidence for tailored clinical trials in the future.
2. Materials and methods
2.1. Experimental reagents
Fetal bovine serum (FBS) and McCoy’s 5A medium were purchased from Gibco (Grand Island, NY, USA); trypsin (1:250) was purchased from Ameresco (Solon, OH, USA); antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH), E-cadherin, and Snail were purchased from Cell Signaling Technology (Danvers, MA, USA); antibodies against COX-2 were purchased from Bioss technology (Woburn, MA, USA); antibodies against Slug and Vimentin were purchased from Abcam (Cambridge, UK). Enhanced chemiluminescence (ECL) detection reagent was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The bicinchoninic acid (BCA) protein assay kit was purchased from Pierce (Rockford, IL, USA). SYBR® Premix Ex Taq™ was purchased from Promega (Madison, WI, USA) and the cell counting kit-8 (CCK-8) was purchased from Dojindo (Dalian, China).
2.2. Experimental methods
2.2.1 Cell culture and establishment of stable COX-2 overexpressing cells
The SKOV3 and ES2 cell lines (purchased from Shanghai Institute of Chinese Academy of Sciences, Shanghai, China) were cultured with McCoy’s 5A medium or Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were carried out in the medium and cultured in a constant temperature, closed incubator at 37 °C with 5% CO2 and a relative humidity of 95%. When cell confluence reached 90%, the cells were passaged and digested once every two to three days with 0.25% trypsin. The lentiviral vector, Lenti-COX-2-enhanced green fluorescent protein (EGFP), was constructed, the SKOV3 and ES2 cell lines were transfected, and an empty vector group was set up to eliminate the influence of the vector on the experiment.
2.2.2 qPCR detection of mRNA expression levels of EMT-related genes
RNA was extracted from OC cells in the logarithmic growth phase using guanidinium isothiocyanate-phenol-chloroform and then was reverse-transcribed into complementary DNA (cDNA), which was stably stored. The relative mRNA expression levels of epithelial-mesenchymal transition (EMT)-related genes were detected by real-time quantitative polymerase chain reaction (qPCR). The forward and reverse primer sequences for the EMT-related genes are shown in Table 1.
Table 1.
Primers used for real-time quantitative polymerase chain reaction (qPCR)
| Gene | Forward primer sequence (5'→3') | Reverse primer sequence (5'–3') |
| GAPDH | GGACCTGACCTGCCGTCTAG | TAGCCCAGGATGCCCTTGAG |
| E-cadherin | ACAATGCCGCCATCGCTTAC | AACTCTCTCGGTCCAGCCCA |
| Snail | TTCTCCTCTACTTCAGTCTCTTCC | GAGGTATTCCTTGTTGCAGTATTT |
| Slug | GCCCCATTAGTGATGAAGAGGAAA | AGCCCAGAAAAAGTTGAATAGGTC |
| Vimentin | AAGCAGGAGTCCACTGAGTA | GCTTCAACGGCAAAGTTCTC |
| COX-2 | TCAAGTCCCTGAGCATCTACGGTT | CTGTTGTGTTCCCGCAGCCAGATT |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; COX-2: cyclooxygenase-2
2.2.3 Western blot analysis
The cells were collected, washed three times with phosphate-buffered saline (PBS), lysed on ice in radio immunoprecipitation assay (RIPA) buffer for 30 min, then centrifuged at 4 °C, 12 000 r/min for 30 min, and the supernatant was collected. The protein concentration was quantified by the BCA method, and then proteins were denatured at 100 °C for 10 min and subjected to 10% (0.1 g/mL) sodium dodecyl sulfate-polyarylamide gel electrophoresis (SDS-PAGE). The proteins were separated and transferred to polyvinylidene difluoride (PVDF) membranes by semidry rotation for 20 min. The membranes were blocked with 5% skim milk at room temperature for 2 h and incubated with the corresponding primary antibody at 4 °C overnight. The blots were washed with Tris‐buffered saline/Tween 20 (TBS-T; 5 min/time, washed 5 times) and incubated with the corresponding secondary antibody for 2 h at room temperature, followed by washing with TBS-T (5 min/time, washed 5 times). An appropriate amount of ECL detection reagent was added, and Image Quant LAS4000 Biomolecular Imager (GE Healthcare Technology, Boston, USA) was used to display the results.
