Abstract
Purpose
To evaluate the efficacy of a new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea on pain and inflammation.
Patients and Methods
Pilot study with one-group pretest–posttest quasi-experimental design in which 50 subjects with moderate knee osteoarthritis (OA) (mean age: 62.46±8.45) were supplied for four weeks with two tablets/day.
Results
Primary outcomes were 1) the evaluation of pain intensity, by a 30-day visual analogue scale (VAS) and 2) the assessment of knee function by WOMAC (Western Ontario and McMaster Universities Arthritis) Index and by Tegner Lysholm Knee Scoring collected at baseline, at 15 and 30 days after treatment. Secondary outcomes were 3) health-related quality of life, by the ShortForm36 (SF-36); 4) inflammation grade by C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR); and 5) body composition by dual-energy X-ray absorptiometry (DXA) measured at baseline and 30 days after treatment. Data showed significant effects of supplement intake for WOMAC (β=−3.27, p<0.0001), Lysholm (β=1.06, p=0.0003), CRP (β=−0.13, p=0.006), ESR (β=−3.09, p=0.004), physical activity (β=4.3, p=0.009) and fat-free mass (β=376.7, p=0.046). A significant VAS’s decrease over time was observed in both knees (left: β=−0.08, p<0.0001; right: β=−0.07, p<0.0001).
Conclusion
The tested formulation seems to be effective and also free of side effects.
Keywords: pain, knee osteoarthritis, dietary supplements, Phytosome®
Introduction
Knee osteoarthritis (OA) is ranked as the 11th highest contributor to global disability in the world; the global prevalence of knee OA is 3.8% (95% uncertainty interval (UI) 3.6% to 4.1%) with no discernible change from 1990 to 2010. Its prevalence is higher in females than males and disability related to knee OA has increased from 10.5 million in 1990 to 17.1 million in 2010. OA is predicted to become the fourth leading cause of disability worldwide by 2020.1 In the US, the prevalence of diagnosed arthritis among adults (more than 18 years), estimated using the annual average from the 2003–2005 NHIS surveys, is 21.6%, or 46.4 million; in particular, arthritis prevalence is higher in adults of more than 65 years but two-thirds of the adults with arthritis are younger than 65 and more than 60% were women. The number of persons with arthritis is projected to increase to nearly 67 million by 2030, an increase of more than 40%. The prevalence of activity limitations was higher in adults of more than 65 years old, affecting more than 22% of them; joint symptoms led to activity limitation in 40% of the adults with arthritis and this outcome is supposed to increase to 25 million (9.3% of the adult population) by 2030. Affected people have a worse health-related quality of life.2 In Europe OA is the most common type of arthritis, it is estimated to affect more than 40 million people, its prevalence is 35% among people aged 50–59 years, and 55% for people over 70 years of age, while the lifetime risk for knee OA is 45%.3 In Italy, knee OA affected 4 million people, its prevalence is 50% among people aged 15–79; in particular, it hurts more women than men among people of more than 55 years old and more men than women among people younger than 45 years old. Joint symptoms affected 9.6% of men and 18% of women over 65 years old.4
Care and management of knee OA pain remains very complex. Despite advances in medicine and numerous agents that counteract knee OA pain, millions of patients continue to suffer from knee OA. Recently, attention has been given in the identification of novel botanical interventions producing both analgesia, by interacting with nociceptive-transducing channels, and anti-inflammatory activity. Ginger and acmella are recognized among botanicals with anti-inflammatory and analgesic activity,5,6 therefore they are dietary botanicals supplements potentially useful in the management and treatment of the OA.
Acmella oleracea is known by traditional medicine for its power against toothache pain.7 The well-recognized anesthetic activity of Acmella is due to one of its active compounds: the pungent alkamide-like spilanthol, that have been related to different processes, studied in vitro and in animal models, including inhibition of prostaglandin synthesis,8 activation of opioidergic,9 serotoninergic and GABAergic systems10 and anesthetic activity through blockage of voltage-gated Na channels.11 Moreover, the anti-inflammatory activity of dried flowers of Acmella has only been proven on the commonly used lipopolysaccharide-activated murine macrophage model, RAW 264.7.12 These findings suggest that spilanthol can be a useful inhibitor of inflammatory mediators and is potentially applicable for COX-2 selective nonsteroidal anti-inflammatory drugs (NSAIDs) non-responders.
Also, gingerols and shogaols of Zingiber officinale have analgesic and anti-inflammatory activity.5 These compounds act as agonist of transient receptor potential cation channel subfamily V member 1 (TRPV1) receptors13 and, at the same time, exhibit inhibitory activity on arachidonic acid metabolism via the COX2 (prostaglandins, thromboxanes).14,15
The aim of this pilot study is to verify the efficacy of a new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea in the care and management of knee OA.
Materials and Methods
Study Design
This is a one-group pretest–posttest quasi-experimental pilot study design, in which all the participants received the treatment (without control group).
Subjects
This study was performed in volunteer outpatients referred to the Rehabilitation Department of Santa Margherita Institute, University of Pavia, Pavia. This is a convenience sampling. Inclusion criteria were as follows: 1) established moderate knee OA (classification 1–3 according to Kellgren and Lawrence system for classification of knee OA); 2) Lequesne Index of 6–10; 3) body mass index (BMI) between 22 and 30 kg/m2; 4) aged 40–75; 5) no drugs for OA, such as nonsteroidal anti-inflammatory drugs (NSAIDs); 6) no use of dietary supplements. Subjects with diabetes, metabolic disease, or neoplasia, as well as the patients with disabling diseases that could directly affect muscle weakness (such as neurological diseases, hip fractures, or amputations), were excluded.
