Abstract
Background:
Racial inequities for patients with heart failure (HF) have been widely documented. HF patients who receive cardiology care during a hospital admission have better outcomes. It is unknown whether there are differences in admission to a cardiology or general medicine service by race. This study examined the relationship between race and admission service, and its effect on 30-day readmission and mortality
Methods:
We performed a retrospective cohort study from 9/2008 to 11/2017 at a single large urban large urban academic referral center of all patients self-referred to the emergency department (ED) and admitted to either the cardiology or general medicine service with a principal diagnosis of HF, who self-identified as white, black, or Latinx. We used multivariable generalized estimating equation models to assess the relationship between race and admission to the cardiology service. We used Cox regression to assess the association between race, admission service and 30-day readmission and mortality.
Results:
Among 1,967 unique patients (66.7% white, 23.6% black, and 9.7% Latinx), black and Latinx patients had lower rates of admission to the cardiology service than white patients (adjusted rate ratio [ARR] 0.91, 95% CI 0.84-0.98, for black; ARR 0.83, 95% CI 0.72-0.97 for Latinx). Female sex and age>75 years were also independently associated with lower rates of admission to the cardiology service. Admission to the cardiology service was independently associated with decreased readmission within 30 days, independent of race.
Conclusions:
Black and Latinx patients were less likely to be admitted to cardiology for HF care. This inequity may, in part, drive racial inequities in HF outcomes.
Inequitable quality of healthcare and access to healthcare by race is a well-documented phenomenon in the United States (US).1 This is, in part, driven by the differential access to the goods, services, and opportunities of society by race, which has been termed structural racism.2 Structural racism in the US is a major impediment to achieving health equity - the opportunity for all people to achieve their full health potential.3 Health disparities are differences in health outcomes between groups within a population, whereas health inequities are differences in health outcomes that are systematic, avoidable, and unjust.4 Racial inequities in mortality and readmission rates for patients with heart failure (HF) have been widely documented.5-10 Some studies suggest that racial inequities in HF care are driven by between-hospital quality differences in the setting of de facto health facility segregation for minority patients,11-16 although there is recent evidence that these differences are derived from a systemic, rather than hospital-specific, effect.17
At our institution, patients admitted with HF may be primarily cared for by a hospitalist (on the general medicine service [GMS]) or cardiologist (on the cardiology service). Patients on GMS may receive a cardiology consult, but this is uncommon at our institution. Observational studies have found that patients receiving specialty cardiology care during an admission for HF have superior outcomes, including lower readmission rates and mortality.18-23 This beneficial outcome may result from a combination of access to cardiology expertise and improved care, and additional supports found on cardiology services (e.g. specialty nursing, pharmacy, post-discharge services). We hypothesized that at our institution there was inequitable access to the cardiology service for patients admitted with HF, and that this inequity could contribute to the previously-mentioned racial inequities in HF outcomes. To address this hypothesis, we performed a single-center retrospective cohort study of patients admitted with a principal diagnosis of HF over a ten-year period to evaluate the relationship between race and admission service assignment, as well as the subsequent relationship between admission service and outcomes.
Methods
Datasets and statistical analysis code are available from the corresponding author upon reasonable request.
Approach
This analysis was one of the first projects undertaken by our institution’s Department of Medicine Health Equity Committee, a multidisciplinary group formed in 2017 to identify and address health equity concerns. The committee chose to focus on patients admitted with HF because this is the most frequent medical admission diagnosis at our institution, the second most common reason for hospital admission in the US among older adults,24 and a condition for which there are known racial inequities in outcomes. The committee also wished to test a hypothesis that leading with a racial equity analysis would uncover additional structural inequities, which could be addressed intersectionally.25 This study was guided by Public Health Critical Race Praxis (PHCRP), an approach utilized by researchers to study and ameliorate instances of structural racism and resultant health inequities and developed out of the legal framework of Critical Race Theory. 26, 27 We considered race to be a social construct that captures the impacts of racism rather than innate biological differences, and, therefore, hypothesized that differences in HF outcomes were due to structural drivers rather than biological causes.2
Study Population and Data Source
We conducted a retrospective cohort study at an urban tertiary care academic hospital with large general medicine and cardiology services. We identified all patients self-referred to the emergency department and admitted with a principal diagnosis of HF to either the general medicine or cardiology service from September 2008 to November 2017.
Self-reported racial identity was our primary exposure and was extracted from the electronic medical record (EMR). We included all black, white, and Latinx patients. Latinx is a gender-neutral term describing a person of Latin American origin or descent. 28 Our EMR did not allow us to distinguish comprehensively between Latinx white and Latinx non-white patients. We excluded patients with other racial identifiers owing to limited numbers (N=131).