2.2.4 CCK-8 assay
2.2.4.1 Toxic effects of CDDP on OC cells
ES2 and SKOV3 cells were seeded in 96-well plates at 5×103 cells/well with 100 µL of medium per well, and three replicate wells in each group were cultured for 24 h at 37 °C and 5% CO2. The culture media containing different concentrations of CDDP (0, 4, 8, 12, and 16 μg/mL) was applied for 24, 48, 72, and 96 h. CCK-8 solution were added to each well at a concentration of 10 μL/100 µL of medium, and then incubated for 4 h. The absorbance (A) of each well was read by a plate reader according to an enzyme-linked immunosorbent assay (ELISA) (λ experiment=450 nm, λ reference=600 nm). The experiment was repeated three times, and the inhibitory rate (IR) was calculated according to the following formula:
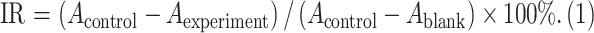 , ,
|
(1) |
The drug treatment time was plotted on the horizontal axis, and the cell inhibition rate was plotted on the vertical axis. Linear regression equations were used to calculate the 50% and 20% maximal inhibitory concentrations (IC50 and IC20) for different drugs.
2.2.4.2 Toxic effects of CXB on OC cells
ES2 and SKOV3 cells were seeded in 96-well plates at 5×103 cells/well with 100 μL of medium per well, and three replicate wells in each group were cultured for 24 h at 37 °C and 5% CO2. The culture media containing different concentrations of CXB (0, 10, 40, 70, and 100 μmol/L) were applied for 24, 48, 72, and 96 h. CCK-8 solution was added to each well at a concentration of 10 μL/100 µL of medium, and then incubated for 4 h. The A value of each well was read by a plate reader according to ELISA (λ experiment=450 nm, λ reference=600 nm). The experiment was repeated three times, and the IR was calculated according to Eq. (1). The drug treatment time was plotted on the horizontal axis, and the cell inhibition rate was plotted on the vertical axis. Linear regression equations were used to calculate the IC50 and IC20 for different drugs.
2.2.4.3 Toxic effects of CXB combined with CDDP on OC cells
ES2 and SKOV3 cells were seeded in 96-well plates at 5×103 cells/well with 100 µL of medium per well, and three replicate wells in each group were cultured for 24 h at 37 °C and 5% CO2. The media containing different concentrations of CDDP (0, 4, 8, 12, and 16 μg/mL) and CXB (40 μmol/L) were applied for 24, 48, 72, and 96 h. CCK-8 solution was added to each well at a concentration of 10 μL/100 μL of medium, and then incubated for 4 h. The A value of each well was read by a plate reader according to ELISA (λ experiment=450 nm, λ reference=600 nm). The experiment was repeated three times, and the IR was calculated according to Eq. (1). The drug treatment time was plotted on the horizontal axis, and the cell inhibition rate was plotted on the vertical axis. A linear regression equation was used to calculate the IC50 of different drug combinations.
2.2.5 Scratch test to observe the effects of COX-2 and CXB on cell mobility
The cells were collected and divided into three groups: Lenti-EGFP, Lenti-COX-2-EGFP, and Lenti-EGFP+CXB (wherein the final CXB concentration was 40 μmol/L). Cells (5×104 cells/well) were seeded in 24-well plates, cultured until the cells were confluent, and a scratch was made on a single layer of cells with a sterile 20 μL pipette tip. The detached cells were washed with PBS and serum-free medium was added to each well. After 0, 12, 24, and 36 h of culture, cell growth was observed and photographed, and the cell migration distance was analyzed using ImageJ software (National Institutes of Health (NIH), Bethesda, USA). Cell mobility was calculated as follows:
healing degree=(SW 0−SWn)/SW 0×100%, (2)
where SW 0 means scratch width at 0 h and SWn is scratch width at different culture time.
2.3. Statistical analysis
Using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA), the measurement data were compared using a t-test and analysis of variance (ANOVA). Experimental mapping was performed using ImageJ and GraphPad Prism 6.0 software (GraphPad Software for Science, San Diego, CA, USA). Differences were considered statistically significant when Ρ value is <0.05.
