Abstract
Fasting blood glucose level is the primary indicator for the diagnosis of diabetes. We aim to conduct a longitudinal study on the association between long-term fine particulate matter (PM2.5) exposure and fasting blood glucose concentrations. We recruited and followed up 1449 participants older than 65 years of age in 2009, 2012, 2014, and 2017 in eight counties in China. Fasting blood glucose was repeatedly measured 3697 times in total among these participants. Data on annual ground-level PM2.5 concentrations with a 0.01° spatial resolution from 2005 to 2016 were used to assess exposures. An increase of 10μg/m3 in 3-year average exposure to PM2.5 was associated with an increase of 0.146 mmol/L (95% confidence interval [CI]: 0.045, 0.248) in fasting blood glucose in all participants. The association was more pronounced among the subgroup with diabetes compared to the subgroup without diabetes (P <0.05). In conclusion, Long-term PM2.5 exposure was associated with an increase in fasting blood glucose levels among elderly people. Elderly individuals with diabetes are particularly vulnerable to high level exposures of PM2.5.
Keywords: fine particulate matter, long-term exposure, fasting blood glucose, elderly
Summary:
Long-term PM2.5 exposure was associated with an increase in fasting blood glucose levels among elderly people. Elderly individuals with diabetes are particularly vulnerable to high level exposures of PM2.5.
Introduction
A total of 383 million people were diagnosed with diabetes in 2016, making it one of the leading causes of years lived with disability in 2016 (GBD-2016-Disease-and-Injury-Incidence-and-Prevalence-Collaborators, 2017). Based on data from the International Diabetes Federation, the global prevalence of diabetes is expected to increase to 592 million by 2035(Guariguata et al., 2014). The elderly population is a vulnerable group that is more susceptible to diabetes and diabetic complications(Mordarska and Godziejewska-Zawada, 2017). According to the sixth population census in China in 2010, there are 176 million people that are over 60 years of age, and that number will continue to grow(Gerland et al., 2014). The prevalence of diabetes in China was 11.6%, and higher in older age groups(Xu et al., 2013). Fasting blood glucose levels are traditionally used to diagnose and manage diabetes(American-Diabetes-Association, 2012; Internal-Clinical-Guidelines-Team, 2015) and is associated with altered risk of other major chronic conditions such as cardiovascular disease and cancer(Coutinho et al., 1999; Liao et al., 2015).
In addition to a number of established causes or risk factors for diabetes such as unhealthy behavior (drinking alcohol, or smoking, etc.), genetic factors, and chronic diseases(Bellou et al., 2018; Kong et al., 2016), ambient air pollution has been also associated with increased diabetes prevalence(Liu et al., 2016; Liu et al., 2019; Yang et al., 2020), and especially particular matter (PM) exposure(Liang et al., 2019; Meo et al., 2015; Park and Wang, 2014; Rao et al., 2015; Yang et al., 2018). Diabetes progresses over time, and even before formal diagnosis of diabetes, impaired fasting blood glucose levels alone may be hazardous to human health(Kong et al., 2016). There are many studies that have showed significant associations between fine particular matter (PM2.5) and fasting blood glucose. However, most of these studies only explored the short-term effects of PM2.5 on fasting blood glucose(Brook et al., 2013; Chen et al., 2016; Li et al., 2018b; Lucht et al., 2018; Ma et al., 2019; Meo et al., 2015; Peng et al., 2016). Studies focusing on long-term exposure of PM2.5 and fasting blood glucose, on the other hand, are still lacking. Most existing long-term studies are cross-sectional studies, which has limited power in verifying causality(Chuang et al., 2011; Liu et al., 2016; Lu et al., 2017; Wolf et al., 2017; Yang et al., 2018; Zhang et al., 2019). Therefore, we conducted this repeated measurement longitudinal study in an elderly cohort to examine the association between long-term exposure to ambient PM2.5 and fasting blood glucose concentrations.
Methods
Study population
We investigated 1449 participants older than 65 years of age in eight Chinese counties (Chengmai, Hainan Province; Sanshui, Guangzhou Province; Yongfu, Guangxi Province; Mayang, Hunan Province; Rudong, Jiangsu Province; Zhongxiang, Hubei Province; Xiayi, Henan Province; Laizhou, Shandong Province) (Figure 1). Some participants (542) were recruited in 2009, and were followed up three times, in 2012, 2014 and 2017. In 2012, new participants (907) were also recruited and followed up two times, in 2014 and 2017. All individuals older than 100 were included; their neighbors younger than 100 were invited to participate in the study. Additional details regarding the participants are described in the previously published articles(Ma et al., 2017; Yin et al., 2012). Written consent forms were provided by each participant, and the ethics committee of Peking University approved this study.