We extracted additional covariates from the EMR at the time of admission: sex, age, date of admission, Medicaid beneficiary/insurance status, address, primary language, number of days since the last outpatient appointment with a cardiologist or primary care physician (PCP) at our institution, whether the patient was seen in follow-up at a cardiology clinic at our institution within thirty days of discharge (EMR encounter during which the patient attended a visit at one the institution’s cardiology clinics), most recent prior admitting service, and readmission and mortality within 30 days after discharge. Post-discharge deaths were identified from a combination of our institutional EMR and the Massachusetts Death Registry. We used ICD codes recorded in the EMR at the time of admission to determine the presence of the following conditions: HF with preserved ejection fraction (HFpEF), chronic pulmonary disease, valvular heart disease, cardiac arrhythmia, hypertension, diabetes, cancer, chronic kidney disease (CKD), end-stage renal disease (ESRD), liver disease, history of substance use, and psychiatric illness. As an overall measure of comorbidity, we calculated the Elixhauser Comorbidity Index (ECI), considering both overall ECI as well as ECI divided into two components - cardiovascular and non-cardiovascular. As a measure of socioeconomic disadvantage, we determined the 2013 Area Deprivation Index (ADI) for each patient’s census block group.29 The ADI is a validated composite index (reported as a national percentile) that uses 17 neighborhood-level indicators for poverty, education, housing, and employment. We considered patients to be Boston Metro residents if they resided in one of 510 zip codes as defined by the US Census Bureau.30 We abstracted the chief complaints of the patients upon arrival to the ED from the EMR, which were only available for admissions from 2010–2015.
Ethical approval was obtained from the Partner’s Healthcare Institutional Review Board.
Statistical Analysis
We presented categorical data using proportions and continuous data using medians (interquartile range). We made statistical comparisons of proportional variables using Chi-squared tests and continuous variables using Student’s t-tests. To assess the relationship between race and admission to the cardiology service, we used generalized estimating equations (GEE) models with an exchangeable correlation structure to account for multiple HF admissions by patients during the study period. We used a Poisson regression approach with robust standard errors to calculate unadjusted rate ratios (RR) with 95% confidence intervals (CI) for admission to the cardiology service by race.31 We developed a multivariable model for admission to the cardiology service, including race and all covariates correlated with admission to cardiology with p<0.2 in univariable models. We again used a Poisson regression approach with robust standard errors to calculate adjusted rate ratios (ARR) with 95% CI.31 We chose a more lenient p-value of 0.2 for model inclusion to ensure identification and incorporation of variables that could be important confounders.32
If continuous variables met criteria for inclusion in our final model, we evaluated the linearity assumption by dividing them into deciles or quartiles and visually inspecting a plot of log (Odds Ratio) of cardiology admission against category. If the resulting plot was not linear, we considered transformation or categorization, yielding categorization for age, ADI, and date of admission.
We calculated the variance inflation factor (VIF) to assess for multicollinearity among model covariates, with a VIF greater than 2.50 considered indicative of multicollinearity. We tested admission date, sex, and age as interaction terms with race in the final model.
As a secondary analysis, we compared presenting chief complaint by race among the sub-cohort with available chief complaint, and whether the addition of chief complaints correlating with cardiology admission with p<0.2 to the final multivariable model changed our primary findings.
Missing Data
We performed a complete case analysis because there were no missing data for any covariate except ADI (331/3133 admissions, 11%). Rates of missing ADI did not differ by race. As a sensitivity analysis, we performed multiple imputation (N=25) by covariate and outcome data using the fully conditional specification method, and generated pooled effect estimates across datasets.
Propensity Score Matched Cohort Analysis
In addition to our primary analysis we performed a propensity score analysis using each admission as the unit of analysis. We built two multivariable logistic regression models to determine the propensity for a patient to be black and Latinx, each with white race as reference. We included covariates that correlated with race with p<0.25 (c statistics: black 0.85; Latinx 0.88). We excluded English as primary language from the Latinx model because of very high collinearity with Latinx identity. Using a five-to-one-digit “greedy matching” approach with the relevant propensity score,33 we formed two cohorts of matched patients: black patients matched to white patients, and Latinx patients matched to white patients. For each cohort, we calculated RR with 95% CI of admission to cardiology using the McNemar’s test. Using a similar method, we generated a propensity score-matched cohort based on sex (c statistic: 0.70).
Outcomes
We calculated rates of the death and readmission within 30 days of discharge by admission service. We evaluated for differences in the outcome between admission services using Cumulative Incidence Function (CIF) plots and the Gray’s tests, with death considered to be a competing risk for readmission. CIF plots were generated using days from discharge as the unit of time. We repeated this process by race and for admission service stratified by race.
We built multivariable Cox proportional hazards models to determine predictors of (1) readmission and (2) mortality within 30 days. We included race, admission to cardiology service, and all covariates that correlated with the outcome with p<0.2 in univariable models. The proportional hazards assumption was tested by Schoenfeld residuals and inspection of hazard ratio plots. We generated cause-specific hazard ratios (HR) for the models, with death considered to be a competing risk for readmission.