3. Results
3.1. Toxic effects of CXB combined with CDDP on OC cells
3.1.1 Toxic effects of CXB on OC cells
The inhibitory effects of CXB on ES2 and SKOV3 cell proliferation were time-and dose-dependent. The IRs of ES2 cells after 48 h at 10, 40, 70, and 100 μmol/L CXB were 5.06%, 16.56%, 30.14%, and 39.05%, respectively (Fig. 1a). The IRs of SKOV3 cells after 48 h at 10, 40, 70, and 100 μmol/L CXB were 8.81%, 34.44%, 48.82%, and 57.92%, respectively (Fig. 1b). With the increase in drug concentration, the IR increased accordingly. With prolonged incubation time, the IR of cells gradually increased under an increasing concentration of CXB. The IRs of ES2 cells after 96 h of incubation with CXB at concentrations of 10, 40, 70, and 100 μmol/L were 18.48%, 39.16%, 56.15%, and 87.04%, respectively (Fig. 1a). The SKOV3 cell IRs after 96 h of incubation reached 47.27%, 59.87%, 71.69%, and 80.29%, respectively (Fig. 1b). According to the linear regression equation, the IC50 of ES2 cells after 48 h of CXB was 121.25 μmol/L, and the IC20 was 46.25 μmol/L; the IC50 of SKOV3 cells after 48 h of CXB treatment was 84.40 μmol/L, and the IC20 was 24.40 μmol/L. The concentration of 40 μmol/L was chosen as the noncytotoxic drug concentration for downstream experiments.
Fig. 1.
Effects of COX-2 on the drug resistance of ovarian cancer cells
(a) Cell inhibition of ES2 cells treated with CXB; (b) Cell inhibition of SKOV3 cells treated with CXB; (c) Cell inhibition of ES2 cells treated with CDDP; (d) Cell inhibition of SKOV3 cells treated with CDDP; (e) Cell inhibition of COX-2-overexpressed ES2 cells treated with CDDP; (f) Cell inhibition of COX-2-overexpressed SKOV3 cells treated with CDDP. Data are expressed as mean±standard deviation (SD), n=3. COX-2: cyclooxygenase-2; CXB: celecoxib; CDDP: cisplatin (cis-dichloro diammine platinum)
3.1.2 Toxic effects of CDDP on OC cells
The inhibitory effect of CDDP on the proliferation of ES2 and SKOV3 cells was time-and dose-dependent. The IRs of ES2 cells after 48 h at 4, 8, 12, and 16 μg/mL CDDP were 25.49%, 36.72%, 54.53%, and 81.91%, respectively (Fig. 1c), and the IRs of SKOV3 cells were 25.10%, 36.66%, 41.91%, and 60.04%, respectively (Fig. 1d). With the increased drug concentration, the IR increased correspondingly. With the prolonged incubation time, the inhibition rate gradually increased. The IRs of ES2 and SKOV3 cells from all groups exceeded 50% after 96 h. According to the linear regression equation, the IC50 values of ES2 and SKOV3 cells after 48 h of CDDP treatment were 10.02 and 13.07 μg/mL, respectively.
3.1.3 Effect of COX-2 on OC cell drug resistance
CDDP still inhibited COX-2-overexpressed ES2 and SKOV3 cells in a time-and dose-dependent manner, but the IR of CDDP-treated cells at each time point was higher than that in non-transfected cells. The IRs of ES2 cells transfected with COX-2 at 48 h after treatment with CDDP at concentrations of 4, 8, 12 and 16 μg/mL were 12.78%, 23.08%, 41.62%, and 70.31%, respectively (Fig. 1e); while the IRs of SKOV3 cells transfected with COX-2 were 21.59%, 25.62%, 35.42%, and 53.26%, respectively (Fig. 1f). According to the linear regression equation, the IC50 of ES2 cells transfected with COX-2 was 12.67 μg/mL after 48 h of CDDP treatment, and the IC50 of the cells transfected with COX-2 was increased by 26.45% compared with the non-transfected cells. The drug resistance index was IC50 (ES2 COX-2)/IC50 (ES2)=12.67/10.02=1.26, indicating that upregulation of COX-2 expression may promote resistance of ES2 cells to CDDP. The IC50 of SKOV3 cells transfected with COX-2 was 16.23 μg/mL after 48 h of CDDP treatment, which was 24.18% higher than that of non-transfected cells; the drug resistance index was IC50 (SKOV3 COX-2)/IC50 (SKOV3)=16.23/13.07=1.24, indicating that upregulated COX-2 expression also promotes SKOV3 cell resistance to CDDP.
