Abstract
Background
Urinary tract infections are the common types of infections in the community and health care settings. Despite the widespread availability of antibiotics, urinary tract infection remains a worldwide therapeutic problem. It is a continuous and significant problem in cancer patients.
Methods
A hospital-based comparative cross-sectional study was conducted on 240 study participants from January to June 2019. Sociodemographic data were collected by a predesigned questionnaire and midstream urine samples collected using simple random sampling technique by using clean, sterile plastic cups and then inoculated onto CLED agar plates and incubated at 37°C for 24 hours. Urine culture was considered significant bacteriuria when colony forming units ≥105/mL of voided urine and a single pure colony suspended in nutrient broth and then subcultured onto a blood agar plate and MacConkey agar plate, incubated at 37°C for 24 hours for identification. Identification was done by using standard microbiological methods. Modified Kirby–Bauer disk diffusion technique was applied for antimicrobial susceptibility testing in accordance with CLSI 2018 criteria. Data were entered, cleared, and checked using Epi Info version 7 and exported to SPSS version 20 for analysis. The results were displayed using tables and figures. p value <0.05 at 95% CI was considered as statistically significant.
Results
The overall prevalence of asymptomatic bacteriuria in cancer patients was 23.3% while 6.7% in apparently healthy blood donors. E. coli (32.1%) was the commonest isolated uropathogenic bacteria followed by Klebsiella species (25.0%), S. aureus (21.4%), Enterococcus species (10.7%), Serratia species (7.1%), and Enterobacter aerogenes (3.6%) in cancer patients. In apparently healthy blood donors, E. coli, Klebsiella species, and S. aureus were isolated from 75%, 12.5%, and 12.5%, respectively. Most Gram-negative bacteria were more sensitive to ceftazidime, cefoxitin, nalidixic acid, nitrofurantoin, norfloxacin, ciprofloxacin, and tobramycin, whereas highly resistant to ampicillin, penicillin, tetracycline, and ceftazidime. S. aureus isolates were 100% susceptible to nitrofurantoin.
Conclusions
This study showed a high prevalence of asymptomatic bacteriuria among cancer patients (23.3%) compared to apparently healthy blood donors (6.7%). E. coli was isolated predominately. Nitrofurantoin and ciprofloxacin should be used to treat asymptomatic bacteriuria in the study area.
1. Background
Cancer is the second cause of death next to cardiovascular diseases worldwide [1]. The most common location and highest mortality rate belong to pulmonary cancer in men and breast cancer in women. The International Agency for Research on Cancer (IARC) reported the most common cancers in both sexes are pulmonary, breast, colorectal, prostate, and gastric cancers [1, 2]. Infection is a continuous and significant problem in cancer patients due to many factors that increase the susceptibility of immunosuppressed cancer patients to infection, such as neutropenia during chemotherapy, altered gut flora because of frequent antibiotic administration, and disruption of skin and damage of epithelial surfaces of the tissues by cytotoxic chemotherapeutic agents [3, 4].
Bacteriuria is the presence of microbial pathogens in the urethra, bladder, ureter, and pelvis of the kidney [5, 6]. Escherichia coli, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus saprophyticus, Enterococcus faecalis, and Streptococcus agalactiae are the leading cause of urinary tract infections [7, 8]. Obstruction of the urinary tract, use of a catheter, immunocompromised condition, estrogen deficiency, genetic predisposition, and sexual intercourse are common risk factors for urinary tract infection [9, 10]. Cancer patients are at high risk of bacterial infections due to the chemotherapy for cancer patients leads to severe and prolonged immunosuppression [11, 12]. The development of infections caused by multidrug-resistant bacteria has become a major health problem worldwide [13–15].
Urinary tract infection is a serious health problem in the community and health care settings. Cancer patients are at high risk of urinary infections due to cancer chemotherapy leads to severe and prolonged immunosuppression such as neutropenia during chemotherapy, altered gut flora because of frequent antibiotic administration, and disruption of skin and damage of epithelial surfaces of the tissues by cytotoxic chemotherapeutic agents [16, 17]. Due to this, it is a serious health problem that involves bacterial invasion and multiplication in the organs of the urinary tract system, and it is a usual problem for patients to visit outpatient departments [18–20]. Despite the widespread availability of antibiotics, urinary tract infection remains a worldwide therapeutic problem [21–24]. In Ethiopia, no study had been carried out before in this area. Hence, it was important to assess asymptomatic bacteriuria and antibiotic susceptibility patterns of uropathogens among cancer patients and apparently healthy blood donors at the University of Gondar comprehensive specialized referral hospital.
2. Methods
2.1. Study Area, Study Design, and Population
The study was conducted at the University of Gondar comprehensive specialized referral hospital. Gondar town has 8 health centers, 21 private clinics, and one referral hospital with a projected population of 323,900. The hospital serves for more than five million people of Gondar town and its surroundings. The hospital has different departments and 500 beds for admitted patients. A hospital-based comparative cross-sectional study was conducted to assess the prevalence of asymptomatic bacteriuria and antibiotic susceptibility patterns of bacterial isolates among cancer patients and apparently healthy blood donors at the University of Gondar comprehensive specialized referral hospital, Northwest Ethiopia, from January to June 2019. Cancer confirmed patients and apparently healthy blood donors were the study population. However, study participants who were unable to give sociodemographic information currently on antibiotic treatment and had a recent history of antibiotic treatment for the last three weeks at the time of data collection were excluded.
2.2. Ethical Approval
Ethical approval was obtained from the University of Gondar ethical review committee. Written legal permission was obtained from medical directors of the University of Gondar compressive specialized hospital. The objectives of the study were explained to the hospital directors, health-care providers, and patients; clarification also was given for patients before starting data collection. To keep confidentiality of information from participants, no personal identifiers were recorded in the client information extraction predesigned form and data secured from participant records were not available to anyone except for the main investigator.