All the participants received the treatment (ie new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea).
The study was carried out in accordance with the relevant guidelines of the Declaration of Helsinki (1964), its amendments and the general principles of ICH Harmonised Tripartite Guidelines for Good Clinical Practice (ICH Topic E6, CPMP/ICH/135/95). At the beginning of the study, the written informed consent, reviewed by the Ethics Committee, was obtained from all individual participants included in the study, according to ICH-GCP, the ethical principles that have their origin in the Declaration of Helsinki and the regulatory and legal requirements in Italy. The study was conducted after approval from the Ethics Committee of the University of Pavia no. 1206/15012017 (clinicaltrial.gov: n. NCT03907787).
Dietary Supplement
The food-grade lecithin-based formulation of Zingiber officinale and Acmella oleracea standardized extracts (Mitidol™) involved in the study was prepared and provided by Indena S.p.A. (Milan, Italy). This formulation consists in the association of the two standardized extracts Zingiber officinale and Acmella oleracea combined with sunflower lecithin in a 1:1 weight ratio (expressed as the sum of the botanical extracts and lecithin, respectively); food additives such as microcrystalline cellulose (E460), sucrose esters of fatty acids (E473), hydroxypropyl cellulose (E463) and silicon dioxide (E551) are also included in the formulation in order to improve physical and technological characteristics of the product.
For the clinical study, 350 mg of the lecithin-based formulation of Zingiber officinale and Acmella oleracea standardized extracts were formulated by Indena S.p.A. as film-coated tablets, corresponding to a content of 37.5 mg of Zingiber officinale and 7.5 mg of Acmella oleracea respectively (HPLC).
Coated tablets contained also food additives suitable for tabletting, namely bibasic calcium phosphate dihydrate (Di-Cafos® D160), polyvinylpolypirrolidone (Polyplasdone® XL), sodium croscarmellose (Solutab® A-IP), silicon dioxide (Syloid® 244FP), magnesium stearate and talc; tablets were also coated with a hydroxypropylmethylcellulose-based film-coating system.
Before releasing, the film-coated tablets containing the food-grade lecithin formulation of Zingiber officinale and Acmella oleracea standardized extracts were tested for appearance, average mass, uniformity of mass, HPLC-content of Zingiber officinale and Acmella oleracea extracts, disintegration time and microbiological quality.
The participants were asked to report any adverse events. Moreover, they were asked to undergo laboratory tests (liver and kidney function, blood count with formula, lipid and glucidic profile) that have been assessed at baseline (t0) and after 30 days of treatment (t2), in order to detect any changes in their health during supplementation.
Primary and Secondary Outcomes
We considered as primary outcomes: 1) the evaluation of pain intensity, by a 30-day VAS and 2) the assessment of knee function by WOMAC Index16 and by Tegner Lysholm Knee Scoring.17 As secondary outcomes were evaluated: 1) the Health-related quality of life, by the SF-36, 2) the inflammation by CRP and ESR, and, 3) the body composition by DXA.
Assessment of Knee Function by WOMAC (Western Ontario and McMaster Universities Arthritis) Index and by Tegner Lysholm Knee Scoring Scale
The WOMAC pain scale consists of five questions that assess pain while walking on a flat surface, going upstairs or downstairs, in bed at night, sitting or lying, and standing upright.18 Responses are recorded on a five-point Likert scale, with a higher score representing a greater level of pain. This scale has been shown to be valid and reliable in hip and knee OA populations. We have used the validated WOMAC Italian version.16
Moreover, each patient completed a self-report questionnaire, Tegner Lysholm Knee Scoring Scale, related to knee symptoms and function.17 Lysholm scores were categorized into poor (<65), fair (65–83), good (84–90) and Excellent (>90).
Both WOMAC and Lysholm scores were collected at baseline (t0), 15 days (t1) and 30 days (t2) during dietary supplements administration.
Visual Analogue Scale (VAS) of Pain
The visual analogue scale (VAS) is an instrument that tries to measure a characteristic or attitude that is believed to range across a continuum of values and cannot easily be directly measured. For example, the amount of pain that a patient feels ranges across a continuum from none to an extreme amount of pain. The VAS score is determined by measuring in millimeters from the left-hand end of the line to the point that the patient marks.19 Our sample underwent a 30-day VAS evaluation.
Health–Related Quality of Life
The participants were tested with the Short-Form 36-Item Health Survey (SF-36)20 to assess their quality of life. This questionnaire is a valid generic measure that is used for rating health-related quality of life in several research fields because of its validity, high internal consistency, and high test–retest reliability. The quality of life SF-36 was administered at t0 and after 30 days of treatment.
Body Composition by Dual-Energy X-Ray Absorptiometry (DXA)
Body composition such as fat-free mass (FFM), fat mass (FM), gynoid and android (subcutaneous or visceral) fat distribution was measured with dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy DXA (GE Medical Systems) both at t0 and after 30 days of treatment. The in vivo CVs were 0.89% and 0.48% for whole-body fat (fat mass) and FFM, respectively. Abdominal subcutaneous fat (SAT) and visceral fat (VAT) were estimated within the android region (Corescan, Lunar Prodigy DXA, GE Medical Systems).
Blood Sample Measurements of Inflammation Markers in Plasma: C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR)
Fasting venous blood samples were drawn between 8 am and 10 am, with the subjects in a sitting position. Blood handling and collection were carried out under strictly standardized conditions. High-sensitivity C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) were measured both at t0 and after 30 days of treatment. High-Sensitivity CRP was determined by immunonephelometry (Dade Behring, Marburg, Germany). ESR was analyzed by capillary photometry (Test 1, Alifax).