3.1.4 Sensitization of CXB to CDDP
The combination of the COX-2 inhibitors CXB and CDDP increased the cell-killing effect of CDDP and reversed drug resistance. This inhibitions in a time- and dose-dependent manner. After the addition of CXB, the IRs of CDDP at various concentrations on OC cells were significantly increased. The IRs of ES2 cells after 48 h with CDDP concentrations of 4, 8, 12 and 16 μg/mL were 47.55%, 52.82%, 79.45%, and 90.29%, respectively (Fig. 2a), while the IRs of SKOV3 cells were 44.27%, 53.57%, 64.89%, and 88.03%, respectively (Fig. 2c). A similar effect was obtained using CXB in combination with CDDP in OC cells transfected with COX-2. After the addition of CXB, the IR of CDDP on OC cells significantly increased. ES2 and SKOV3 cells transfected with COX-2 were treated with CDDP at concentrations of 4, 8, 12 and 16 μg/mL. The IRs of ES2 cells transfected with COX-2 after 48 h were 37.45%, 50.32%, 75.05%, and 87.20%, respectively (Fig. 2b), while the IRs of SKOV3 cells transfected with COX-2 after treatment under the same conditions were 30.77%, 40.31%, 44.59%, and 66.48%, respectively (Fig. 2d). The IR of each treatment group increased with increasing drug concentration, and the difference was statistically significant (Ρ<0.05). After COX-2 transfection and incubation at the same drug concentration, the IRs of SKOV3 and ES2 cells decreased, and the differences were statistically significant (Ρ<0.05) compared with the control group IRs. Compared to CDDP alone, the IRs of the two OC cell lines combined with CDDP and CXB were the highest, and the difference was statistically significant (Ρ<0.05), indicating that the addition of CDDP to CXB can have synergistic anticancer effects (Figs. 2e and 2f).
Fig. 2.
Sensitization of CXB to CDDP
(a) Cell inhibition of ES2 cells treated with a combination of CDDP and CXB; (b) Cell inhibition of COX-2-overexpressed ES2 cells by combined CDDP and CXB treatment; (c) Cell inhibition of SKOV3 cells treated with a combination of CDDP and CXB; (d) Cell inhibition of COX-2-overexpressed SKOV3 cells by combined CDDP and CXB treatment; (e) Cell inhibition of ES2 cells in different drug treatment groups; (f) Cell inhibition of SKOV3 cells in different drug treatment groups. Data are expressed as mean±SD, n=3. * Ρ<0.05, compared to the control group. CXB: celecoxib; CDDP: cisplatin (cis-dichloro diammine platinum)
According to the linear regression equation, the IC50 of ES2 cells after 48 h incubation with a combination of CDDP and CXB was 5.43 μg/mL, which reversed the resistance trend. The reversal multiple was IC50 (ES2)/IC50 (ES2 (CDDP+CXB))=10.02/5.43=1.84, indicating partially reversed resistance. The IC50 of COX-2-expressed ES2 cells after CDDP and CXB combined incubation for 48 h was 7.05 μg/mL, which indicated reversal of resistance trend compared to ES2 cells transfected with COX-2. The reversal multiple was IC50 (ES2 COX-2)/IC50 (ES2 COX-2 (CDDP+CXB))=12.67/7.05=1.80, indicating partially reversed resistance. The IC50 of SKOV3 cells after 48 h incubation with a combination of CDDP and CXB was 6.39 μg/mL, which indicated reversal of the resistance trend. The reversal multiple was IC50 (SKOV3)/IC50 (SKOV3 (CDDP+CXB))=13.07/6.39=2.05, indicating completely reversed resistance. The IC50 of SKOV3 cells transfected with COX-2 after combined CDDP and CXB incubation for 48 h was 11.54 μg/mL, which also indicated drug resistance reversal compared to SKOV3 cells transfected with COX-2. The reversal multiple was IC50 (SKOV3 COX-2)/IC50 (SKOV3 COX-2 (CDDP+CXB))=16.23/11.54=1.41, indicating partially reversed resistance. The IC50 of SKOV3 and ES2 cells after combined CDDP and CXB incubation significantly decreased compared to single drug groups regardless of the transfection of COX-2. Our findings indicate that the combined use of CXB and CDDP can inhibit CDDP resistance and even partially reverse the drug resistance. This can shorten the administration time and reduce the dosage.
3.2. Promotion of OC cell migration by COX-2
The results of the scratch test showed that compared to the migration rate of control cells, the migration rate of ES2 cells overexpressing COX-2 was significantly increased (Ρ<0.05), and the healing degree was greater than 80% after 24 h in ES2 cells overexpressing COX-2. After treatment with CXB, the migration rate significantly decreased and the difference was statistically significant (Ρ<0.05; Figs. 3a and 3b). The migration rate of SKOV3 cells overexpressing COX-2 was more than 60% after 24 h. This was significantly greater than that of the control cells (Ρ<0.05). The migration rate of SKOV3 cells decreased slightly with CXB, but the difference was not statistically significant (Ρ>0.05; Figs. 3a and 3c).