2.3. Sample Size and Sampling Technique
A total of 120 cancer patients and 120 apparently healthy blood donors were enrolled using simple random sampling technique, and we took a 1 : 1 ratio of cancer patients and apparently healthy blood donors.
2.4. Sociodemographic Data and Urine Specimen Collection
A pretested questionnaire based on postulated risk factors was developed and modified to explore the objectives of the study. Then, sociodemographic characteristics and other relevant information were collected. Urine specimens were collected by a laboratory technologist by instructing the patients to collect approximately 10 ml to 15 ml midstream urine in clean, leak-proof sterile plastic cups at the University of Gondar compressive specialized referral hospital reception. Then, midstream urine samples were collected from each study participants after obtaining written informed consent and assent from the families of children and then transported to the Medical Microbiology Laboratory immediately.
2.5. Laboratory Identification Procedures
Each urine samples were inoculated onto a Cysteine-Lactose-Electrolyte Deficient agar (CLED) (Oxoid Ltd., England) by using a calibrated, sterile, nonreusable plastic loop 1 μl (0.001 ml) and incubated aerobically at 37°C for 18 to 24 hours to check the growth, and urine cultures were considered as significant bacteriuria when colony forming units (CFUs) were ≥105/ml of voided urine, and a single colony was picked and suspended in nutrient broth and then subcultured onto blood agar plate and MacConkey agar plate, finally incubated at 37°C for 24 hours for further identification. Bacterial identification was then done using standardized biochemical tests, namely, indole production, lactose fermentation, hydrolysis of urea, citrate utilization, lysine decarboxylation, and motility test for Gram-negative bacteria and for Gram-positive bacteria, mannitol fermentation, and catalase and coagulase tests.
2.6. Antimicrobial Susceptibility Testing
A suspension of a pure colony from each confirmed culture isolate was performed by using 0.85% sterile normal saline, and the suspension was adjusted at 0.5% MacFarland standard. Using a sterile cotton applicator stick, the suspension was distributed evenly on Muller-Hinton agar. Modified Kirby-Bauer disk diffusion technique was implemented for antibiotic susceptibility pattern using different antibiotics such as ampicillin (10 μg), amoxicillin/clavulanate (30 μg), ceftazidime (30 μg), tobramycin (10 μg), cefoxitin (30 μg), vancomycin (30 μg), tetracycline (30 μg), penicillin (10 μg), ciprofloxacin (5 μg), norfloxacin (10 μg), nitrofurantoin (300 μg), nalidixic acid (30 μg), and rifampicin (5 μg). Then, we applied those antibiotics on Mueller-Hinton agar plate and incubated for 18–24 hours at 37°C. The zones of inhibition were measured, recorded, and interpreted as sensitive, intermediate, and resistant using the CLSI 2018 performance standards for antimicrobial susceptibility testing interpretation table. MDR isolates are bacterial strains which are nonsusceptible to greater than or equal to one antimicrobial agent in three or more antimicrobial categories [25].
2.7. Data and Laboratory Quality Control
The questionnaire was pretested before the actual study begins to make sure whether the questionnaire was appropriate and understandable. The collected data were checked daily for consistency and accuracy. Investigators were also following standard data collection process. Five percent (5%) of the prepared culture media were randomly selected and incubated aerobically for 24 hours at 37°C to cheek the sterility of the prepared culture media, and also known strains of Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) were inoculated onto the prepared culture media to check the performance of the prepared culture media and antibiotic susceptibility test. Laboratory identification procedures such as inoculation of culture media, colony characterization, and measuring of antibiotic susceptibility testing were checked. Reagents for Gram stain and biochemical tests were checked using Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922).
2.8. Data Entry and Analysis
Data were entered to EPI Info version 7 to check data completeness and data clearance and then transferred to SPSS version-20 for analysis. The characteristics of the study populations were summarized using frequencies, mean, and standard deviation. Binary logistic regression was used to determine the strength of the association between variables. Moreover, adjusted odds ratio was computed using multivariate logistic regression for variables with p value ≤0.2 to control confounding variables. p value ≤0.05 was considered statistically significant at 95% CI.
3. Results
3.1. Sociodemographic Characteristics
In this study, a total of 240 study participants were included. Of these, 50% (120/240) were cancer patients and 50% (120/240) were apparently healthy blood donors. Among cancer patients, 58.3% (70/120) were females and 41.7% (50/120) were males, and also among apparently healthy blood donors, 68.3% (82/120) were males and 31.7% (38/120) were females. The majority, 61.7% (74/120) of cancer confirmed patients were from an urban resident, while the rest, 67.5% (81/120) apparently healthy blood donors were from a rural resident. Most of the cancer confirmed patients, 32 (26.7%), had breast cancer followed by colon cancer, 30 (25%). The mean age of the study subjects was 42 years with a range of 3–80 years. 35% (42/120) of cancer patients belonged to 41–60 years of age while 38.3% (46/120) of apparently healthy blood donors belong to 21–30 years of age (Table 1).
Table 1.