Statistical Analysis
To evaluate statistically significant pre- and post-treatment changes (time), each continuous outcome was analyzed using linear mixed model effect21,22 (nlme R package); while for ordinal categorical variable, a cumulative link mixed model (CLMM)23 (implemented in the clmm function of the ordinal R package) was fitted. A random intercept for each subject (1|subject) was added to all the models in order to account for intra-subject correlation produced by the repeated outcome’s measurements (at different pre- and post-treatment times) carried out on the same subjects. All models were adjusted for sex, age and height. Normality of residuals was assessed graphically and with Shapiro–Wilk test. P-values <0.05 on 2-sided test are considered as statistically significant.
To evaluate VAS’s means change over time, we fitted a linear mixed model for analyzing longitudinal data,24,25 separately in the two knees, taking into account intra-subject correlation and temporal effect for sampling/measuring, thus including in the model an autocorrelation term (corAR1) and a random intercept in the form of time|subject.
Pearson’s pairwise partial correlations (r), adjusted for sex, age and height, and their corresponding p-values, were also calculated to investigate the linear relationship between selected variables both at pre- and post-treatment. Data are presented as mean±standard deviation (SD), and all the analyses were performed using R 3.4.2 software.22
Results
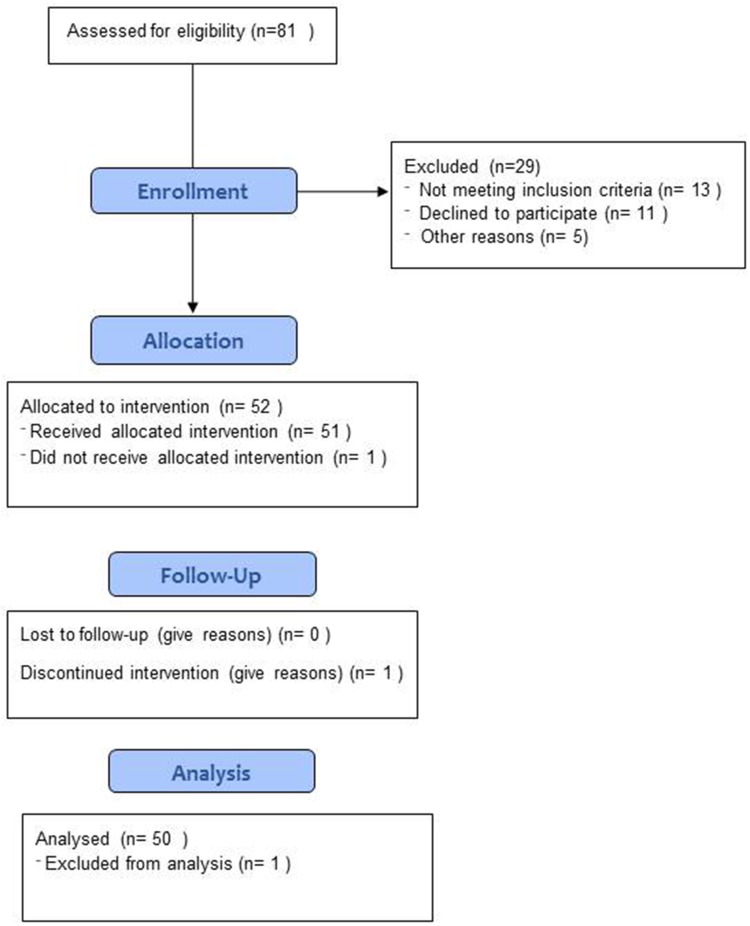
We have analyzed 50 subjects (20 males and 30 females) with a mean age of 62.46±8.45 years and a mean height of 1.62±0.10 meters. Figure 1 shows the flow chart of the subjects studied. Eighty-one subjects were assessed for eligibility and 51 were enrolled in the study, but one subject discontinued the treatment for personal reasons, no patients complained of adverse events during the supplementation.
Figure 1.
Flow diagram of the study.
Table 1 reports the descriptive statistics of each variable collected both at t0 considered as baseline and after 30 days of treatment (t2).
Table 1.
Descriptive Statistics of the Studied Variables Measured in the Sample at Baseline (T0) and After 30 Days of Treatment (T2)
| N=50 (20 Males): (Mean Age 62.46±8.45 SD): (Mean Height 1.62±0.10 SD): |
Baseline (t0): Mean±SD: |
After 30 Days (t2): Mean±SD: |
|---|---|---|
| BMI (kg/m2) | 28.26±3.71 | 26.93±6.44 |
| Fat-free mass (g) | 43,317.86±8812.937 | 43,535.31±8991.567 |
| Fat mass (g) | 29,387.54±12,330.77 | 28,420.94±11,485.22 |
| VAT (g) | 2029.36±5895.902 | 2029.625±5978.721 |
| ESR (mm/h) | 24.78±13.65 | 21.64±14.85 |
| CRP (mg/L) | 0.33±0.65 | 0.21±0.47 |
| SF-36 | ||
| Physical activity | 74.5±23.54 | 78.96±22.51 |
| Role limitations due to physical health | 58.08±36.67 | 63.16±33.97 |
| Pain | 50.68±28.18 | 54.55±30.81 |
| General health | 53.68±16.91 | 53.84±17.87 |
| Vitality | 53.76±15.08 | 54.94±16.39 |
| Social functioning | 62.36±19.97 | 63.94±23.44 |
| Role limitations due to emotional problems | 66.48±33.31 | 71.92±30.71 |
| Emotional well-being | 63.38±29.31 | 61.64±29.39 |
Abbreviations: BMI, body mass index; VAT, visceral fat; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; SF-36, Short Form36; SD, standard deviation.