We performed statistical analysis using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). CIF plots were generated using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
There were 7,629 total admissions with a principal diagnosis of HF during the study period. Of these, 3,133 admissions (1,967 unique patients) met criteria for inclusion in our analysis (Figure 1). Table 1 shows the characteristics of the included patients at the time of their first admission during the study period. Nearly three-quarters of included patients were white. White patients were older, more likely to be male, more likely to have been seen in a cardiology clinic within the past year, and lived in neighborhoods with higher percentile ADI. Black and Latinx patients were more likely to have been seen by a PCP at our institution, live in the Boston Metro Area, and be Medicaid beneficiaries. There was no difference in percentage of patients with HFpEF or ECI by race, although white patients were more likely to have valvular heart disease and cancer and black and Latinx patients were more likely to have CKD or ESRD. Of the patients’ first admission during the time period, 874 (67%) of the white patients were admitted to cardiology as compared to 247 (53%) of the black patients and 100 (53%) of the Latinx patients (p<0.0001).
Figure 1.
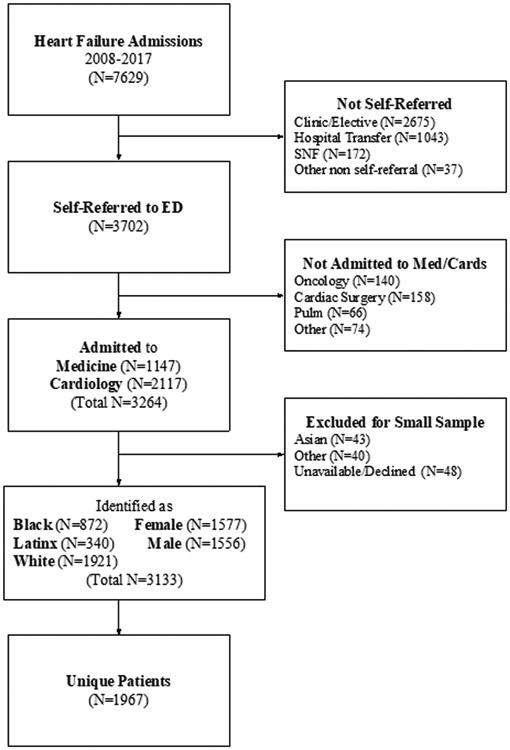
Flow diagram showing selection of study cohort.
Table 1.
Characteristics of people admitted with a principal admission diagnosis of HF for the first time to the general medicine or cardiology service after self-referral to the Emergency Department of the Brigham and Women’s Hospital from 2008-2017 (N=1967*)
| White | Black | Latinx | p-value | |
|---|---|---|---|---|
| n=1312 | n=465 | n=190 | ||
| Age at first admission | 77 (66-84) | 66 (56-76) | 71 (58-79) | <.0001 |
| Female | 601 (46) | 264 (57) | 114 (60) | <.0001 |
| Boston Metro Resident † | 1031 (79) | 440 (95) | 176 (93) | <.0001 |
| English as Primary Language | 1239 (94) | 437 (94) | 54 (28) | <.0001 |
| Medicaid | 54 (4) | 61 (13) | 53 (28) | <.0001 |
| Area Deprivation Index National Percentile, (N=1754) | 13 (7-23) | 30 (21-40) | 36 (22-98) | <.0001 |
| Seen in institutional cardiology clinic in last year | 672 (51) | 188 (40) | 93 (49) | 0.0003 |
| Seen by institutional PCP in last year | 407 (31) | 210 (45) | 95 (50) | <.0001 |
| HFpEF | 464 (35) | 156 (34) | 63 (33) | 0.70 |
| Specific Comorbidity | ||||
| Arrhythmia | 178 (14) | 63 (14) | 15 (8) | 0.088 |
| Valvular Disease | 468 (36) | 111 (24) | 45 (24) | <.0001 |
| Chronic Pulmonary Disease | 363 (28) | 138 (30) | 59 (31) | 0.50 |
| Diabetes | 25 (2) | 7 (2) | 3 (2) | 0.83 |
| Psychiatric disease | 195 (15) | 67 (14) | 30 (16) | 0.90 |
| Cancer | 138 (11) | 39 (8) | 10 (5) | 0.04 |
| Drug Use Disorder | 14 (1) | 10 (2) | 1 (1) | 0.13 |
| Alcohol Use Disorder | 8 (1) | 5 (1) | 2 (1) | 0.54 |
| Hypertension | 940 (72) | 373 (80) | 153 (81) | 0.0002 |
| Chronic Kidney Disease | 523 (40) | 222 (48) | 76 (40) | 0.01 |
| End-Stage Renal Disease | 37 (3) | 47 (10) | 15 (8) | <.0001 |
| Chronic Liver Disease | 56 (4) | 25 (5) | 15 (8) | 0.08 |
| Elixhauser Comorbidity Index | ||||
| Overall | 12 (8-17) | 12 (9-16) | 12 (8-15) | 0.10 |
| Cardiovascular | 7 (7-10) | 7 (7-10) | 7 (7-7) | 0.0005 |
| Non-cardiovascular | 5 (0-8) | 5 (0-8) | 3 (1-6) | 0.78 |
| Year of first admission | ||||
| 2008-2010 | 506 (39) | 177 (38) | 73 (38) | 0.97 |
| 2011-2013 | 479 (36) | 167 (36) | 72 (38) | |
| 2014-2017 | 336 (26) | 121 (26) | 45 (24) | |
| Admitted to cardiology service | 874 (67) | 247 (53) | 100 (53) | <.0001 |
Data are presented as N (%) unless otherwise specified. For continuous variables, median (IQR) are shown.