Figure 1.
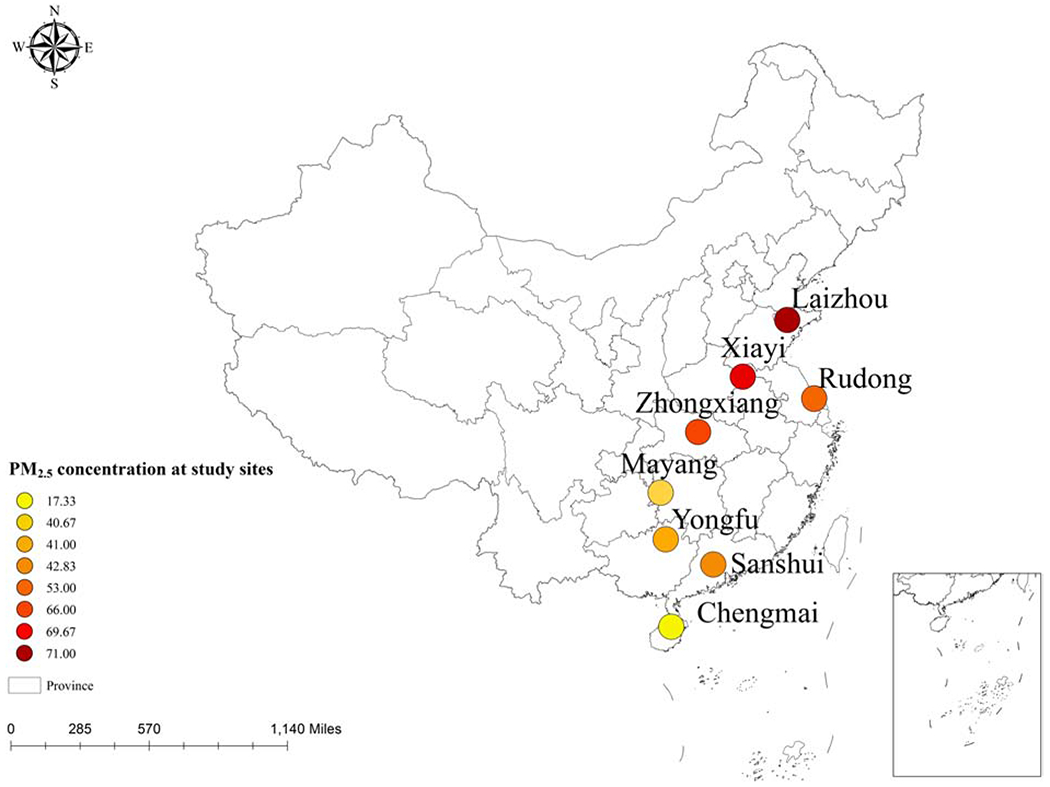
Map of the median of PM2.5 concentration in eight counties
FBG assessment
Participants were asked not to eat for at least 8 hours prior to the morning of blood collection day to accurately determine fasting glucose. Among the 1449 participants, two, three and four serial fasting blood glucose measurements were available for 769, 561 and 119 participants, respectively. We defined diabetes using the data from the baseline survey. Self-reported physician-diagnosed diabetes or fasting glucose levels greater than 7.0 mmol/L were defined as diabetes. In total, we analyzed 3697 fasting blood glucose measurements and questionnaire data acquired over an 8-year timespan.
Assignment of exposure data
Ambient annual PM2.5 data from 2005–2016 were obtained from the Atmospheric Composition Analysis Group, Dalhousie University (Nova Scotia, Canada). Ground-level PM2.5 concentrations with a 0.01° spatial resolution were estimated by applying geographically weighted regression using information from satellite-, simulation- and monitor-based sources. The R2 of the satellite-based estimation and PM2.5 concentrations from monitors is 0.81(van Donkelaar et al., 2016). PM2.5 concentrations were assigned to each participant by home address. Because the cohort was followed up in 3 years averages, PM2.5 concentrations were assigned to the previous year (lag1), and we then calculated the previous year to 2-year (lag1-2) and the previous year to 3-year (lag1-3) moving averages, based on the year of survey.