Table 2 reports the descriptive statistics of the two variables (ie Lysholm and WOMAC) collected at t0 and two times after treatment (ie, t1 and t2, respectively, during 15 and 30 days).
Table 2.
Descriptive Statistics of the Lhysolm and WOMAC Variables Measured (T0), After 15 Days of Treatment (T1) and After 30 Days of Treatment (T2)
| N=50 (20 Males): (Mean Age 62.46±8.45 SD): (Mean Height 1.62±0.10 SD): |
Baseline (t0): Mean±SD: |
After 15 Days (t1): Mean±SD: |
After 30 Days (t2): Mean±SD: |
|---|---|---|---|
| Lhysolm | 65.06±15.55 | 70.31±13.84 | 73.46±14.53 |
| WOMAC | 44.31±15.28 | 39.79±15.19 | 38.14±15.19 |
Abbreviations: WOMAC, Western Ontario and McMaster Universities Arthritis; SD, standard deviation
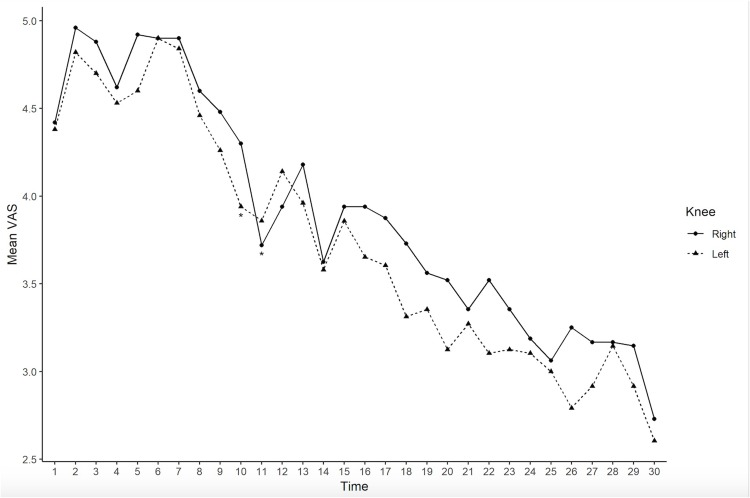
In Figure 2 VAS’s means over time in both the knees are plotted, showing a general improvement, in term of VAS’s decrease. A linear mixed model for analyzing longitudinal data shows a statistically significant VAS’s means change over time both in the left (β = −0.08, 95% CI = −0.09; −0.06, p<0.0001) and in the right knee (β =−0.07, 95% CI = −0.08; −0.06, p<0.0001). In the left knee a significant VAS’s decrease (β=−0.44, p= 0.02) is observed after 10 days, while in the right knee a significant VAS’s decrease (β=−0.70, p= 0.0003) is observed after 11 days.
Figure 2.
Plot of the VAS’s mean over time in the left knee (dashed line) and in the right knee (solid line).
Note: *Significant time in which we observe a significant decrease of VAS (VAS (time2) –VAS (time1)) both in right and left knee, taking into account correlations between measurements and times.
Linear mixed models, adjusted for sex, age and height, were fitted to evaluate significant pre- and post-treatment changes (time) on the analyzed continuous primary and secondary outcomes (full results are reported in Table 3).
Table 3.
Estimate (β), Standard Error and p-value of the Treatment Effect on the Primary and Secondary Endpoints, Evaluated as Difference Between Pre-Post Treatments, Using a Linear Mixed Model
| Outcomes | β | Stand. Error | p-value |
|---|---|---|---|
| BMI (kg/m2) | −0.091 | 0.095 | 0.34 |
| Fat-free mass (g) | 376.732 | 184.232 | 0.046: |
| Fat mass (g) | −481.37 | 255.214 | 0.065 |
| VAT (g) | −31.089 | 30.698 | 0.316 |
| ESR (mm/h) | −3.092 | 1.014 | 0.004: |
| CRP (mg/L) | −0.129 | 0.045 | 0.006: |
| WOMAC | −3.271 | 0.469 | <0.0001: |
| SF-36: | |||
| Physical activity | 4.303 | 1.592 | 0.009: |
| Role limitations due to physical health | 5.014 | 4.513 | 0.272 |
| Pain | 3.721 | 3.497 | 0.293 |
| General health | 0.002 | 2.044 | 0.999 |
| Vitality | 1.129 | 2.199 | 0.61 |
| Social functioning | 2.104 | 1.983 | 0.294 |
| Role limitations due to emotional problems | 4.99 | 4.061 | 0.225 |
| Emotional well-being | −2.459 | 1.706 | 0.16 |
Notes: Analysis was performed using linear mixed model effect. A random intercept for each subject (1|subject) was added to all the models in order to account for intra-subject correlation produced by the repeated outcome’s measurements (at different pre- and post-treatment times) carried out on the same subjects. All models were adjusted for sex, age and height. In bold – significant results (p<0.05).
Abbreviations: BMI, Body mass index, VAT, Visceral fat, ESR, Erythrocyte sedimentation rate, CRP, C-reactive protein, WOMAC, Western Ontario and McMaster Universities Arthritis, SF-36, ShortForm36.
The analysis to evaluate if the supplement intake significantly affected the knee function (evaluating by Lysholm) was carried out using the CLMM approach.