Unless otherwise noted
Per US Census Bureau
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; PCP, primary care provider
In univariable analysis, black patients had an RR 0.84 (95% CI 0.77–0.91, p<0.0001) and Latinx patients had an RR 0.81 (95% CI 0.72–0.91, p=0.0006) for admission to the cardiology service compared to white patients. Table 2 shows the final multivariable model with all included covariates. Compared to white patients, black patients had an ARR 0.91 (95% CI 0.84–0.98, p=0.019) and Latinx patients had an ARR of 0.83 (95% CI 0.72–0.97, p=0.017) for admission to the cardiology service. Female sex, age over 75 years, chronic pulmonary disease, ESRD, and being seen by a PCP at our institution within the past year were independently associated with admission to GMS. Cardiac valvular disease, arrhythmia, and being seen in a cardiology clinic at our institution within the past year were independently associated with admission to the cardiology service. Results were similar using multiply imputed datasets.
Table 2.
Multivariable Generalized Estimating Equations (GEE) analysis* showing factors associated with admission to the cardiology service for people admitted with a principal diagnosis of HF after self-referral to the Emergency Department of the Brigham and Women’s Hospital from 2008-2017. (Complete case analysis, N=2802; multiply imputed analysis, N=3133).
| Complete Case Analysis | Multiply Imputed Analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Adjusted RR |
95% CI | p-value | Adjusted RR |
95% CI | p-value |
| Race | ||||||
| White | ref | ref | ||||
| Black | 0.91 | 0.84, 0.98 | 0.019 | 0.91 | 0.84, 0.98 | 0.015 |
| Latinx | 0.83 | 0.72, 0.97 | 0.017 | 0.84 | 0.73, 0.96 | 0.012 |
| Age | ||||||
| <50 | ref | ref | ||||
| 50-75 | 0.98 | 0.89, 1.08 | 0.71 | 0.98 | 0.89, 1.08 | 0.66 |
| >75 | 0.85 | 0.77, 0.95 | 0.003 | 0.87 | 0.78, 0.96 | 0.0056 |
| Female | 0.91 | 0.86, 0.97 | 0.003 | 0.90 | 0.85, 0.95 | 0.0003 |
| Boston Metro Resident† | 0.93 | 0.87, 1.00 | 0.056 | 0.95 | 0.89, 1.01 | 0.11 |
| English as primary language | 0.91 | 0.81, 1.01 | 0.085 | 0.93 | 0.84, 1.03 | 0.16 |
| Area Deprivation Index National Percentile‡ | 0.99 | 0.97, 1.01 | 0.48 | 1.00 | 0.98, 1.02 | 0.98 |
| Seen in institutional cardiology clinic in last year | 1.31 | 1.23, 1.39 | <.0001 | 1.32 | 1.25, 1.41 | <.0001 |
| Seen by institutional PCP in last year | 0.88 | 0.82, 0.93 | <.0001 | 0.87 | 0.82, 0.92 | <.0001 |
| HFpEF | 0.93 | 0.87, 0.99 | 0.029 | 0.93 | 0.87, 0.99 | 0.017 |
| Comorbidity | ||||||
| Chronic Pulmonary Disease | 0.85 | 0.80, 0.91 | <.0001 | 0.85 | 0.80, 0.91 | <.0001 |
| Valvular Disease | 1.11 | 1.05, 1.18 | 0.0002 | 1.11 | 1.05, 1.17 | 0.0001 |
| Arrhythmia | 1.14 | 1.03, 1.27 | 0.014 | 1.15 | 1.04, 1.27 | 0.0053 |
| Hypertension | 0.95 | 0.90, 1.01 | 0.08 | 0.95 | 0.90, 1.00 | 0.051 |
| End-Stage Renal Disease | 0.47 | 0.36, 0.61 | <.0001 | 0.47 | 0.37, 0.60 | <.0001 |
| Diabetes | 1.08 | 0.92, 1.27 | 0.34 | 1.05 | 0.90, 1.24 | 0.53 |
| Psychiatric Disease | 0.96 | 0.88, 1.04 | 0.33 | 0.95 | 0.88, 1.03 | 0.20 |
| Elixhauser Index, cardiovascular | 1.00 | 0.98, 1.01 | 0.81 | 1.00 | 0.98, 1.01 | 0.67 |
| Admission Year | ||||||
| 2008-2010 | ref | ref | ||||
| 2011-2013 | 1.17 | 1.09, 1.25 | <.0001 | 1.16 | 1.09, 1.24 | <.0001 |
| 2014-2017 | 1.19 | 1.11, 1.28 | <.0001 | 1.19 | 1.11, 1.28 | <.0001 |
The multivariable model includes race, age, sex, Boston metro residence, primary language, year of admission, being seen in a primary care clinic at our institution within the last year, being seen in cardiology clinic at our institution within the last year, ADI, HFpEF, history of chronic pulmonary disease, cardiac valvular disease, cardiac arrhythmia, hypertension, ESRD, diabetes, psychiatric disease, and the ECI (cardiovascular component)
Per US Census Bureau
Per increase in 10%
Abbreviations: GEE, generalized estimating equations; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; PCP, primary care provider; RR, rate ratio
There was no evidence of multicollinearity in the final model, and no significant interaction between race and admission date, age, or sex.