Meteorological data were obtained from the European Centre for Medium-Range Weather Forecasts (https://cds.climate.copernicus.eu/cdsapp#!/search?type=dataset). Annual temperature and humidity data were matched by the home address of the participant and the investigation year.
Assessment of potential confounders
Potential confounders related to PM2.5 exposure and fasting blood glucose were collected by questionnaire from face to face interviews, including sociodemographic characteristics (such as sex, age, education years, marital status, and home address), smoking and drinking status, exercise habits, and dietary intake. We defined former and current smokers as ever smokers, and similarly defined former and current drinkers as ever drinkers.
Statistical analysis
Linear mixed models in the package “lme4” were used for the analysis between PM2.5 and fasting blood glucose concentrations using the statistical software R, version 3.4.2. First, we conducted a nonlinear analysis. We introduced a basis matrix generated by “dlnm” package for PM2.5 concentration (lag1-3), modeled using a natural spline with 2 degrees of freedom. The model adjusted for age, years of education, body mass index (BMI), family income, staple food intake linearly; sex, marital status, residence, smoking and drinking status as indicator/categorical variables; exercise status as fixed effects; and participant and county as random effects. We found that the shape of the curve was almost linear (Figure 2.). We then remodeled PM2.5 concentration as a linear variable, and adjusted for the same covariates mentioned above as with our non-linear model. We also tested various lag effects of PM2.5 concentrations. The correlations of independent variables are shown in Table S1.
Figure 2.
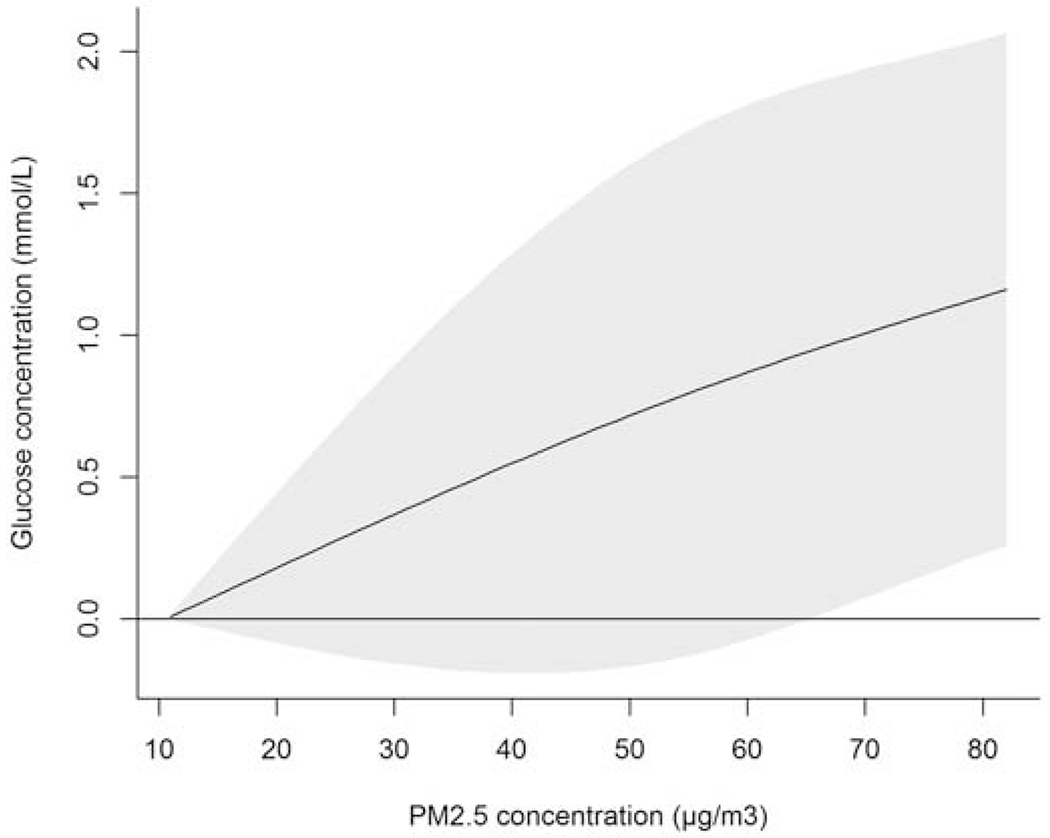
Increase of glucose concentration (mmol/L) associated with increase of previous 3 years average PM2.5 exposure (compared with the minimum concentration of PM2.5 exposure)
We then conducted stratified analyses by sex, age groups, smoking status, and drinking status. We used the following formula(Di et al., 2017) to determine whether the risk estimates of PM2.5 in subgroup a versus subgroup b were statistically different (H0: βa = βb):
As sensitivity analyses, we removed the covariates one-by-one from the main analysis model to determine the stability of our model. Furthermore, we replaced smoking and drinking status (ever vs never) in the main analysis model with current smoking and drinking status. To determine whether fruit intake is a potential confounder, we added the intake of fruit to the main analysis model (Du et al., 2017). In addition, we introduced temperature and humidity into the main analysis model. Finally, we used a linear regression model instead of a linear mixed model to determine the change in the effect estimate without adjusting for the random effects. In the linear regression model, we removed the random effect of participants from the main analysis model, and changed the random effect of the county to a fixed effect, keeping all other covariates identical to that of the main analysis model.