Frequency of sociodemographic characteristics of cancer patients and apparently healthy blood donors at the University of Gondar comprehensive specialized hospital, Gondar, Northwest Ethiopia, 2019.
| Variables | Cancer patients, n = 120 (%) | Blood donors, n = 120 (%) | |
|---|---|---|---|
| Sex | Male | 50 (41.7) | 82 (68.3) |
| Female | 70 (58.3) | 38 (31.7) | |
|
| |||
| Age category in years | <10 | 8 (6.7) | 0 (0) |
| 11–20 | 9 (7.5) | 11 (9.2) | |
| 21–30 | 15 (12.5) | 46 (38.3) | |
| 31–40 | 28 (23.3) | 39 (32.5) | |
| 41–60 | 42 (35) | 20 (16.7) | |
| 61–70 | 11 (9.2) | 4 (3.3) | |
| >70 | 7 (5.8) | 0 (0) | |
|
| |||
| Marital status | Married | 91 (78.8) | 82 (68.0) |
| Unmarried | 27 (22.5) | 38 (31.7) | |
| Divorced | 1 (0.8) | 0 (0) | |
| Widowed | 1 (0.8) | 0 (0) | |
|
| |||
| Residences | Urban | 74 (61.7) | 81 (67.5) |
| Rural | 46 (38.3) | 39 (32.5) | |
|
| |||
| Educational status | Illiterate | 50 (41.7) | 19 (15.8) |
| Read and write | 9 (41.7) | 8 (6.7) | |
| Elementary | 20 (16.7) | 9 (7.5) | |
| Secondary | 21 (17.5) | 23 (19.2) | |
| Twelve and above | 20 (16.7) | 61 (50.8) | |
|
| |||
| Occupation | Governmental | 22 (18.3) | 60 (50) |
| Nongovernmental | 1 (0.8) | 0 (0) | |
| Farmer | 43 (35.8) | 30 (25) | |
| Merchant | 22 (18.3) | 2 (1.7) | |
| Student | 15 (12.5) | 20 (16.7) | |
| Unemployed | 17 (14.2) | 0 (0) | |
|
| |||
| Monthly income | <17 dollar | 53 (44.2) | 29 (24.2) |
| 18–33 dollar | 27 (22.5) | 16 (13.3) | |
| 34–50 dollar | 12 (10) | 10 (8.3) | |
| 51–67 dollar | 6 (5.0) | 8 (6.7) | |
| >68 dollar | 22 (18.3) | 57 (47.5) | |
3.2. Prevalence of Asymptomatic Bacteriuria
The overall prevalence of asymptomatic bacteriuria in cancer patients attending the University of Gondar comprehensive specialized hospital was 23.3% (28/120) while the prevalence of asymptomatic bacteriuria in apparently healthy blood donors was 6.7% (8/120). Of the 28 bacterial isolates in cancer patients, 35.7% (10/28) of them were isolated from male participants while 64.3% (18/28) of them from female participants. Moreover, of the 8 bacterial isolates in apparently healthy blood donors, 50% (4/8) of isolates were from females and 50% (4/120) of them were isolated from male participants.
The predominant bacterial isolate in cancer patients was E. coli (32%), Klebsiella species (25%), S. aureus (21.4%), Enterococcus species (10.7%), Serratia species (7.14%), and Enterobacter aerogenes (3.57%) (Figure 1). In apparently healthy blood donors, E. coli (75%), K. pneumonia (12.5%), and S. aureus (12.5%) were the most frequently isolated bacteria (Figure 2). E. coli was the frequently isolated bacteria in both cancer patients and apparently healthy blood donors, 32% (9/28) and 75% (6/8), respectively. The prevalence of asymptomatic bacteriuria in females was 15% (18/120) in cancer patients while 3.3% (4/120) in apparently healthy blood donors. High prevalence of asymptomatic bacteriuria was observed in females as compared to males. From a total of cancer patients with asymptomatic bacteriuria, 32.1% (9/120) were in the age group of 40–60 years while 25% (7/120) were in the age group of above 60 years (Table 2).
Figure 1.
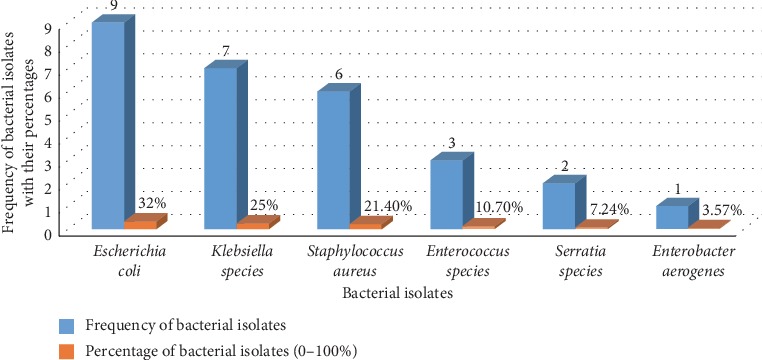
Frequency of bacterial isolates among cancer patients at the University of Gondar comprehensive specialized hospital, 2019.
Figure 2.
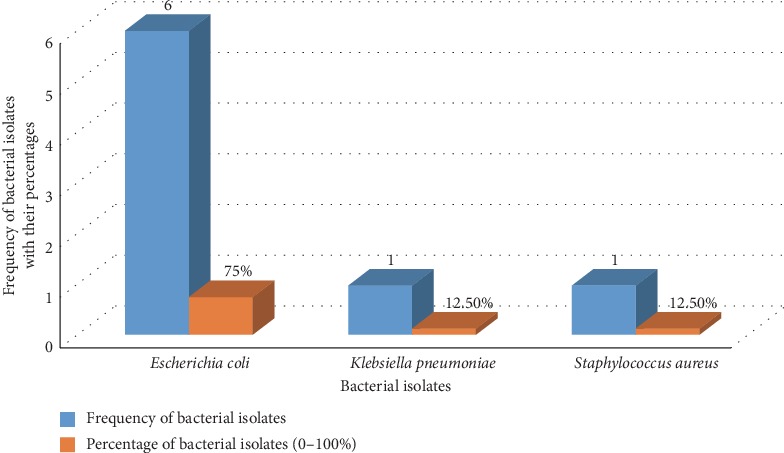
Frequency of bacterial isolates among apparently healthy blood donors at the University of Gondar comprehensive specialized hospital, 2019.
Table 2.