Chief Complaint Analysis
Data for chief complaint at presentation were available for 1,983 (63%) admissions. There were no differences in chief complaint by race with the exception of chest pain, which was more prevalent in Latinx patients (Supplementary Table 10). Shortness of breath and chest pain correlated with admission to GMS in univariable analyses; adding these to our primary model did not alter our findings (Supplementary Table 11).
Propensity Score Matched Cohorts
Details about the development of the propensity scores are not shown (Supplementary Tables 1-6). All covariates are balanced between the cohort matched by propensity to be black (N=782), propensity to be Latinx (N=336), and propensity to be female (N=1558) with the exception of primary language for the cohort matched by propensity to be Latinx (Supplementary Tables 7-9). ORs for admission to cardiology were consistent with our primary analysis for all matched cohorts (Table 3).
Table 3.
Rate ratios for admission to cardiology for propensity-matched cohorts.
| Rate Ratio of Admission to Cardiology |
95% CI | p-value | |
|---|---|---|---|
| Black vs White | 0.74 | 0.63, 0.87 | 0.0001 |
| Latinx vs White | 0.75 | 0.60, 0.95 | 0.014 |
| Female vs Male | 0.86 | 0.77-0.96 | 0.0055 |
Abbreviations: CI, confidence interval
Outcomes
Figure 2 shows unadjusted CIF plots of readmission and mortality within 30 days by service - overall and stratified by race. There were 38/1092 (3%) patients with HF who died within 30 days of discharge from GMS and 67/2041 (3%) from cardiology (p=0.68). There were 299/1092 (27%) patients with HF readmitted within 30 days from GMS and 507/2041 (25%) from cardiology (p=0.13). After stratification by race, black patients discharged after admission to GMS had higher risk of death within 30 days compared to black patients discharged after admission to cardiology (3% vs <1%, p=0.01), but there were no differences for white (4% vs 4%, p=0.82) or Latinx patients (3% vs 3%, p=0.85). There were no unadjusted differences in readmissions within 30 days for patients discharged after admission to medicine compared to cardiology: overall (27% vs 25%,p=0.13), white (25% vs 23%, p=0.32), black (30% vs 28%, p=0.34), and Latinx (28% vs 30%, p=0.78).
Figure 2.