Results reported in Table 4 show that the supplement intake significantly (p=0.0003) increases the odds to be rated in the highest Lysholm’s categories with respect to the combined lower ones by a factor of exp (β=1.062; p=2.89). In other words, it indicates that it is more likely to obtain a higher Lysholm score after the treatment.
Table 4.
Estimate (β), Standard Error and p-value of the Treatment Effect on Lysholm Using a Cumulative Link Mixed Model
| Outcome | β | Stand. Error | p-value |
|---|---|---|---|
| Lysholm | 1.062 | 0.295 | 0.0003: |
Notes: Analysis was performed using a cumulative link mixed model for ordinal categorical variable. A random intercept for each subject (1|subject) was added to the model in order to account for intra-subject correlation produced by the repeated outcome’s measurements (at different pre- and post-treatment times) carried out on the same subjects. The model was adjusted for sex, age and height. In bold – significant results (p<0.05).
Lastly, we calculated the pairwise partial correlations, adjusted for age, sex and height at pre-treatment between VAT and ESR (r=−0.07, p=0.65), VAT and CRP (r=−0.08, p=0.60), fat-free mass and ESR (r=−0.13, p=0.39), fat-free mass and CRP (r=−0.12, p=0.42), WOMAC and CRP (r=0.04, p=0.77), Lysholm and CRP (r=−0.33, p=0.02). The same pairwise partial correlations were also calculated at post-treatment: between VAT and ESR (r=−0.19, p=0.21), VAT and CRP (r=−0.08, p=0.58), fat-free mass and ESR (r=−0.20, p=0.19), fat-free mass and CRP (r=−0.11, p=0.47), WOMAC and CRP (r=−0.06, p=0.69), Lysholm and CRP (r=−0.29, p=0.05). Pairwise partial correlations between Lysholm (both pre- and post-treatment) and VAS (both at 0 and 30 days) and between WOMAC (both pre- and post-treatment) and VAS (both at 0 and 30 days) were calculated separately in the two knees (Table 5).
Table 5.
Pairwise Partial Correlation Between Lysholm and VAS and Between WOMAC and VAS
| Variables 1 | Variables 2 | r | p-value |
|---|---|---|---|
| Lysholm (pre treatment) | VAS (time 0 – right knee) | −0.22 | 0.13 |
| Lysholm (pre treatment) | VAS (time 0 – left knee) | −0.35 | 0.02: |
| Lysholm (post treatment) | VAS (time 30 – right knee) | 0.02 | 0.88 |
| Lysholm (post treatment) | VAS (time 0 – left knee) | −0.08 | 0.61 |
| WOMAC (pre treatment) | VAS (time 0 – right knee) | 0.32 | 0.03: |
| WOMAC (pre treatment) | VAS (time 0 – left knee) | 0.001 | 0.99 |
| WOMAC (post treatment) | VAS (time 30 – right knee) | 0.35 | 0.02: |
| WOMAC (post treatment) | VAS (time 30 – left knee) | −0.08 | 0.61 |
Notes: Pearson’s pairwise partial correlations (r), adjusted for sex, age and height, and their corresponding p-values were performed to investigate the linear relationship between selected variables both at pre- and post-treatment. In bold significant results (p<0.05).
Abbreviations: WOMAC, Western Ontario and McMaster Universities Arthritis; VAS, visual analogue scale.
Discussion
This is the first study presented in the literature showing that a 30-days supplementation with the new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea is effective in decreasing the sensation of pain in individuals with moderate knee OA, as shown by the significant improvement in pre-and post-treatment VAS scale of pain. This pilot study also showed that the dietary supplementation was associated with an amelioration of knee function, as from statistically significant improvement in WOMAC and Lysholm scale scores.16,17 It is important to note that in addition to the statistically significant improvement of subjective pain and function, a statistically significant objective decrease of the knee main inflammatory indices, such as CRP and ESR, has also been shown.
As far as the secondary end-points of the study, after supplementation, significant improvements were observed for SF-36 physical activity and fat-free mass, assessed by DXA. These results are very appealing and new, because we can hypothesize that, thanks to the decrease of pain and to the improvement of the knee function, the subjects have performed more physical movement, as demonstrated by the physical activity of the SF-36 test, which has led to an increase in muscle mass.
The results of our study appear of relevant clinical interest due to the fact that OA is a leading cause of musculoskeletal pain worldwide and the knee is one of the most commonly affected joints. As there is currently no cure for OA, treatment has focused on symptomatic relief with the aim of reducing pain and disability and maintaining or improving joint mobility.26 Drug treatments such as simple analgesics and NSAIDs are associated with adverse events.27,28 The potential use of a supplementation based on a food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea as a treatment of OA could represent an interesting alternative to the use of NSAIDs avoiding the risk of unwanted symptoms and complications. Furthermore, it could be also an alternative option in subjects NSAIDs non-responders. As a matter of fact, the intervention with this dietary supplement appears to be safe: no relevant side effects were observed in the intervention group.
These interesting results on pain and inflammation are due to synergic combination of the two extracts because ginger has significant anti-inflammatory and anti-oxidant activity, while acmella has painkiller activity.