Independent variables examined for relations to urinary tract infections on cancer patients at the University of Gondar comprehensive specialized hospital, Gondar, Northwest Ethiopia, 2019.
| Characteristics | Number (%) | Urinary tract infection | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) +ve for UTI | No. (%) −ve for UTI | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |||
| Sex | Female | 70 (53.8) | 18 (64.3) | 52 (56.5) | 1.4 (0.58–3.74) | 0.47 | — | — |
| Male | 50 (41.7) | 10 (35.7) | 40 (43.5) | 1 (ref) | 1 (ref) | — | ||
|
| ||||||||
| Residences | Urban | 74 (61.7) | 17 (60.7) | 57 (62) | 0.95 (0.40–2.26) | 0.91 | — | — |
| Rural | 46 (38.3) | 11 (39.3) | 35 (38) | 1 (ref) | 1 (ref) | — | ||
|
| ||||||||
| Age in years | <20 years | 17 (14.3) | 4 (14.3) | 13 (14.1) | 2.07 (0.48–8.97) | 0.52 | — | — |
| 21–30 years | 15 (12.5) | 2 (7.1) | 13 (14.1) | 4.14 (0.71–24.12) | 0.33 | — | — | |
| 31–40 years | 28 (21.4) | 6 (21.4) | 22 (23.9) | 2.33 (0.63–8.64) | 0.12 | 0.30 (0.156–4.46) | 0.18 | |
| 41–60 years | 42 (35) | 9 (32.1) | 33 (35.9) | 0.41 (0.18–0.9) | 0.20 | 0.25 (0.529–19.47) | 0.09 | |
| >60 years | 18 (15) | 7 (25) | 11 (12) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| Educational status | Illiterate | 50 (41.7) | 13 (46.4) | 37 (40.2) | 0.71 (0.20–2.5) | 0.59 | — | — |
| Read and write | 9 (7.5) | 4 (14.3) | 5 (5.4) | 0.3 (0.05–1.7) | 0.18 | 0.24 (1.05–124.54) | 0.16 | |
| Primary | 20 (16.7) | 2 (7.1) | 18 (19.6) | 2.3 (0.36–13.9) | 0.38 | — | — | |
| Secondary | 21 (17.5) | 5 (17.9) | 16 (17.4) | 0.8 (0.18–3.54) | 0.77 | — | — | |
| 12 and above | 20 (16.7) | 4 (14.3) | 16 (17.4) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| Occupation | Farmer | 43 (35.8) | 12 (42.9) | 31 (33.7) | 0.25 (0.05–1.21) | 0.08 | 0.53 (0.327–2.967) | 0.089 |
| Merchant | 22 (18.3) | 7 (25) | 15 (16.3) | 0.20 (0.04–1.12) | 0.07 | 4.14 (0.458–6.454) | 0.13 | |
| Students | 15 (12.5) | 4 (14.3) | 11 (12) | 0.26 (0.04–1.66) | 0.26 | — | — | |
| Unemployed | 17 (14.2) | 3 (10.7) | 14 (15.2) | 0.44 (0.07–3.00) | 0.41 | 2.10 (0.177–1.646) | 0.54 | |
| Employed | 23 (19.2) | 2 (7.1) | 20 (22.8) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| Income | <17 dollar | 53 (44.2) | 15 (53.6) | 38 (41.3) | 0.25 (0.05–1.22) | 0.08 | 8.64 (0.909–11.882) | 0.12 |
| 18–33 dollar | 27 (22.5) | 5 (17.9) | 22 (23.9) | 0.44 (0.08–2.53) | 0.36 | — | — | |
| 34–50 dollar | 12 (10) | 3 (10.7) | 9 (9.8) | 0.30 (0.04–2.12) | 0.23 | — | — | |
| 51–67 dollar | 6 (5) | 3 (10.7) | 3 (3.3) | 0.10 (0.01–0.0.87) | 0.04 | 0.25 (0.542–15.58) | 0.09 | |
| >68 dollar | 22 (18.3) | 2 (7.1) | 20 (21.7) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| Marital status | Married | 91 (75.8) | 22 (78.6) | 69 (75) | 0.82 (0.30–2.23) | 0.70 | — | — |
| Unmarried | 29 (24.2) | 6 (21.4) | 23 (25) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| History of hospitalization | Yes | 104 (86.7) | 23 (82.1) | 81 (88) | 0.63 (0.20–2.00) | 0.42 | — | — |
| No | 16 (13.3) | 5 (17.9) | 11 (12) | 1 (ref) | 1 (ref) | — | — | |
|
| ||||||||
| History of surgery | Yes | 64 (53.3) | 14 (50) | 50 (54.3) | 0.84 (0.36–2.00) | 0.68 | — | — |
| No | 56 (46.7) | 14 (50) | 42 (45.7) | 1 (ref) | 1 (ref) | — | — | |
|
| ||||||||
| History of catheterization | Yes | 8 (6.7) | 2 (7.1) | 6 (6.5) | 1.10 (0.21–5.80) | 0.91 | — | — |
| No | 112 (93.3) | 26 (92.9) | 86 (93.5) | 1 (ref) | 1 (ref) | — | — | |
|
| ||||||||
| History of previous UTI | Yes | 4 (3.3) | 2 (7.1) | 2 (2.2) | 0.29 (0.40–2.20) | 0.22 | — | — |
| No | 116 (96.7) | 26 (92.9) | 90 (97.8) | 1 (ref) | 1 (ref) | — | — | |
|
| ||||||||
| Type of cancer | Blood | 12 (10) | 1 (3.6) | 11 (12) | 0.92 (0.30–2.23) | 0.38 | — | — |
| Colon | 22 (18) | 4 (14.3) | 18 (19.6) | 1.20 (0.21–5.80) | 0.45 | — | ||
| Bladder | 9 (7.5) | 2 (7.1) | 7 (7.6) | 0.41 (0.03–5.30) | 0.38 | — | ||
| Breast | 32 (26.7) | 9 (32.1) | 21 (22.8) | 0.30 (0.01–0.87) | 0.17 | 0.47 (0.094–2.486) | 0.49 | |
| Lymph | 22 (18.3) | 7 (25) | 15 (16.3) | 0.44 (0.08–2.53) | 0.15 | 0.70 (0.049–3.189) | 0.64 | |
| Thyroid | 23 (19.2) | 5 (17.9) | 20 (21.7) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
|
| ||||||||
| Cancer treatment follow-up | <1 year | 97 (80.8) | 25 (89.3) | 72 (78.2) | 0.26 (0.03–2.13) | 0.2 | 3.32 (0.542–15.58) | 0.33 |
| 1–2 years | 11 (9.2) | 2 (7.1) | 9 (9.8) | 0.41 (0.03–5.30) | 0.4 | — | — | |
| >2 years | 12 (10) | 1 (3.6) | 11 (12) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
3.3. Associated Risk Factors for Asymptomatic Bacteriuria
Sociodemographic data such as age, sex, residence, occupation, educational status, marital status, and monthly income of the patients had been analyzed to assess their contribution for asymptomatic bacteriuria. Similarly, history of hospitalization, history of surgery, history of catheterization, history of UTIs, type of cancer, and duration of cancer chemotherapy (follow-up time) had also been assessed for the association. Among the risk factors assessed in cancer patients, there were no statistically significant risk factors for asymptomatic bacteriuria (P > 0.05). However, MDR isolates are associated with a history of antimicrobial usage.