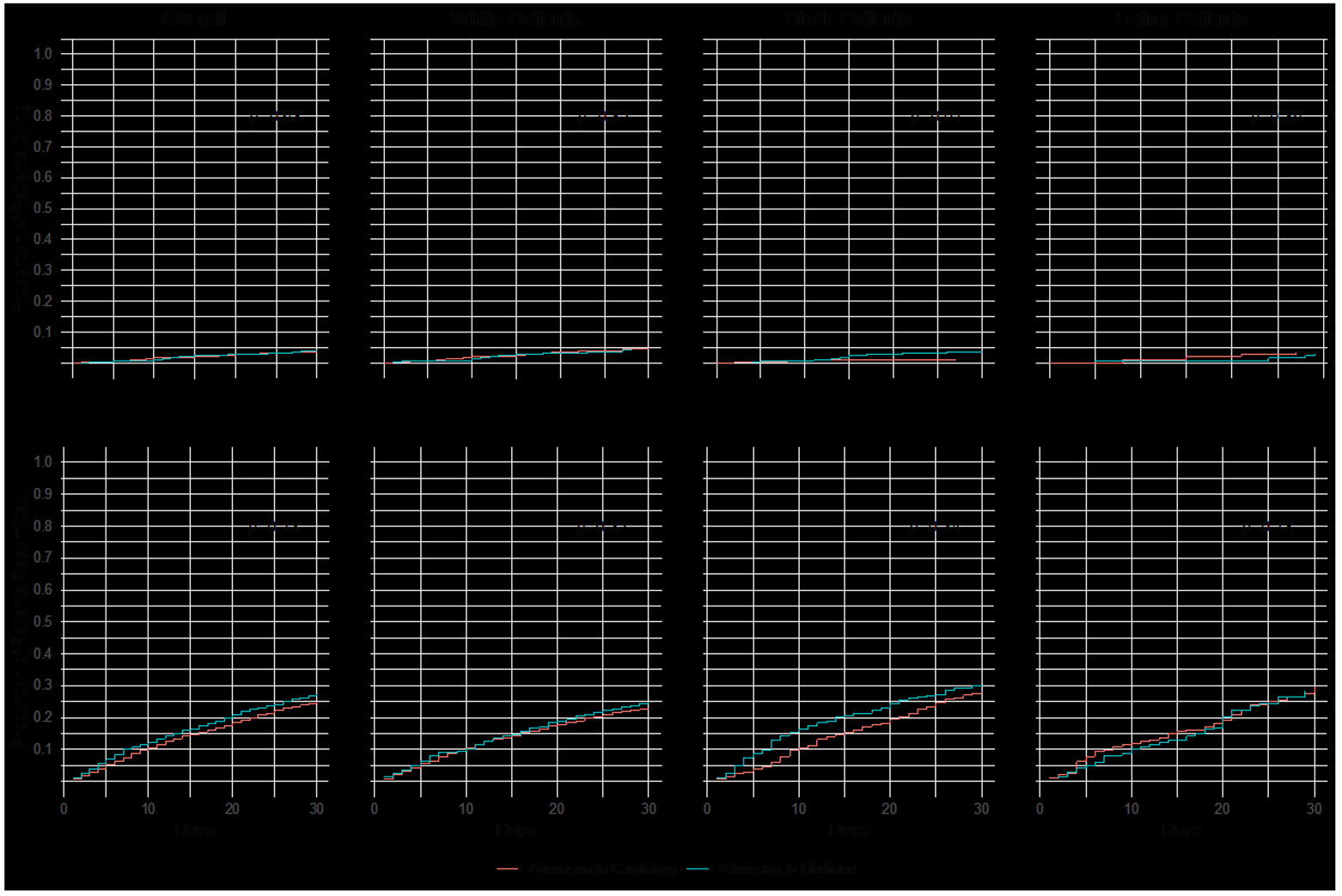
Unadjusted Cumulative Incident Function (CIF) plots with Gray’s tests comparing rates of readmission and death within 30 days after discharge for (A) all patients (B) white patients (C) black patients and (D) Latinx patients.
There were 78/192 (4%) white patients who died within 30 days of discharge compared to 17/872 (2%) black patients and 10/330 (3%) Latinx patients (p=0.0076). There were 452/1921 (26%) white patients who were readmitted within 30 days after discharge compared to 254/872 (29%) black patients and 100/340 (29%) Latinx patients (p=0.0043).
Multivariable cox regression models including admission service, race, and other covariates with p<0.2 in univariate analyses are shown in Supplementary Table 12 for mortality within 30 days and Table 4 for readmission within 30 days. After adjustment, black race was independently associated with a reduced risk of death within 30 days (HR 0.52, 95% CI 0.30–0.91, p=0.02), while there was no significant difference for Latinx patients compared to white patients (HR 0.89, 95% CI 0.46–1.73, p=0.73). Admission to cardiology was not associated with death within 30 days (HR 0.83, 95% CI 0.55–1.24, p=0.36). Admission to cardiology was independently associated with readmission within 30 days (HR 0.84, 95% CI 0.72–0.97, p=0.018), whereas race was not (black vs white HR 1.09, 95% CI 0.92–1.29, p=0.31; Latinx vs white HR 1.14, 95% CI 0.91–1.42,p=0.27)(Table 4).
Table 4.