It has been shown in vitro and in animal studies that alkamides like spilanthol, the main bioactive component of acmella, exert a spectrum of different biological activities. With regards to pain and chronic joint inflammatory disorders, the most promising one is the penetration enhancing effect on model drugs,29 strong local anesthetic,11 analgesic,6,12,30-39 antinociceptive,40,41 antioxidant,36 and antinflammatory12 without causing adverse effects.42
Ginger may have anti-inflammatory effects by inhibiting COX and lipoxygenase. It may also affect tumor necrosis factor and decrease the synthesis of inflammatory prostaglandins.43
The use of ginger in pain relief is based on anti-inflammatory and analgesic properties. In fact, several in vitro and animal studies showed that gingerols and shogaols from fresh ginger root have inhibitory activity on arachidonic acid metabolism via the COX-2 (prostaglandins, thromboxanes)44 and lipoxygenases (leukotrienes) pathways.45 However, a direct inhibitory action on genes encoding for pro-inflammatory substances (eg cytokines) may also play a role.46 Therefore, ginger has been used as an anti-inflammatory acting by inhibition of prostaglandin synthesis.47 Furthermore, preliminary studies support the analgesic effect48 for both 6-shogaol and 6-gingerol.45 Gingerols are potent vanilloid receptor (VR1) agonists which may in part explain ginger’s analgesic effects.13
In human studies, the positive effect of intake of ginger on pain has shown in athletes49,50 and in women with dysmenorrhea.51 In particular, regarding dysmenorrhea, it has been shown that ginger is as effective as mefenamic acid and ibuprofen on pain relief in primary dysmenorrhea.52,53
Our study is based on a one-group pretest–posttest research design. The choice of this kind of study was driven by the small sample of volunteers. The major limitations are given by the lack of control group, the small sample size and the presence of threats to internal validity (eg regression to the mean). These limitations do not allow to draw causal conclusions on the effect of the treatment on the outcomes analyzed, meaning that other factors could influence the outcomes other than the investigated treatment.54,55
Another limitation is that the study did not evaluate the safety of the dietary supplement. Despite the limitations reported above, this is the first study aiming at evaluating the efficacy of a new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea on decreasing the sensation of pain in individuals with moderate knee OA. Furthermore, the interval between pre- and post-measurements is quite short (ie 30 days) and this could improve the internal validity, in the sense that the within-subject variation due to chance might be limited.
Conclusion
In conclusion, the new food-grade lecithin formulation of standardized extracts of Zingiber officinale and Acmella oleracea had a statistically significant effect on reducing symptoms of knee OA (pain and knee function) due to the anti-inflammatory activity showed by a reduction of markers of inflammation (CRP and ESR), so this study demonstrates the efficacy of the tested formula. Moreover, a significant improvement was observed for SF-36 physical activity and fat-free mass, assessed by DXA. These results are very appealing and new, because we can hypothesize that, thanks to the decrease of pain and to the improvement of the knee function, the subjects have performed more physical movement, as shown by the physical activity of the SF-36 test, which has led to an increase in muscle mass. Finally, this new formulation of standardized extracts of Zingiber officinale and Acmella oleracea showed no sides effects on recruited subjects. The results obtained prompt for a further experimental study with a randomized control group, which will allow us to draw causal conclusions on the effect of this formulation on pain and inflammation. Moreover, the safety of the supplementation must be studied.
Funding Statement
Non-financial competing interests.
Abbreviations
OA, Osteoarthritis; UI, Uncertainty interval; NSAIDs, Nonsteroidal anti-inflammatory drugs; TRPV1, Transient receptor potential channel subfamily V member 1; COX2, Ciclooxigenase 2; BMI, Body mass index; VAT, Visceral adipose tissue; ESR, Erythrocyte sedimentation rate; CRP, c-reactive protein; VAS, Visual analogue scale; DXA, Dual-energy X-ray absorptiometry; WOMAC, Western Ontario and McMaster Universities Arthritis; VR1, Vanilloid receptor 1; ICH-GCP, International conference on harmonization-good clinical practice; CLMM, Cumulative link mixed model.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177 [DOI] [PubMed] [Google Scholar]
- 3.Paoloni M, Bernetti A, Belelli A, et al. Appropriateness of clinical and organizational criteria for intra-articular injection therapies in osteoarthritis. A delphi method consensus initiative among experts in Italy. Ann Ist Super Sanita. 2015;51(2):131–138. doi: 10.4415/ANN_15_02_11 [DOI] [PubMed] [Google Scholar]
- 4.De Filippis L, Gulli S, Caliri A, et al. [Epidemiology and risk factors in osteoarthritis: literature review data from “OASIS” study]. Reumatismo. 2014;56(3):169–184. [DOI] [PubMed] [Google Scholar]
- 5.Yong-liang JIA, Jun-ming Z, Lin-hui Z, Bao-shan SUN, Meng-jing BAO, Fen-fen LI. Analgesic and anti-inflammatory effects of ginger oil. Chin Herbal Med. 2011;3(2):150–155. doi: 10.3969/j.issn.1674-6384.2011.02.011 [DOI] [Google Scholar]