3.4. Antimicrobial Susceptibility Patterns of Bacterial Isolates
Bacterial antimicrobial susceptibility tests were performed for bacterial isolates; the antimicrobial susceptibility test result showed that majority of the isolates were sensitive for tobramycin (88.8% for Escherichia coli, 57.1% for Klebsiella species, 100% for Serratia species, and 100% for Enterobacter aerogenes), ciprofloxacin (77% for Escherichia coli, 71.4% for Klebsiella species, 100% for Enterobacter aerogenes, and 66.6% for Staphylococcus aureus), nitrofurantoin (77.8% for Escherichia coli, 85.7% for Klebsiella species, and 100% for Serratia species, Enterobacter aerogenes, and Staphylococcus aureus) and cefoxitin (100% for E. coli, 71.4% for Klebsiella species, and 100% for E. aerogenes) which were found to be efficient antibiotics (Table 3). However, most of the isolates were resistant to tetracycline (77.8% for E. coli, 42.9% for Klebsiella species, 66.7% for Enterococcus species, and 100% for E. aerogenes and Serratia species). S. aureus isolates were 100% susceptible to nitrofurantoin.
Table 3.
Antibiotic susceptibility patterns of pathogenic bacterial isolates among cancer patients at the University of Gondar comprehensive specialized hospital, Gondar, Northwest Ethiopia, 2019.
| Antibiotics | Cancer patients | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli (9) | Klebsiella species (7) | S. aureus (6) | Enterococcus species (3) | Serratia species (2) | E. aerogenes (1) | |||||||||||||
| S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | S (%) | I (%) | R (%) | |
| Ampicillin | 11.1 | 11.1 | 77.8 | 14.3 | 0 | 85.7 | N/A | N/A | N/A | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 |
| Amoxicillin/clavulanate | 44.5 | 22.2 | 33.3 | 57.1 | 0 | 42.9 | N/A | N/A | N/A | N/A | N/A | N/A | 50 | 0 | 50 | 100 | 0 | 0 |
| Ceftazidime | 100 | 0 | 0 | 57.1 | 0 | 42.9 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 100 | 100 | 0 | 0 |
| Tobramycin | 88.8 | 0 | 11.2 | 57.1 | 0 | 42.9 | N/A | N/A | N/A | N/A | N/A | N/A | 100 | 0 | 0 | 100 | 0 | 0 |
| Cefoxitin | 100 | 0 | 0 | 71.4 | 0 | 28.9 | 83.3 | 18.7 | 0 | N/A | N/A | N/A | 50 | 0 | 50 | 100 | 0 | 0 |
| Vancomycin | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 100 | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Tetracycline | 22.2 | 0 | 77.8 | 42.9 | 14.2 | 42.9 | 33.3 | 33.3 | 33.3 | 33.3 | 0 | 66.7 | 0 | 0 | 100 | 0 | 0 | 100 |
| Penicillin | N/A | N/A | N/A | N/A | N/A | N/A | 16.7 | 0 | 83.3 | 0 | 0 | 100 | N/A | N/A | N/A | N/A | N/A | N/A |
| Ciprofloxacin | 77.8 | 0 | 22.2 | 71.4 | 0 | 28.9 | 66.6 | 16.7 | 16.7 | 66.7 | 0 | 33.3 | 50 | 0 | 50 | 100 | 0 | 0 |
| Norfloxacin | 77.8 | 0 | 22.2 | 71.4 | 0 | 28.9 | 83.3 | 0 | 18.7 | 0 | 33.3 | 66.7 | 50 | 0 | 50 | 100 | 0 | 0 |
| Nitrofurantoin | 77.8 | 0 | 22.2 | 85.7 | 0 | 14.3 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| Nalidixic acid | 100 | 0 | 0 | 85.7 | 0 | 14.3 | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 100 | 100 | 0 | 0 |
| Rifampicin | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 33.3 | 0 | 66.7 | N/A | N/A | N/A | N/A | N/A | N/A |
S = susceptible; I = intermediate; R = resistance; N/A = not applicable.
3.5. Prevalence of Multidrug-Resistant Isolates
Among the isolates, 46.4% (13/28) showed multidrug-resistance pattern in cancer patients, while in apparently healthy blood donors, 25% (2/8) showed multidrug-resistance pattern. The proportion of multidrug-resistant isolates among cancer patients for E. coli was 44.4% (4/9), Klebsiella species 57.1% (4/7), S. aureus 16.7% (1/6), Enterococcus species 100% (3/3), and Serratia species 50% (1/2) and among apparently healthy blood donors, E. coli was 16.7% (1/6) and S. aureus 100% (1/1).