Results
The ages of the participants ranged from 65 to 112 years. Almost half of the participants were women (52.6%), and more than half had not received education in school (53.1%). Most of the participants lived in rural areas (82.9%) (Table 1). The 3-year average PM2.5 concentration ranged from 11 to 82 μg/m3 with a median concentration of 56 μg/m3; the interquartile range of PM2.5 exposure was 27 μg/m3. The PM2.5 exposure of participants in the baseline survey in each county is shown in Figure 1. The participants with the highest exposure resided in Laizhou County, Shandong Province, and the lowest exposure was in Chengmai, Hainan Province.
Table 1.
Characteristics of the study population and PM2.5 concentration
| Variables | Value |
|---|---|
| Total | 1449 |
| Age (mean±SD) | 83±12 |
| Sex | |
| Men (n) | 687 |
| Women (n) | 762 |
| Education in Years (n, median(Q1-Q3)) | 0(0-4) |
| BMI(kg/m2, mean±SD) | 21.4±13.2 |
| County | |
| Chengmai, Hainan Province (n) | 84 |
| Sanshui, Guangdong Province (n) | 123 |
| Yongfu, Guangxi Province (n) | 98 |
| Mayang, Hunan Province (n) | 97 |
| Rudong, Jiangsu Province (n) | 177 |
| Zhongxiang, Hubei Province (n) | 214 |
| Xiayi, Henan Province (n) | 417 |
| Laizhou, Shandong Province (n) | 239 |
| Smoking status | |
| Current smoker (n) | 296 |
| Former smoker (n) | 413 |
| Drinking status | |
| Current drinker(n) | 285 |
| Former drinker (n) | 350 |
| Residence | |
| Rural (n) | 1201 |
| Exercise status | |
| Exercises regularly (n) | 279 |
| Diabetes | |
| Prevalence (%) | 10.1 |
| Fasting glucose (mmol/L, mean±SD) | 5.11±1.82 |
| PM2·5 (μg/L, median (Q1-Q3)) | 56 (43-70) |
Table 2 shows the variation in estimated fasting blood glucose levels with each 10 μg/m3 increase in annual PM2.5 exposure. An increase of 10 μg/m3 in previous 3 years average exposure to PM2.5 was associated with a blood glucose increase of 0.146 mmol/L (95% confidence interval [CI]: 0.045, 0.248) in the main analysis for all participants. For participants with an increase in annual exposure of PM2.5, subgroups analyses showed significant differences among those with and without diabetes, age ≥ 85, without regular exercise, never smoking or never drinking. The subgroup with diabetes had a higher estimated increase in fasting blood glucose levels than the subgroup without diabetes (Z=1.99, P=0.047). Details comparing estimated value between subgroups are shown in Table S3. After conducting relevant sensitivity analyses (changing the covariates and estimation approach), the estimated effects were stable (Table 3), suggesting that our models were robust.
Table 2.