Fig. 3.
Effects of COX-2 on the migration of ovarian cancer cells
(a) Ovarian cell migration after 24 h (scale bar=200 µm); (b) Healing rates of ES2 cells; (c) Healing rates of SKOV3 cells. Data are expressed as mean±SD, n=3. * Ρ<0.05, compared to the control group. COX-2: cyclooxygenase-2; EGFP: enhanced green fluorescent protein; CXB: celecoxib
3.3. qPCR and western blot analysis
mRNA levels of EMT-related genes were analyzed by qPCR (Figs. 4a–4d). E-cadherin expression was downregulated in SKOV3 and ES2 cells transfected with Lenti-COX-2-EGFP compared to the control group (Ρ<0.05). On the contrary, Vimentin, Snail, and Slug expression levels were upregulated in SKOV3 and ES2 cells transfected with Lenti-COX-2-EGFP compared to the control group (Ρ<0.05). The results indicated that increased COX-2 expression could stimulate SKOV3 and ES2 cells to EMT.
Fig. 4.
EMT-related gene expression in SKOV3 and ES2 cells
(a) E-cadherin expression in SKOV3 and ES2 cells; (b) Slug expression in SKOV3 and ES2 cells; (c) Snail expression in SKOV3 and ES2 cells; (d) Vimentin expression in SKOV3 and ES2 cells; (e) EMT-related protein expression in SKOV3 and ES2 cells. Data are expressed as mean±SD, n=3. * Ρ<0.05, compared to the control group. EMT: epithelial-mesenchymal transition; EGFP: enhanced green fluorescent protein; COX-2: cyclooxygenase-2; GAPDH: glyceraldehyde-3-phosphate dehydrogenase
The effect of COX-2 on EMT-related protein expression was assessed by western blot analysis (Fig. 4e). E-cadherin protein expression was slightly downregulated while protein expression levels of Snail and Slug were upregulated in SKOV3 and ES2 cells transfected with Lenti-COX-2-EGFP compared to the controls (Ρ<0.05). Vimentin protein expression in ES2 cells was slightly upregulated but did not have a significant increase in SKOV3 cells compared to the controls. These results demonstrated that COX-2 could regulate Snail and Slug expression, resulting in OC cell EMT and subsequently migration.
4. Discussion
OC mortality ranks first in gynecologic malignancies. The application of multidrug combinations remains the regular mode of treatment. The use of platinum combined with paclitaxel is the preferred OC treatment option. Metastasis and drug resistance are the key issues and are the main factors affecting patient prognosis. Previous studies have shown that the upregulation of COX-2 expression in OC cells is an important factor in the development of OC (Ferrandina et al., 2004), and that COX-2 plays a crucial role in tumor invasion and metastasis (Soslow et al., 2000; Sangoi et al., 2008). Patients with high expression of COX-2 are insensitive to chemotherapy response and have short postoperative recurrence (Raspollini et al., 2005). Barnes et al. (2007) used in vitro experiments with OC cell lines to show the effectiveness of COX-2 inhibitors and found that COX-2 dose-dependently developed platinum’s cytotoxic effect of on OC cells. The present study obtained similar results. The expression level of COX-2 had a significant effect on the cytotoxicity of CDDP. In the COX-2-overexpressed group, the inhibitory effect of CDDP on OC cells at each time point was significantly lower than that in the control group. The IC50 of ES2 cells increased 26.45% compared to the control group and the drug resistance index was 1.26. The IC50 of SKOV3 cells was also higher than that of the control group. The IC50 of the COX-2-overexpressed group increased 24.18%, and the drug resistance index was 1.24. These results indicated that COX-2 could reduce the sensitivity of OC cells to CDDP and promote CDDP resistance. This is similar to the results from studies on other epithelial cancers such as head and neck squamous cell carcinoma, esophageal cancer, and lung cancer. This suggests that COX-2 has mutual CDDP resistance-promoting effects (Chung et al., 2012; Okamura et al., 2013; Neumann et al., 2015). Therefore, COX-2 is speculated to be a molecular marker for predicting chemotherapy resistance in OC.