4. Discussion
This study was carried out to assess the burden of asymptomatic bacteriuria and associated risk factors which could be the possible causes for bacteriuria in cancer patients attending at the University of Gondar comprehensive specialized hospital. In this study, the overall prevalence of asymptomatic bacteriuria in cancer confirmed patients was 23.3%. This finding of bacteriuria was in line with the reports in Korea (23.5%) [26] and India (20.1%) [27]. However, a higher prevalence of bacteriuria was reported in this study than the studies conducted in Texas (15%), Sweden (5%), and Japan (15%) [28–30]. On the other hand, this finding showed a lower prevalence of bacteriuria than those reported in Saudi Arabia (35.8%), India (34.7%, 32%), and Sudan (31.6%) [31–34]. This variation of bacteriuria from other studies might be due to the difference in the characteristics of the study population, quality of sampling, culturing techniques, geographical distribution, and diagnostic techniques.
The frequency of Gram-negative bacteria isolated from cancer patients in Egypt was 17.2% [35] from a urine sample, which is higher than our study. However, in our study (15.83%), Gram-negative bacteria were found from the urine of cancer patients, which is similar to the frequency of bacteriuria (15%) reported in a Japanese study [36]. Among Gram-negative isolates, Escherichia coli and Klebsiella species were isolated from 32.1% and 25.0%, respectively. In this finding, E. coli was the predominant isolate which is compatible with a study conducted in India (E. coli (40%) and K. pneumonia (25%)) [33], another study in India (E. coli (38.1%)) [34], and in Sudan (E. coli (39.2%) and Klebsiella pneumonia (19%)) [37]. Among Gram positives, S. aureus was the predominantly isolated bacteria followed by Enterococcus species, which is in line with a study conducted in Tamil Nadu, India [32], and in Nigeria [38]. This variation might be due to sample size variation, and we had included all types of cancer patients in our study, but other studies include specific cases of cancer patients.
The prevalence of asymptomatic bacteriuria among cancer patients (23.3%) was higher than that of apparently healthy blood donors (6.7%). This higher prevalence of asymptomatic bacteriuria in cancer patients might be due to the immunocompromised state of cancer patients by cancer chemotherapeutic agents. Moreover, in cancer patients, the prevalence of asymptomatic bacteriuria in females (64.3%) was higher than that of males (35.7%). The higher prevalence of asymptomatic bacteriuria in females might be due to women's urethra is short and located near the anus that allows relatively easy passage of bacteria into the bladder and urethral opening.
Many factors were assessed as risk factors for bacteriuria in cancer patients. However, no evidence was found to support the association between asymptomatic bacteriuria and sex, age, and cancer types (P > 0.05). Correspondingly, a previous study by Fukushima et al. showed that neither of the above factors mentioned in our study had no effect on the occurrence of bacteriuria [36]. On the contrary, bladder cancer had shown statistically significant association with urinary tract infection in a study done by Richards et al. [39]. A study by Fan et al. in 2017 had also identified an association between prostate cancer and bacteriuria [40]. Another study by Sun et al. in 2013 had also confirmed the association among urinary tract cancers and asymptomatic bacteriuria [8]. This difference might be due to the variation in study design and characteristics of the study population. In our study, catheterization and previous surgery and other independent variables were not significantly associated with bacteriuria. Consistently, surgery had been found to be less important in a study by Kim et al. in patients with bladder cancer [41].
In our study, the effect of ampicillin, augmentin, tetracycline, and penicillin were minimal while cefoxitin, nitrofurantoin, norfloxacin, nalidixic acid, and ciprofloxacin were found to be the most efficient antibiotics for bacterial isolates from both cancer patients and apparently healthy blood donors. Among cancer patients, Enterococcus species were resistant to five antimicrobials, one Serratia species isolate showed resistance to four antimicrobials, and three klebsiella species were resistant to three antimicrobials. Among the apparently healthy blood donors, one bacterial isolate of S. aureus showed multidrug resistance to three antimicrobials and one E. coli isolate was also resistant to three antimicrobials. The differences in susceptibility pattern of the isolates might be due to the differences in the management of antibiotics and geographical area. MDR isolates are associated with a history of antimicrobials; this might be due to the development of specific mechanisms of resistance through time.
5. Conclusion
In this study, the prevalence of asymptomatic bacteriuria among cancer patients (23.3%) was found to be higher than that of apparently healthy blood donors (6.7%). Escherichia coli was the most frequent isolate in both cancer patients and apparently healthy blood donors. S. aureus was the most commonly isolated Gram-positive bacteria in cancer patients. Cefoxitin, nitrofurantoin, norfloxacin, nalidixic acid, and ciprofloxacin were found to be the most efficient antibiotics. Based on the study findings, if asymptomatic bacteriuria is suspected and laboratory tests are not available, it is recommended that nitrofurantoin and ciprofloxacin should be used in preference to penicillin, augmentin, and tetracycline in the study area. Ministry of health should think about ampicillin, augmentin, and penicillin since they are inefficient to isolates.
Acknowledgments
The authors would like to thank all participants of this k, and hospital directors for their participation and cooperation.
Abbreviations
- ATCC:
American Type Culture Collection
- BAP:
Blood agar plate
- CLED:
Cysteine-Lactose-Electrolyte Deficient
- CLSI:
Clinical and Laboratory Standards Institute
- CFU:
Colony forming unit
- EPI:
Epidemiological information
- IARC:
International Agency for Research on Cancer
- MAC:
MacConkey agar
- MUH:
Muller-Hinton agar
- SPSS:
Statistical package for the social sciences
- UTI:
Urinary tract infection
- USD:
United States dollar
- WHO:
World Health Organization.