Changes in fasting blood glucose levels (mmol/L) associated with a 10μg/m3 increase of PM2.5; exposure
| Groups | Lag1a | Lag1-2a | Lag1-3a | |
|---|---|---|---|---|
| Total | 0.096(0.027,0.164) * | 0.109(0.023,0.195) * | 0.146(0.045,0.248) * | |
| Diabetes Status | Non-diabetic | 0.064(0.022,0.107) * | 0.079(0.028,0.130) * | 0.111(0.052,0.170) * |
| Diabetic | 0.437(0.121,0.753) * | 0.494(0.123,0.865) * | 0.542(0.121,0.962) * | |
| Sex | Men | 0.073(−0.022,0.167) | 0.095(−0.022,0.212) | 0.127(−0.009,0.262) |
| Women | 0.074(−0.013,0.161) | 0.046(−0.055,0.148) | 0.060(−0.057,0.177) | |
| Age | <75 | 0.010(−0.084,0.103) | 0.010(−0.102,0.122) | 0.006(−0.120,0.132) |
| 75 to 84 | 0.033(−0.130,0.196) | 0.085(−0.122,0.292) | 0.196(−0.057,0.448) | |
| ≥85 | 0.041(−0.052,0.134) | 0.082(−0.038,0.203) | 0.147(0.000,0.294) * | |
| Exercise status | Exercise | −0.018(−0.208,0.173) | −0.039(−0.259,0.182) | −0.018(−0.269,0.234) |
| No-exercise | 0.086(0.022,0.149) * | 0.096(0.017,0.174) * | 0.128(0.036,0.220) * | |
| Smoking status | Never-smoke | 0.127(0.045,0.209) * | 0.156(0.055,0.258) * | 0.191(0.073,0.310) * |
| Smoker | 0.064(−0.043,0.171) | 0.049(−0.081,0.18) | 0.070(−0.079,0.218) | |
| Drinking status | Never-drink | 0.109(0.025,0.193) * | 0.134(0.029,0.240) * | 0.176(0.050,0.301) * |
| Drinker | 0.024(−0.062,0.109) | 0.005(−0.092,0.103) | 0.011(−0.096,0.119) |
Lag 1 is previous year average of PM2.5 exposure; Lag 1-2 is previous year to 2-year average of PM2.5 exposure; Lag 1-3 is previous year to 3-year average of PM2.5 exposure;
P<0.05
Table 3.
Results of the sensitivity analyses for a 10μg/m3 increase in previous 3 years average of PM2.5 exposure (lag1-3)
| No. | Model* | B (95%CI)[mmol/L] |
|---|---|---|
| Model 1 | Main analysis | 0.146 (0.045,0.248) |
| Model 2 | Main analysis excluding age variable | 0.148 (0.047,0.250) |
| Model 3 | Main analysis excluding sex variable | 0.151 (0.049,0.253) |
| Model 4 | Main analysis excluding smoking status | 0.148 (0.047,0.250) |
| Model 5 | Main analysis excluding drinking status | 0.146 (0.044,0.248) |
| Model 6 | Main analysis excluding marital status | 0.148 (0.047,0.249) |
| Model 7 | Main analysis excluding income | 0.141 (0.040,0.242) |
| Model 8 | Main analysis excluding education years | −0.053 (−0.136,0.030) |
| Model 9 | Main analysis excluding exercise status | 0.149 (0.046,0.252) |
| Model 10 | Main analysis excluding staple food intake | 0.151 (0.050,0.252) |
| Model 11 | Main analysis excluding residence | 0.135 (0.037,0.233) |
| Model 12 | Main analysis excluding BMI | 0.146 (0.045,0.247) |
| Model 13 | Current smoking and drinking status | 0.144 (0.042,0.246) |
| Model 14 | Adding the intake of fruit | 0.144 (0.043,0.246) |
| Model 15 | Adding temperature and humidity | 0.260(0.144,0.375) |
| Model 16 | Linear regression model | 0.216 (0.112,0.319) |
In the main analysis we controlled for age, sex, marital status, education years, family income, residence, smoking and drinking status, exercise status, staple food intake, and BMI as the fixed effect. In the linear regression model, we excluded the random effects of individual from the main analysis, and changed the county as the fixed effect; other covariates were as the same as the main analysis.
Discussion
To the best of our knowledge, this is the first multi-center longitudinal study focused on the association between fasting blood glucose levels and long-term PM2.5 exposure. We observed an increase in fasting blood glucose level in the elderly population exposed long-term to PM2.5. Furthermore, elderly participants with diabetes were more likely to have increased fasting blood glucose levels under high exposure to PM2.5.