CXB, a selective COX-2 inhibitor, is widely used clinically as an anti-inflammatory treatment. In recent years, studies have found that CXB has synergistic anticancer effects when combined with chemotherapy drugs (al-Wadei et al., 2012; Cervello et al., 2013). Kim et al. (2014) found that the combination of CXB and paclitaxel significantly increased the toxicity of paclitaxel to OC cells, inhibited cell growth, and promoted apoptosis. Legge et al. (2011) combined CXB (400 mg/d) and carboplatin in patients with recurrent OC and found that CXB had a synergistic anticancer effect with carboplatin. The current study reached similar conclusions. In this study, we combined CXB with CDDP and found a significantly higher cell IR for each concentration of CDDP at each time point compared to the control group and the COX-2-overexpressed group. This effect increased over time. Interestingly, if combined with CXB, a lower concentration of CDDP achieved the same effect as a high concentration of CDDP alone at the same time point. The IR of ES2 and SKOV3 cell combination treatment group was significantly increased, showing a reversed drug resistance trend compared to the CDDP alone group. It has been reported that a high dose of COX-2 inhibitors (400–800 mg/d) can cause serious adverse cardiovascular reactions during chemoprevention and treatment, and small doses of <400 mg/d have greater safety. If long-term CXB use is necessary for tumor chemoprevention, the dose should be under 200 mg (Madan et al., 2007; Bertagnolli et al., 2009). According to the conversion formula of in vitro and in vivo drug concentrations, the dose calculated in vitro should be between 115 and 190 μmol/L (Konstan et al., 1995). Based on these studies, we used 100 μmol/L as the maximum dose of CXB in in vitro studies. A small dose of 40 μmol/L CXB was used in combination with CDDP to reduce its side effects as well. Even a small dose of CXB, if combined with CDDP, could also reverse drug resistance.
Migration is a key factor in the metastasis of OC cells. In this experiment, the migration ability of both OC cell lines was significantly increased after COX-2 overexpression. However, the migration rate of OC cells was significantly inhibited after 24 h of treatment with CXB compared to the control. The current research results show that CXB has a significant effect in inhibiting OC cell migration and reversing CDDP resistance (Hu et al., 2013; Giaginis et al., 2015). EMT refers to the transformation of epithelial cells into mesenchymal cells under specific physiological and pathological conditions. This process is closely related to embryonic development, tumor invasion, metastasis, drug resistance, and other malignant behaviors (Denkert et al., 2004; Li et al., 2004; al-Wadei et al., 2012; Cervello et al., 2013). EMT transformation of epithelial cells mainly showed upregulated Snail, Slug, and Vimentin expression and downregulated E-cadherin expression. E-cadherin expression is closely related to histological classification, differentiation, and peritoneal metastasis of OC (Cho et al., 2006; Zhai et al., 2014). Lower E-cadherin is directly related to a higher degree of tumor malignant, easier cancer transference, and worsened prognosis (Cho et al., 2006; Zhang et al., 2015; Yim et al., 2017). Vimentin is a marker of mesenchymal cells that can promote cell migration and invasion and participate in the tumor metastasis process. Its expression level is relevant to the degree of tissue differentiation, distant metastasis, and poor prognosis (Chen et al., 2008; Wei et al., 2008; Zhao et al., 2008). Vimentin can be used as a predictor of tumor invasion and metastasis. This study found that COX-2 can regulate the expression levels of Slug and Snail and ultimately promote the EMT of OC cells, suggesting that OC drug resistance may be related to the EMT induced by COX-2. This study provides laboratory evidence for the safe and rational use of chemotherapeutic drugs to reduce complications and dosage, and prevent drug resistance and OC recurrence. Combined with the scratch test results, the migration rate of OC cells with a high COX-2 expression level increased significantly. This suggests that COX-2 may promote OC migration by promoting OC cell EMT. CXB may reverse EMT transformation, thus inhibiting the migration and metastasis of OC cells. Our results further confirmed that COX-2 is a crucial factor affecting the migration and metastasis of OC and that CXB is a potential anticancer drug.
5. Conclusions
COX-2 plays a crucial role in the resistance of OC cells to CDDP by inducing cells EMT. OC cells with high COX-2 expression have a low response to CDDP. CXB plays a similar role in inhibiting cell migration as does CDDP. The combination of CXB and CDDP in OC treatment inhibits cell migration, reverse drug resistance, and offer synergistic anticancer effects, providing a new research direction for OC treatment.