- 6.Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. High therapeutic potential of Spilanthes acmella: a review. EXCLI J. 2013;12:291–312. [PMC free article] [PubMed] [Google Scholar]
- 7.Boonen J, Baert B, Burvenich C, Blondeel P, De Saeger S, De Spiegeleer B. LC–MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J Pharm Biomed Anal. 2010;53(3):243–249. doi: 10.1016/j.jpba.2010.02.010 [DOI] [PubMed] [Google Scholar]
- 8.Ratnasooriya WD, Pieris KPP, Samaratunga U, Jayakody JRAC. Diuretic activity of Spilanthes acmella flowers in rats. J Ethnopharmacol. 2004;91(2–3):317–320. doi: 10.1016/j.jep.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Ong HM, Mohamad AS, Adilah MN, et al. Antinociceptive activity of methanolic extract of Acmella uliginosa (Sw.) cass. J Ethnopharmacol. 2011;133(1):227–233. doi: 10.1016/j.jep.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 10.Acosta-García J, Hernández-Chan N, Paz-Bermúdez F, et al. D4 and D1 dopamine receptors modulate [3H]GABA release in the substantia nigra pars reticulata of the rat. Neuropharmacology. 2009;57(7–8):725–730. doi: 10.1016/j.neuropharm.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty A, Devi BRK, Sanjebam R, Khumbong S, Thokchom IS. Preliminary studies on local anesthetic and antipyretic activities of Spilanthes acmella Murr. in experimental animal models. Indian J Pharmacol. 2010;42(5):277–279. doi: 10.4103/0253-7613.70106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L-C, Fan N-C, Lin M-H, et al. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. J Agric Food Chem. 2008;56(7):2341–2349. doi: 10.1021/jf073057e [DOI] [PubMed] [Google Scholar]
- 13.Dedov VN, Tran VH, Duke CC, et al. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol. 2002;137(6):793–798. doi: 10.1038/sj.bjp.0704925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vennekens R, Vriens J, Nilius B. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6(1):79–96. doi: 10.2174/157015908783769644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semwal RB, Semwal DK, Combrinck S, Viljoen AM. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry. 2015;117:554–568. doi: 10.1016/j.phytochem.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 16.Salaffi F, Blasetti P, Del Medico P, Stancati A, Carotti M. Affidabilità e validità della versione italiana del WOMAC nella valutazione della gonartrosi sintomatica. Reumatismo. 2000;52(Suppl 2):602.:. [Google Scholar]
- 17.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150–154. doi: 10.1177/036354658201000306 [DOI] [PubMed] [Google Scholar]
- 18.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 19.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Pinheiro J, Bates D. Mixed-Effects Models in S and S-Plus. New York: Springer; 2000. Available from: https://scholar.google.it/scholar?q=Mixed-Effects+Models+in+S+and+S-PLUS+2000&hl=it≈sdt=0%2C5≈ylo=2000≈yhi=2001. Accessed July10, 2018. [Google Scholar]
- 22.R Core Team. R: A Language and Environment for Statistical Computing. Austria: R Foundation for Statistical Computing; 2017. doi: 10.1038/sj.hdy.6800737 [DOI] [Google Scholar]
- 23.Agresti A. Modelling ordered categorical data: recent advances and future challenges. Stat Med. 1999;18(17–18):2191–2207. doi: [DOI] [PubMed] [Google Scholar]
- 24.Molenberghs G, Verbeke G. A review on linear mixed models for longitudinal data, possibly subject to dropout. Stat Model an Int J. 2001;1(4):235–269. doi: 10.1177/1471082X0100100402 [DOI] [Google Scholar]
- 25.Molenberghs G, Verbeke G. Linear Mixed Models for Longitudinal Data. New York: Springer New York; 2000. doi: 10.1007/978-1-4419-0300-6 [DOI] [Google Scholar]
- 26.Harvey WF, Hunter DJ. The role of analgesics and intra-articular injections in disease management. Med Clin North Am. 2009;93(1):201–211. doi: 10.1016/j.mcna.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 27.Bjordal J, Ljunggren A, Klovning LS. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ. 2004;4(4):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, min LY, mao YS, yun DX. [Mechanism of exacerbation of colonic damage in experimental colitis treated with celecoxib]. Beijing Da Xue Xue Bao. 2008;40(2):195–199. [PubMed] [Google Scholar]
- 29.De Spiegeleer B, Boonen J, Malysheva SV, et al. Skin penetration enhancing properties of the plant N-alkylamide spilanthol. J Ethnopharmacol. 2013;148(1):117–125. doi: 10.1016/j.jep.2013.03.076 [DOI] [PubMed] [Google Scholar]
- 30.Paulraj J, Govindarajan R, Palpu P. The genus Spilanthes ethnopharmacology, phytochemistry, and pharmacological properties: a review. Adv Pharmacol Sci. 2013;2013:510298. doi: 10.1155/2013/510298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rios MY, Olivo HF. Natural and synthetic alkamides: applications in pain therapy. Stud Nat Prod Chem. 2014;43:79–121. doi: 10.1016/B978-0-444-63430-6.00003-5 [DOI] [Google Scholar]
- 32.Molina-Torres J, Salazar-Cabrera CJ, Armenta-Salinas C, Ramírez-Chávez E. Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: affinin and reduced amides. J Agric Food Chem. 2004;52(15):4700–4704. doi: 10.1021/jf034374y [DOI] [PubMed] [Google Scholar]
- 33.Hind N, Biggs N. Plate 460. Acmella oleracea compositae. Curtis’s Bot Mag. 2003;20(1):31–39. doi: 10.1111/1467-8748.00368 [DOI] [Google Scholar]
- 34.Cilia-López VG, Juárez-Flores BI, Aguirre-Rivera JR, Reyes-Agüero JA. Analgesic activity of Heliopsis longipes and its effect on the nervous system. Pharm Biol. 2010;48(2):195–200. doi: 10.3109/13880200903078495 [DOI] [PubMed] [Google Scholar]