Data Availability
All data generated or analyzed during this study are included within this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.
Ethical Approval
Ethical clearance was obtained from the University of Gondar ethical review committee. Written legal permission was obtained from hospital directors prior to data collection. Written informed consent from adults and assent from parents/legal guardians of children were obtained. Additionally, the confidentiality of information was assured.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
AT and WF were responsible for the conception of the research idea, data collection, and analysis and interpretation; AT, GB, and BG were involved in the manuscript write-up and review and data collection; AT was involved in the study design; GB and BG were involved in the supervision, interpretation, and thesis preparation; GB was involved in the analysis. All authors read and approved the final manuscript.
References
- 1.Mofid B., Novin K., Roointan E. S., Forouzanfar M. M. Epidemiology and death-related factors of oncology patients in emergency department. Emergency (Tehran, Iran) 2016;4(4):145–150. [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Ashour H. M., El-Sharif A. Species distribution and antimicrobial susceptibility of gram-negative aerobic bacteria in hospitalized cancer patients. Journal of Translational Medicine. 2009;7(1):p. 14. doi: 10.1186/1479-5876-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolar M., Htoutou Sedlakova M., Pudova V., et al. Incidence of fecal Enterobacteriaceae producing broad-spectrum beta-lactamases in patients with hematological malignancies. Biomedical Papers. 2015;159(1):100–103. doi: 10.5507/bp.2014.042. [DOI] [PubMed] [Google Scholar]
- 5.Seifu W. D., Gebissa A. D. Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infectious Diseases. 2018;18(1):p. 30. doi: 10.1186/s12879-017-2911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manik C., Amsath A. Prevalence and distribution of bacteria and fungi isolated from patients with urinary tract infections in pattukkottai, tamilnadu, India. International Journal of Pure and Applied Zoology. 2013;1(3) [Google Scholar]
- 7.Raad E. B. Prevalence and antibiotic susceptibility patterns of bacteria causing urinary tract infections in Youssef Hospital Center: frst report from Akkar governorate, North Lebanon. The International Arabic Journal of Antimicrobial Agents. 2017;7(1) doi: 10.3823/802. [DOI] [Google Scholar]
- 8.Sun L.M., Lin C.L., Liang J.A., et al. Urinary tract infection increases subsequent urinary tract cancer risk: A population-based cohort study. Cancer Science. 2013;104(5):619–623. doi: 10.1111/cas.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manikandan S., Ganesapandian S., Singh M., Kumaraguru A. Antimicrobial susceptibility pattern of urinary tract infection causing human pathogenic bacteria. Asian Journal of Medical Sciences. 2011;3(2):56–60. [Google Scholar]
- 10.Ifeanyichukwu I., Emmanuel N., Chika E., et al. Frequency and antibiogram of uropathogens isolated from urine samples of HIV infected patients on Antiretroviral Therapy. American Journal of BioScience. 2013;1(3):50–53. doi: 10.11648/j.ajbio.20130103.11. [DOI] [Google Scholar]
- 11.Merga Duffa Y., Terfa Kitila K., Mamuye Gebretsadik D., Bitew A. Prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients at yekatit 12 hospital medical college, Addis Ababa, Ethiopia. International Journal of Microbiology. 2018;2018 doi: 10.1155/2018/8492309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores-Mireles A. L., Walker J. N., Caparon M., Hultgren S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology. 2015;13(5):269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangaraj G., Granwehr B. P., Jiang Y., Hachem R., Raad I. Perils of quinolone exposure in cancer patients. Cancer. 2010;116(4):967–973. doi: 10.1002/cncr.24812. [DOI] [PubMed] [Google Scholar]
- 14.Rosa R. G., Goldani L. Z., dos Santos R. P. Risk factors for multidrug-resistant bacteremia in hospitalized cancer patients with febrile neutropenia: a cohort study. American Journal of Infection Control. 2014;42(1):74–76. doi: 10.1016/j.ajic.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Cornejo-Juárez P., Vilar-Compte D., Pérez-Jiménez C., Ñamendys-Silva S. A., Sandoval-Hernández S., Volkow-Fernández P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. International Journal of Infectious Diseases. 2015;31:31–34. doi: 10.1016/j.ijid.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Gul N., Mujahid T. Y., Ahmad S. Isolation, identification and antibiotic resistance profile of indigenous bacterial isolates from urinary tract infection patients. Pakistan Journal of Biological Sciences. 2004;7(12):2051–2054. doi: 10.3923/pjbs.2004.2051.2054. [DOI] [Google Scholar]
- 17.Rezaei-Tavirani M., Ghafourian S., Sayehmiri F., Pakzad R., Safiri S., Pakzad I. Prevalence of cotrimoxazole resistance uropathogenic bacteria in Iran: a systematic review and meta-analysis. Archives of Clinical Infectious Diseases. 2018;13(5) doi: 10.5812/archcid.63256. [DOI] [Google Scholar]
- 18.Carratala J., Fernandez-Sevilla A., Tubau F., Callis M., Gudiol F. Emergence of quinolone-resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clinical Infectious Diseases. 1995;20(3):557–560. doi: 10.1093/clinids/20.3.557. [DOI] [PubMed] [Google Scholar]
- 19.Sandoval C., Sinaki B., Weiss R., et al. Urinary tract infections in pediatric oncology patients with fever and neutropenia. Pediatric Hematology and Oncology. 2012;29(1):68–72. doi: 10.3109/08880018.2011.617809. [DOI] [PubMed] [Google Scholar]