Acknowledgments
The authors would like to thank Prof. Zhong-jun DONG and Dr. Juan DU (Tsinghua University, Beijing, China) for technical support.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81372777)
Contributors: Lin DENG and Bin LING designed experiments. Lin DENG carried out experiments, analyzed experimental results, and wrote the manuscript. Bin LING and Ding-qing FENG revised the manuscript. All authors have read and approved the final manuscript, have full access to all the data in the study, and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Lin DENG, Ding-qing FENG, and Bin LING declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.JNCI (Journal of the National Cancer Institute) Ovarian cancer, five-year stage-specific relative survival rates (2004–2008) J Natl Cancer Inst. 2011;103(17):1287. doi: 10.1093/jnci/djr342. [DOI] [PubMed] [Google Scholar]
- 2.al-Wadei HAN, al-Wadei MH, Ullah MF, et al. Celecoxib and GABA cooperatively prevent the progression of pancreatic cancer in vitro and in xenograft models of stress-free and stress-exposed mice. PLoS ONE. 2012;7(8):e43376. doi: 10.1371/journal.pone.0043376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes AP, Miller BE, Kucera GL. Cyclooxygenase inhibition and hyperthermia for the potentiation of the cytotoxic response in ovarian cancer cells. Gynecol Oncol. 2007;104(2):443–450. doi: 10.1016/j.ygyno.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the adenoma prevention with celecoxib trial. Cancer Prev Res. 2009;2(4):310–321. doi: 10.1158/1940-6207.Capr-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305(22):2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 6.Cervello M, Bachvarov D, Lampiasi N, et al. Novel combination of sorafenib and celecoxib provides synergistic anti-proliferative and pro-apoptotic effects in human liver cancer cells. PLoS ONE. 2013;8(6):e65569. doi: 10.1371/journal.pone.0065569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen FL, Wen X, Lin PF, et al. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol Lett. 2019;301:1–10. doi: 10.1016/j.toxlet.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Chen MHS, Yip GWC, Tse GMK, et al. Expression of basal keratins and vimentin in breast cancers of young women correlates with adverse pathologic parameters. Mod Pathol. 2008;21(10):1183–1191. doi: 10.1038/modpathol.2008.90. [DOI] [PubMed] [Google Scholar]
- 9.Chen XL, Lingala S, Khoobyari S, et al. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55(4):838–845. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho EY, Choi Y, Chae SW, et al. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006;56(2):62–70. doi: 10.1111/j.1440-1827.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 11.Chung LY, Tang SJ, Sun GH, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18(15):4037–4047. doi: 10.1158/1078-0432.Ccr-11-3348. [DOI] [PubMed] [Google Scholar]
- 12.Denkert C, Fürstenberg A, Daniel PT, et al. Induction of G0/G1 cell cycle arrest in ovarian carcinoma cells by the anti-inflammatory drug NS-398, but not by COX-2-specific RNA interference. Oncogene. 2003;22(54):8653–8661. doi: 10.1038/sj.onc.1206920. [DOI] [PubMed] [Google Scholar]
- 13.Denkert C, Weichert W, Pest S, et al. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res. 2004;64(1):189–195. doi: 10.1158/0008-5472.CAN-03-1987. [DOI] [PubMed] [Google Scholar]
- 14.Ferrandina G, Lauriola L, Distefano MG, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002;20(4):973–981. doi: 10.1200/jco.2002.20.4.973. [DOI] [PubMed] [Google Scholar]
- 15.Ferrandina G, Zannoni GF, Ranelletti FO, et al. Cyclooxygenase-2 expression in borderline ovarian tumors. Gynecol Oncol. 2004;95(1):46–51. doi: 10.1016/j.ygyno.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Gartung A, Yang J, Sukhatme VP, et al. Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci USA. 2019;116(5):1698–1703. doi: 10.1073/pnas.1803999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giaginis C, Alexandrou P, Tsoukalas N, et al. Hu-antigen receptor (HuR) and cyclooxygenase-2 (COX-2) expression in human non-small-cell lung carcinoma: associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. Tumour Biol. 2015;36(1):315–327. doi: 10.1007/s13277-014-2637-y. [DOI] [PubMed] [Google Scholar]