Multivariable Cox Regression analysis* showing factors associated with readmission within 30 days after discharge for people admitted to the general medical or cardiology services with a principal diagnosis of HF after self-referral to the Emergency Department of the Brigham and Women’s Hospital from 2008-2017. (N=3133)
| Characteristic | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Admission to Cardiology | 0.84 | 0.72, 0.97 | 0.018 |
| Race | |||
| White | ref | ||
| Black | 1.09 | 0.92, 1.29 | 0.31 |
| Latinx | 1.14 | 0.91, 1.42 | 0.27 |
| Age | |||
| <50 | ref | ||
| 50-75 | 0.61 | 0.49, 0.76 | <.0001 |
| >75 | 0.54 | 0.43, 0.69 | <.0001 |
| Boston Metro Resident† | 1.09 | 0.88, 1.35 | 0.43 |
| Seen in institutional cardiology clinic in last year | 1.27 | 1.09, 1.49 | 0.003 |
| Seen by institutional PCP in last year | 1.17 | 1.01, 1.36 | 0.041 |
| HFpEF | 0.81 | 0.70, 0.94 | 0.007 |
| Comorbidity | |||
| Valvular Disease | 1.24 | 1.07, 1.44 | 0.005 |
| Psychiatric Disease | 1.15 | 0.96, 1.38 | 0.14 |
| Chronic Kidney Disease | 1.36 | 1.15, 1.60 | 0.0003 |
| Chronic Liver Disease | 1.11 | 0.80, 1.54 | 0.55 |
| Elixhauser Index | 1.01 | 0.99, 1.02 | 0.33 |
Multivariable model includes admission to cardiology, race, age, Boston metro resident, whether the patient was seen in institutional cardiology clinic in last year, whether the patient was seen by institutional PCP in last year, diagnosis of HFpEF, valvular disease, psychiatric disease, chronic kidney disease, chronic liver disease, and Elixhauser Comorbidity Index.
Per US Census Bureau
Of patients admitted to GMS for their first admission during the study period, 189 (25%) were seen in follow up at a cardiology clinic within thirty days compared to 557 (46%) on the cardiology service (p<.0001). Of 1312 white patients admitted for the first time during the study period, 502 (38%) white patients, were seen in follow up at a cardiology clinic within 30 days compared to 159/465 (34%) black patients and 85/190 (45%) Latinx patients (p=0.04).
Discussion
This retrospective single-center study is one of the first to demonstrate that admission service for patients admitted with HF is an intra-hospital driver of racial inequities in HF outcomes. Despite adjustment for neighborhood disadvantage, comorbidity, diagnosis of HFpEF, and having seen a cardiologist or PCP at our institution within the past year, black and Latinx patients admitted with HF remained significantly more likely to be admitted to GMS compared to white patients. Admission to GMS was independently associated with higher rates of readmission within 30 days, and there was no difference in mortality by admission service after multivariable adjustment. Taken together, our findings suggest that racial inequities in admission patterns may contribute, in part, to the well-documented racial inequities in heart failure readmissions in the U.S.11-16
Across the US, HF readmission rates are higher for black and Latinx patients compared to white patients.11-16 Specialty cardiology care for patients admitted to the hospital with HF is associated with better outcomes, including lower readmission rates and improved mortality.18-23, 34 In this study, black and Latinx patients admitted with HF were less likely than white patients to be admitted to the cardiology service. This pattern—decreased likelihood of receiving specialty care in the hospital with lower rates of subsequent outpatient follow up and higher 30-day readmission rates on GMS—precipitates a cycle in which inequities are compounded. Our findings are consistent with a recent study of patients with HF admitted to an intensive care unit, which found that black patients were less likely than whites to receive cardiology consultation.35 The higher readmission rates for HF patients on GMS identified in this study suggest that inequities in admission service could be one driver for higher admissions among black patients in the US.10 We also found that black race was independently associated with lower 30-day mortality, a finding noted in other US settings and of unclear significance.5 Regardless, more focus should be given to differences in access to specialized care within institutions as a potential root cause of racial inequities in HF readmissions.
We found that having seen an outpatient cardiologist at our institution was the strongest positive predictor of admission to cardiology, and there were significant differences in the proportion of patients who had seen a cardiologist within the past year by race. It is possible that our outpatient facilities are less accessible to black and Latinx patients. Prior studies have shown racial differences in referral patterns to outpatient specialty cardiology care for patients with HF.36 We found that patients with HF admitted to GMS were less likely to be seen by a cardiologist for outpatient follow-up than those admitted to the cardiology service, consistent with a prior study.34 While we did not identify differential rates of outpatient cardiology follow up by race, large differences in cardiology follow up by admission service combined with a greater probability of black and Latinx patients being admitted to GMS are likely to perpetuate inequities. Because patients with HF tend to be admitted repeatedly, improving rates of cardiology referral and designing programs to reduce barriers for patients to see a cardiologist after discharge from GMS may both improve care for these patients and reduce inequities in subsequent admission service assignment for HF.
At our institution, the admission service for a patient with HF is determined in collaboration between the attending emergency medicine physician and the on-call cardiologist and/or general medicine hospitalist. We hypothesize that a number of factors could influence admission service decisions for patients with HF, including perceived clinical uncertainty or complexity; active co-morbid conditions; bed availability; prior admission service; whether the patient has been followed previously by a cardiologist at our hospital; advocacy by outpatient provider(s); patient preference and self-advocacy; and provider bias.