- 35.Tiwari KL, Jadhav SK, Joshi V. An updated review on medicinal herb genus Spilanthes. Zhong Xi Yi Jie He Xue Bao. 2011;9(11):1170–1178. doi: 10.3736/jcim20111103 [DOI] [PubMed] [Google Scholar]
- 36.Abeysiri GRPI, Dharmadasa RM, Abeysinghe DC, et al. Screening of phytochemical, physico-chemical and bioactivity of different parts of Acmella oleraceae Murr. (Asteraceae), a natural remedy for toothache. Ind Crops Prod. 2013;50:852–856. doi: 10.1016/j.indcrop.2013.08.043 [DOI] [Google Scholar]
- 37.Dias AMA, Santos P, Seabra IJ, RNC J, Braga MEM, de Sousa HC. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. J Supercrit Fluids. 2012;61:62–70. doi: 10.1016/J.SUPFLU.2011.09.020 [DOI] [Google Scholar]
- 38.Sharma V, Boonen J, Chauhan NS, Thakur M, De Spiegeleer B, Dixit VK. Spilanthes acmella ethanolic flower extract: LC–MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine. 2011;18(13):1161–1169. doi: 10.1016/J.PHYMED.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 39.Dubey S, Maity S, Singh M, Saraf SA, Saha S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: a review. Adv Pharmacol Sci. 2013;2013:1–9. doi: 10.1155/2013/423750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rios MY, Aguilar-Guadarrama AB, Gutiérrez M Del C. Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae). J Ethnopharmacol. 2007;110(2):364–367. doi: 10.1016/j.jep.2006.09.041 [DOI] [PubMed] [Google Scholar]
- 41.Déciga-Campos M, Arriaga-Alba M, Ventura-Martínez R, Aguilar-Guadarrama B, Rios MY. Pharmacological and toxicological profile of extract from heliopsislongipes and affinin. Drug Dev Res. 2012;73(3):130–137. doi: 10.1002/ddr.21002 [DOI] [Google Scholar]
- 42.Nomura ECO, Rodrigues MRA, da Silva CF, et al. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J Ethnopharmacol. 2013;150(2):583–589. doi: 10.1016/j.jep.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 43.Philip J, Sperry M, Wilson A, Wilson AF. Dietary supplements for osteoarthritis. American family physician. [Cited January 15, 2008]. Available from: https://www.aafp.org/afp/2008/0115/p177.html#afp20080115p177-b4. Accessed July11, 2018. [PubMed]
- 44.Sang S, Hong J, Wu H, et al. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57(22):10645–10650. doi: 10.1021/jf9027443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young H-Y, Luo Y-L, Cheng H-Y, Hsieh W-C, Liao J-C, Peng W-H. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96(1–2):207–210. doi: 10.1016/j.jep.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 46.Fouda A-M-M, Berika MY. Evaluation of the effect of hydroalcoholic extract of Zingiber officinale rhizomes in rat collagen-induced arthritis. Basic Clin Pharmacol Toxicol. 2009;104(3):262–271. doi: 10.1111/j.1742-7843.2008.00363.x [DOI] [PubMed] [Google Scholar]
- 47.Grzanna R, Lindmark L, Frondoza CG. Ginger–an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–132. doi: 10.1089/jmf.2005.8.125 [DOI] [PubMed] [Google Scholar]
- 48.Ojewole JAO. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (roscoe) rhizomes (zingiberaceae) in mice and rats. Phytother Res. 2006;20(9):764–772. doi: 10.1002/ptr.1952 [DOI] [PubMed] [Google Scholar]
- 49.Wilson PB. Ginger (Zingiber officinale) as an analgesic and ergogenic aid in sport: a systemic review. J Strength Cond Res. 2015;29(10):2980–2995. doi: 10.1519/JSC.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 50.Sellami M, Slimeni O, Pokrywka A, et al. Herbal medicine for sports: a review. J Int Soc Sports Nutr. 2018;15(1):14. doi: 10.1186/s12970-018-0218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahnama P, Montazeri A, Huseini HF, Kianbakht S, Naseri M. Effect of Zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: a placebo randomized trial. BMC Complement Altern Med. 2012;12(1):92. doi: 10.1186/1472-6882-12-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med. 2009;15(2):129–132. doi: 10.1089/acm.2008.0311 [DOI] [PubMed] [Google Scholar]
- 53.Shirvani MA, Motahari-Tabari N, Alipour A. The effect of mefenamic acid and ginger on pain relief in primary dysmenorrhea: a randomized clinical trial. Arch Gynecol Obstet. 2015;291(6):1277–1281. doi: 10.1007/s00404-014-3548-2 [DOI] [PubMed] [Google Scholar]
- 54.Schweizer ML, Braun BI, Milstone AM. Research methods in healthcare epidemiology and antimicrobial stewardship—quasi-experimental designs. Infect Control Hosp Epidemiol. 2016;37(10):1135–1140. doi: 10.1017/ice.2016.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shadish WR, Cook TD, Campbell DT Experimental and quasi-experimental designs for generalized causal inference.jr-*"-** fr houghton mifflin company Boston New York; 2002. Available from: https://pdfs.semanticscholar.org/9453/f229a8f51f6a95232e42acfae9b3ae5345df.pdf. Accessed November15, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Philip J, Sperry M, Wilson A, Wilson AF. Dietary supplements for osteoarthritis. American family physician. [Cited January 15, 2008]. Available from: https://www.aafp.org/afp/2008/0115/p177.html#afp20080115p177-b4. Accessed July11, 2018. [PubMed]
- Shadish WR, Cook TD, Campbell DT Experimental and quasi-experimental designs for generalized causal inference.jr-*"-** fr houghton mifflin company Boston New York; 2002. Available from: https://pdfs.semanticscholar.org/9453/f229a8f51f6a95232e42acfae9b3ae5345df.pdf. Accessed November15, 2018.