- 18.Harris RE, Casto BC, Harris ZM. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol. 2014;5(4):677–692. doi: 10.5306/wjco.v5.i4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z, Liu XJ, Tang ZF, et al. Possible regulatory role of Snail in NF-κB-mediated changes in E-cadherin in gastric cancer. Oncol Rep. 2013;29(3):993–1000. doi: 10.3892/or.2012.2200. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Siegel R, Xu JQ, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Yim GW, Nam EJ, et al. Synergistic effect of COX-2 inhibitor on paclitaxel-induced apoptosis in the human ovarian cancer cell line OVCAR-3. Cancer Res Treat. 2014;46(1):81–92. doi: 10.4143/crt.2014.46.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kısmet K, Akay MT, Abbasoglu O, et al. Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev. 2004;28(2):127–142. doi: 10.1016/j.cdp.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Konstan MW, Byard PJ, Hoppel CL, et al. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–854. doi: 10.1056/nejm199503303321303. [DOI] [PubMed] [Google Scholar]
- 24.Koti M, Siu A, Clément I, et al. A distinct pre-existing inflammatory tumour microenvironment is associated with chemotherapy resistance in high-grade serous epithelial ovarian cancer. Br J Cancer. 2015;112(7):1215–1222. doi: 10.1038/bjc.2015.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landen CN, Jr, Mathur SP, Richardson MS, et al. Expression of cyclooxygenase-2 in cervical, endometrial, and ovarian malignancies. Am J Obstet Gynecol. 2003;188(5):1174–1176. doi: 10.1067/mob.2003.284. [DOI] [PubMed] [Google Scholar]
- 26.Legge F, Paglia A, D'Asta M, et al. Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients. BMC Cancer, 11:214. 2011 doi: 10.1186/1471-2407-11-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SF, Miner K, Fannin R, et al. Cyclooxygenase-1 and 2 in normal and malignant human ovarian epithelium. Gynecol Oncol. 2004;92(2):622–627. doi: 10.1016/j.ygyno.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 28.Li SS, Ma J, Wong AST. Chemoresistance in ovarian cancer: exploiting cancer stem cell metabolism. J Gynecol Oncol. 2018;29(2):e32. doi: 10.3802/jgo.2018.29.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madan RA, Xia Q, Chang VT, et al. A retrospective analysis of cardiovascular morbidity in metastatic hormone-refractory prostate cancer patients on high doses of the selective COX-2 inhibitor celecoxib. Expert Opin Pharmacother. 2007;8(10):1425–1431. doi: 10.1517/14656566.8.10.1425. [DOI] [PubMed] [Google Scholar]
- 30.Neumann W, Crews BC, Sárosi MB, et al. Conjugation of cisplatin analogues and cyclooxygenase inhibitors to overcome cisplatin resistance. ChemMedChem. 2015;10(1):183–192. doi: 10.1002/cmdc.201402353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura H, Fujiwara H, Umehara S, et al. COX-2 overexpression induced by gene transfer reduces sensitivity of TE13 esophageal carcinoma cells to 5-fluorouracil and cisplatin. Anticancer Res. 2013;33(2):537–542. [PubMed] [Google Scholar]
- 32.Raspollini MR, Amunni G, Villanucci A, et al. Increased cyclooxygenase-2 (COX-2) and p-glycoprotein-170 (MDR1) expression is associated with chemotherapy resistance and poor prognosis. Analysis in ovarian carcinoma patients with low and high survival. Int J Gynecol Cancer. 2005;15(2):255–260. doi: 10.1136/ijgc-00009577-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Sangoi AR, Soslow RA, Teng NN, et al. Ovarian clear cell carcinoma with papillary features: a potential mimic of serous tumor of low malignant potential. Am J Surg Pathol. 2008;32(2):269–274. doi: 10.1097/PAS.0b013e31814fa9b0. [DOI] [PubMed] [Google Scholar]
- 34.Shigemasa K, Tian X, Gu L, et al. Expression of cyclooxygenase-2 and its relationship to p53 accumulation in ovarian adenocarcinomas. Int J Oncol. 2003;22(1):99–105. [PubMed] [Google Scholar]
- 35.Soslow RA, Dannenberg AJ, Rush D, et al. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89(12):2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::aid-cncr17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Subbaramaiah K, Hart JC, Norton L, et al. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000;275(20):14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 37.Wei J, Xu G, Wu M, et al. Overexpression of vimentin contributes to prostate cancer invasion and metastasis via SRC regulation. Anticancer Res. 2008;28(1A):327–334. [PubMed] [Google Scholar]
- 38.Yim GW, Kim HJ, Kim LK, et al. Long non-coding RNA HOXA11 antisense promotes cell proliferation and invasion and predicts patient prognosis in serous ovarian cancer. Cancer Res Treat. 2017;49(3):656–668. doi: 10.4143/crt.2016.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai XL, Zhu HJ, Wang W, et al. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med Oncol. 2014;31(6):970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]
- 40.Zhang RR, Zhang P, Wang H, et al. Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44+CD117+ ovarian cancer stem cells. Stem Cell Res Ther. 2015;6(1):262. doi: 10.1186/s13287-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Yan QM, Long X, et al. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008;26(5):571–577. doi: 10.1002/cbf.1478. [DOI] [PubMed] [Google Scholar]