A few studies have explored the relationship between long-term PM2.5 exposure and fasting blood glucose concentrations, and most of the studies have been cross-sectional. In a survey conducted in southern Germany with 2944 participants. Wolf et al.(Wolf et al., 2017) found no statistically significant association of PM2.5 exposure and fasting blood glucose concentrations in the entire population (β=0.308 mmol/L; 95%CI: 0, 0.634). Studies have also been conducted in China. For example, Lu et al.(Lu et al., 2017) observed a significant association (β=0.305mmol/L; 95%CI: 0.22, 0.39) between blood glucose level and PM2.5 exposure in 3288 pregnant women with an increment of 10 μg/m3. Chuang et al.(Chuang et al., 2011) found positive results in 1023 elderly individuals, and estimated an increase of 0.994 mmol/L (95%CI: 0.522, 1.466) in glucose per 10 μg/m3 PM2.5. In mainland China, Liu et al. (Liu et al., 2016) performed a nation-wide baseline survey with 11847 participants, revealing an increase of 0.063 mmol/L (95%CI: 0.049, 0.078) in glucose in type 2 diabetes patients exposed to PM2.5. A cross-sectional study with 15477 participants in 33 communities in Liaoning province in Northeastern China by Yang et al. (Yang et al., 2018) revealed an increase of 0.031 mmol/L (95%CI: 0.015, 0.046) in glucose per 10 μg/m3 PM2.5 exposure in participants aged 18–74(Yang et al., 2018). Our results differed from those of cross-sectional studies. In addition to different study designs, the differences may have been due to the different study populations; Wolf et al.(Wolf et al., 2017) focused on the entire population, Lu et al.(Lu et al., 2017) focused on women at midterm pregnancy, and Yang et al.(Yang et al., 2018) studied adults 50 years or older. In addition, because with the eight study counties spread in China our study evaluated a wider range of annual PM2.5 exposure than previous studies, the dose-response relationship derived from our study has wider applicability, and yielded results related to high concentration exposure levels not seen in previous studies.(Lu et al., 2017; Wolf et al., 2017)
Fasting blood glucose of the elderly with diabetes were increased more than that of the elderly without diabetes. It may be caused by the weaker glycemic regulation of diabetics, and it is potentially more difficult to resist the harmful effects of PM2.5 pollution. The increase in fasting glucose level in the oldest population may be caused by oxidative stress, systemic inflammation, alterations in insulin signaling and β-cell function deficiency (Liu et al., 2019), and oxidative stress was suggested as the key factor among the mechanisms(Lim and Thurston, 2019).
The covariate selection methods used in air pollution and health not only include traditional criteria such as Akaike’s Information Criteria (AIC), Bayesian information criterion (BIC)(Jones, 2011) and least absolute shrinkage and selection operator (LASSO) (Zhang et al., 2017), but also references existing literature(Li et al., 2018a). Covariate selection is often done by the reviewing of relevant published studies, such as a study of long-term PM2.5 exposure and diabetes(Liang et al., 2019), and a study using mixed effects models in exploring the association between PM2.5 exposure and fasting blood glucose(Lucht et al., 2018).
This study had several strengths. First, our longitudinal design provides stronger causal validation than cross-sectional studies. Second, we conducted subgroup analyses to assess vulnerable populations such as participants with diabetes. These analyses facilitate development of more targeted preventative measures. However, this study had several limitations. First, information on medication intake was not collected in the cohort. Since only 32 participants reported suffering from diabetes, the awareness of diabetes was extremely low (about one fifth) in our study; it is reasonable to assume that most diabetic participants did not take prescribed drugs to regulate blood glucose, possibly because the study area was remote and almost all of the participants were illiterate. Furthermore, we have conducted subgroup analysis about participants with or without diabetes, and the results showed both significant association between PM2.5 and fasting blood glucose in the two subgroups. Second, we used the estimated ambient PM2.5 concentration instead of individual exposure to PM2.5; thus, actual PM2.5 exposure may have differed from the ambient concentration(Zhou et al., 2018). Third, due to the low education status of our old age participants and most of them living in the rural areas, the generalization of our study participants is limited. Fourth, without controlling for district-level social economic status (SES), it may result the potential residual confounding. Nonetheless, we have controlled for county as a random effect in our main model, which controls for a portion of potential spatial confounding.
Conclusions
This study adds significant evidence on the increase of fasting blood glucose level with long-term PM2.5 exposure within the elderly population. Elderly individuals with diabetes are more likely to experience an increase in fasting blood glucose levels with high PM2.5 exposure. The results suggested that elderly individuals, especially those with diabetes, should take protection measures during high PM2.5 polluted periods.
Supplementary Material
High Lights.
A longitudinal study was conducted in eight Chinese counties.
Long-term PM2.5 exposure was associated with an increase in fasting blood glucose.
Elderly individuals with diabetes were more vulnerable to high exposure of PM2.5.