- 20.Bodey G. P., Middleman E., Umsawadi T., Rodriguez V. Infections in cancer patients. Results with gentamicin sulfate therapy. Cancer. 1972;29(6):1697–1701. doi: 10.1002/1097-0142(197206)29:6<1697::aid-cncr2820290638>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Huoi C., Vanhems P., Nicolle M.-C., Michallet M., Bénet T. Incidence of hospital-acquired pneumonia, bacteraemia and urinary tract infections in patients with haematological malignancies, 2004–2010: a surveillance-based study. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058121.e58121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marín M., Gudiol C., Garcia-Vidal C., Ardanuy C., Carratalà J. Bloodstream infections in patients with solid tumors: epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine. 2014;93(3) doi: 10.1097/md.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisht R., Katiyar A., Singh R., Mittal P. Antibiotic resistance-A global issue of concern. Asain Journal of Pharmaceutical and Clinical Research. 2009;2(2):34–39. [Google Scholar]
- 24.Ayoade F., Moro D. D., Ebene O. L. Prevalence and antimicrobial susceptibility pattern of asymptomatic urinary tract infections of bacterial and parasitic origins among university students in redemption camp, Ogun state, Nigeria. Open Journal of Medical Microbiology. 2013;3(4):219–226. doi: 10.4236/ojmm.2013.34033. [DOI] [Google Scholar]
- 25.Magiorakos A.-P., Srinivasan A., Carey R. B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim K. H., Yoon H. S., Yoon H., Chung W. S., Sim B. S., Lee D. H. Febrile urinary tract infection after radical cystectomy and ileal neobladder in patients with bladder cancer. Journal of Korean Medical Science. 2016;31(7):1100–1104. doi: 10.3346/jkms.2016.31.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Der Auwera P., Meunier F., Ibrahim S., Kaufman L., Derde M. P., Tulkens P. M. Pharmacodynamic parameters and toxicity of netilmicin (6 milligrams/kilogram/day) given once daily or in three divided doses to cancer patients with urinary tract infection. Antimicrobial Agents and Chemotherapy. 1991;35(4):640–647. doi: 10.1128/aac.35.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolston K. V. I., Bodey G. P., Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clinical Infectious Diseases. 2007;45(2):228–233. doi: 10.1086/518873. [DOI] [PubMed] [Google Scholar]
- 29.Russell B., Garmo H., Beckmann K., Stattin P., Adolfsson J., Van Hemelrijck M. A case-control study of lower urinary-tract infections, associated antibiotics and the risk of developing prostate cancer using pcbase 3.0. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195690.e0195690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukushima H., Kobayashi M., Kawano K., Morimoto S. Effect of preoperative bacteriuria and pyuria on intravesical recurrence in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy. In Vivo. 2017;31(6):1215–1220. doi: 10.21873/invivo.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirkhazi M., Sarriff A., Aziz N. A., Almana F., Arafat O., Shorman M. Bacterial spectrum, isolation sites and susceptibility patterns of pathogens in adult febrile neutropenic cancer patients at a specialist hospital in Saudi Arabia. World Journal of Oncology. 2014;5(5-6):p. 196. doi: 10.14740/wjon850w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manisha S. M., Bhat S., Priya R. L., Magdalene J. Prevalence and antibiotic susceptibility pattern of bacterial isolates from urinary tract infections in a tertiary care hospital in Tamilnadu. IOSR Journal of Dental and medical Sciences. 2015;14(7):59–65. [Google Scholar]
- 33.Husain N., Shumo A., Mekki S., Dawi N., Elsid M. A clinicopathological study of urinary bladder neoplasms in patients at three centers in Khartoum, Sudan. Sudan Journal of Medical Sciences. 2009;4(3) doi: 10.4314/sjms.v4i3.48317. [DOI] [Google Scholar]
- 34.Kantor A. F., Hartge P., Hoover R. N., Narayana A. S., Sullivan J. W., Fraumeni J. F. Urinary tract infection and risk of bladder cancer. American Journal of Epidemiology. 1984;119(4):510–515. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 35.Akortha E., Ibadin O. Incidence and antibiotic susceptibility pattern of Staphylococcus aureus amongst patients with urinary tract infection (UTI) in UBTH Benin City, Nigeria. African Journal of Biotechnology. 2008;7(11) doi: 10.5897/ajb08.176. [DOI] [Google Scholar]
- 36.Fukushima H., Kobayashi M., Kawano K., Morimoto S. Effect of preoperative bacteriuria and pyuria on intravesical recurrence in patients with upper tract urothelial carcinoma undergoing radical nephroureterectomy. In Vivo. 2017;31(6) doi: 10.21873/invivo.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards K. A., Ham S., Cohn J. A., Steinberg G. D. Urinary tract infection-like symptom is associated with worse bladder cancer outcomes in the Medicare population: implications for sex disparities. International Journal of Urology. 2016;23(1):42–47. doi: 10.1111/iju.12959. [DOI] [PubMed] [Google Scholar]
- 38.Onifade A. K., Anibijuwon I. I., Azariah E. J. Urinary tract infection in apparently healthy individuals in Ile-Ife, Nigeria: detection of predominant microorganisms and antibiotics susceptibility profile. African Journal of Microbiology Research. 2011;5(20):3233–3236. doi: 10.5897/ajmr11.279. [DOI] [Google Scholar]
- 39.Richards K. A., Ham S., Cohn J. A., Steinberg G. D. Urinary tract infection‐like symptom is associated with worse bladder cancer outcomes in the Medicare population: Implications for sex disparities. International Journal of Urology. 2016;23(1):42–47. doi: 10.1111/iju.12959. [DOI] [PubMed] [Google Scholar]
- 40.Fan C. Y., Huang W. Y., Lin K. T., et al. Lower urinary tract infection and subsequent risk of prostate cancer: a nationwide population-based cohort study. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168254.e0168254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K. H., Yoon H. S., Yoon H., Chung W. S., Sim B. S., Lee D. H. Febrile urinary tract infection after radical cystectomy and ileal neobladder in patients with bladder cancer. Journal of Korean Medical Science. 2016;31(7):1100–1104. doi: 10.3346/jkms.2016.31.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.