Acknowledgments:
This work was supported by grants from the National Natural Science Foundation of China (Grant: 81273160, 81573247, 91543111), the National High-level Talents Special Support Plan of China for Young Talents, the National Institutes of Health Institutional Research T32 Training Grant (T32 ES023770), the National Institute of Environmental Health Sciences (NIEHS) Individual Fellowship Grant (F31 ES029372) and the National Institutes of Health (NIH) P30 NIH/NIA P30-AG028716 (to VBK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
No author has any potential conflict of interest relevant to this article.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- American-Diabetes-Association, 2012. Diagnosis and classification of diabetes mellitus. Diabetes Care 35 Suppl 1, S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellou V, Belbasis L, Tzoulaki I, Evangelou E, 2018. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS One 13, e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, Sun Q, Harkema J, Rajagopalan S, 2013. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 448, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, Trigo E, Gilliland FD, 2016. Ambient Air Pollutants Have Adverse Effects on Insulin and Glucose Homeostasis in Mexican Americans. Diabetes Care 39, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ, 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68, 64–68. [DOI] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, Yusuf S, 1999. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22, 233–240. [DOI] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, Dominici F, Schwartz JD, 2017. Air Pollution and Mortality in the Medicare Population. N Engl J Med 376, 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Li L, Bennett D, Guo Y, Turnbull I, Yang L, Bragg F, Bian Z, Chen Y, Chen J, Millwood IY, Sansome S, Ma L, Huang Y, Zhang N, Zheng X, Sun Q, Key TJ, Collins R, Peto R, Chen Z, China Kadoorie Biobank, s., 2017. Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: A 7-y prospective study of 0.5 million Chinese adults. PLoS Med 14, e1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD-2016-Disease-and-Injury-Incidence-and-Prevalence-Collaborators, 2017. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerland P, Raftery AE, Sevcikova H, Li N, Gu D, Spoorenberg T, Alkema L, Fosdick BK, Chunn J, Lalic N, Bay G, Buettner T, Heilig GK, Wilmoth J, 2014. World population stabilization unlikely this century. Science 346, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, 2014. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103, 137–149. [DOI] [PubMed] [Google Scholar]
- Internal-Clinical-Guidelines-Team, 2015. Type 2 Diabetes in Adults: Management. 2015 National Institute for Health and Care Excellence, London. [PubMed] [Google Scholar]
- Jones RH, 2011. Bayesian information criterion for longitudinal and clustered data. Stat Med 30, 3050–3056. [DOI] [PubMed] [Google Scholar]
- Kong AP, Luk AO, Chan JC, 2016. Detecting people at high risk of type 2 diabetes- How do we find them and who should be treated? Best Pract Res Clin Endocrinol Metab 30, 345–355. [DOI] [PubMed] [Google Scholar]
- Li T, Zhang Y, Wang J, Xu D, Yin Z, Chen H, Lv Y, Luo J, Zeng Y, Liu Y, Kinney PL, Shi X, 2018a. All-cause mortality risk associated with long-term exposure to ambient PM2.5 in China: a cohort study. Lancet Public Health 3, e470–e477. [DOI] [PubMed] [Google Scholar]
- Li W, Dorans KS, Wilker EH, Rice MB, Kloog I, Schwartz JD, Koutrakis P, Coull BA, Gold DR, Meigs JB, Fox CS, Mittleman MA, 2018b. Ambient air pollution, adipokines, and glucose homeostasis: The Framingham Heart Study. Environ Int 111, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Yang X, Liu F, Li J, Xiao Q, Chen J, Liu X, Cao J, Shen C, Yu L, Lu F, Wu X, Zhao L, Wu X, Li Y, Hu D, Huang J, Liu Y, Lu X, Gu D, 2019. Long-term exposure to ambient fine particulate matter and incidence of diabetes in China: A cohort study. Environ Int 126, 568–575. [DOI] [PubMed] [Google Scholar]
- Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL, 2015. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ 350, g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CC, Thurston GD, 2019. Air Pollution, Oxidative Stress, and Diabetes: a Life Course Epidemiologic Perspective. Curr Diab Rep 19, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Chen R, Kan H, 2016. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int 92-93, 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chen G, Huo W, Wang C, Liu S, Li N, Mao S, Hou Y, Lu Y, Xiang H, 2019. Associations between long-term exposure to ambient air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Environ Pollut 252, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Lu MC, Wang P, Cheng TJ, Yang CP, Yan YH, 2017. Association of temporal distribution of fine particulate matter with glucose homeostasis during pregnancy in women of Chiayi City, Taiwan. Environ Res 152, 81–87. [DOI] [PubMed] [Google Scholar]
- Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, Jockel KH, Jakobs H, Hoffmann B, 2018. Air Pollution and Glucose Metabolism: An Analysis in Non-Diabetic Participants of the Heinz Nixdorf Recall Study. Environ Health Perspect 126, 047001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Yin Z, Zhu P, Luo J, Shi X, Gao X, 2017. Blood cholesterol in late-life and cognitive decline: a longitudinal study of the Chinese elderly. Mol Neurodegener 12, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Zhang Y, Sun Z, Xu D, Li T, 2019. Effects of ambient particulate matter on fasting blood glucose: A systematic review and meta-analysis. Environ Pollut 258, 113589. [DOI] [PubMed] [Google Scholar]
- Meo SA, Memon AN, Sheikh SA, Rouq FA, Usmani AM, Hassan A, Arian SA, 2015. Effect of environmental air pollution on type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 19, 123–128. [PubMed] [Google Scholar]
- Mordarska K, Godziejewska-Zawada M, 2017. Diabetes in the elderly. Prz Menopauzalny 16, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Wang W, 2014. Ambient Air Pollution and Type 2 Diabetes: A Systematic Review of Epidemiologic Research. Curr Environ Health Rep 1, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Bind MC, Colicino E, Kloog I, Byun HM, Cantone L, Trevisi L, Zhong J, Brennan K, Dereix AE, Vokonas PS, Coull BA, Schwartz JD, Baccarelli AA, 2016. Particulate Air Pollution and Fasting Blood Glucose in Nondiabetic Individuals: Associations and Epigenetic Mediation in the Normative Aging Study, 2000-2011. Environ Health Perspect 124, 1715–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Patel P, Puett R, Rajagopalan S, 2015. Air pollution as a risk factor for type 2 diabetes. Toxicol Sci 143, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Hsu NC, Kahn RA, Levy RC, Lyapustin A, Sayer AM, Winker DM, 2016. Global Estimates of Fine Particulate Matter using a Combined Geophysical-Statistical Method with Information from Satellites, Models, and Monitors. Environ Sci Technol 50, 3762–3772. [DOI] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, Herder C, Roden M, Koenig W, Meisinger C, Peters A, 2017. Association Between Long-term Exposure to Air Pollution and Biomarkers Related to Insulin Resistance, Subclinical Inflammation, and Adipokines. Diabetes 2016;65:3314–3326. Diabetes 66, 2725. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G, China Noncommunicable Disease Surveillance, G., 2013. Prevalence and control of diabetes in Chinese adults. JAMA 310, 948–959. [DOI] [PubMed] [Google Scholar]
- Yang BY, Fan S, Thiering E, Seissler J, Nowak D, Dong GH, Heinrich J, 2020. Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res 180, 108817. [DOI] [PubMed] [Google Scholar]
- Yang BY, Qian ZM, Li S, Chen G, Bloom MS, Elliott M, Syberg KW, Heinrich J, Markevych I, Wang SQ, Chen D, Ma H, Chen DH, Liu Y, Komppula M, Leskinen A, Liu KK, Zeng XW, Hu LW, Guo Y, Dong GH, 2018. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 Communities Chinese Health Study. Lancet Planet Health 2, e64–e73. [DOI] [PubMed] [Google Scholar]
- Yin ZX, Shi XM, Kraus VB, Fitzgerald SM, Qian HZ, Xu JW, Zhai Y, Sereny MD, Zeng Y, 2012. High normal plasma triglycerides are associated with preserved cognitive function in Chinese oldest-old. Age Ageing 41, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cavallari JM, Fang SC, Weisskopf MG, Lin X, Mittleman MA, Christiani DC, 2017. Application of linear mixed-effects model with LASSO to identify metal components associated with cardiac autonomic responses among welders: a repeated measures study. Occup Environ Med 74, 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dong B, Li S, Chen G, Yang Z, Dong Y, Wang Z, Guo Y, Ma J, 2019. Particulate matter air pollution and blood glucose in children and adolescents: A cross-sectional study in China. Sci Total Environ 691, 868–873. [DOI] [PubMed] [Google Scholar]
- Zhou X, Cai J, Zhao Y, Chen R, Wang C, Zhao A, Yang C, Li H, Liu S, Cao J, Kan H, Xu H, 2018. Estimation of residential fine particulate matter infiltration in Shanghai, China. Environ Pollut 233, 494–500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