Provider bias against minority patients is pervasive,37-40 and may have been a factor in admission decisions for patients with HF at our institution. For example, while complex social or psychiatric histories are often thought of as reasons to admit to GMS preferentially, we found no difference in alcohol, drug use, or psychiatric disease by race. A perceived importance of these factors for admission service may reflect providers’ beliefs that patients of color have higher likelihood of risky behavior and worse adherence to medical advice.41Patient self-advocacy might also impact admission decisions if white patients more frequently advocate for admission to the cardiology service. Racial differences in self-advocacy have been previously observed, and can be understood in the context of historical and ongoing discrimination against black and brown people in the US healthcare system, with patient-provider interactions involving patients of color frequently characterized by fewer requests for patient input about treatment decisions and less patient-centered care.38, 42
Our institution frequently operates at near-maximal census, pressuring the admission service decision. Depending on the distribution of available hospital beds, patients with uncomplicated HF exacerbations are sometimes preferentially admitted to general medicine. This may exacerbate racial inequities in admission service, particularly if there is a racial difference in prior admitting service or perceived medical complexity.40, 43
While our objective was to explore the presence of racial inequities in HF admission decisions, we also found evidence of differential admission decisions based on age and sex. Implicit gender bias has been shown to affect clinical decision-making in cardiovascular disease, with providers rating cardiovascular testing of higher utility for males versus females.44 Similarly, a recent study found that while women and men have similar symptoms when presenting with acute myocardial infarction, providers are less likely to attribute women’s symptoms to heart disease compared to men.45 It is plausible that gender bias similarly explains decreased access to inpatient cardiology care for patients with heart failure.
Our study is limited by its observational nature, and unmeasured confounders are likely present. For example, we could not account specifically for the severity of heart failure or variability in clinician practice – these should be addressed in future work. Given that the ECI is derived from ICD coding, it is possible that this measure is less accurate for patients with less engagement with our healthcare system, or in general, because of undercoding. The ECI also might not accurately reflect secondary active admission diagnoses that could drive admission to GMS. While there is integration between our inpatient and outpatient EMR, we were unable to evaluate the impact of having a cardiologist or PCP outside of our system on admission decisions, and our readmission rates only reflect readmissions to our institution. Follow-up rates only reflect cardiology-specific visits as data regarding overall rates of post-discharge follow-up were unavailable. There were few deaths overall within our cohort, and, thus, findings around 30-day mortality should be interpreted with caution. We were unable to evaluate for differential cardiology consultation on the medical service based on race, which has been previously demonstrated to play an important role in the intensive care setting.35 Although this is a single-center study at a high-volume quaternary referral center, we suspect the identified inequities are not unique to our institution.
This analysis reflects our institution’s strong tradition of self-reflection and transparency to make care better for all our patients. We are using these findings to inform further studies, including detailed chart reviews and surveys of patients’ and providers’ experience of the admission decision process to better understand better the observed inequities and their consequences. We are utilizing a PHCRP framework to devise appropriate interventions to address these findings.27 By assuming the existence of institutional racism across all American institutions, we can turn from research focused on documenting disparities and inequities to implementation research directed towards correcting them whilst ensuring that institutions like ours are accountable to the communities they serve. Strategies under discussion include strengthened standardized admission guidelines or decision tools, racial equity training for clinicians, standardization of HF care on GMS to ensure high-quality treatment, and methods to ensure higher rates of cardiology follow-up after discharge. It is likely that these interventions and more will be needed to address the inequities described in this study. Ongoing institutional insistence on self-critique and recognition of the pervasiveness of structural racism and bias will increase the likelihood of success in achieving health equity at all US institutions.
Supplementary Material
WHAT IS NEW?
We retrospectively analyzed 10 years of HF admissions using multivariable models. We demonstrated that black or Latinx race were independently associated with decreased likelihood of admission to the cardiology service for HF care.
Female gender and older age were also independently associated with lower likelihood of admission to the cardiology service.
Admission to the cardiology service was independently associated with lower readmission rates.
WHAT ARE THE CLINICAL IMPLICATIONS?
Our analysis demonstrates the presence of structural racism in admission service for HF patients, as well as important inequities based on gender and age. Differential access to cardiology for admission service is an important driver of inequities in readmission rates.
Our findings suggest that differential access to specialty care within institutions may be an important driver of health inequities. Future disparities research in other clinical realms should explore inequities in access to subspecialty care as a causal driver.
Acknowledgments
We acknowledge the Department of Medicine Health Equity Committee’s partners in health equity work: The Southern Jamaica Plain Health Center and Institute for Healthcare Improvement (IHI). We are thankful to our patients with heart failure and the leadership of the Brigham and Women’s Hospital Department of Medicine, Division of Cardiology, and Department of Emergency Medicine for their commitment to health equity.
Sources of Funding: None
Non-standard Abbreviations and Acronyms
- ADI
Area Deprivation Index
- CIF
Cumulative Incidence Function
- ECI
Elixhauser Comorbidity Index
- EMR
electronic medical record
- GEE
generalized estimating equations
- GMS
general medicine service
- PCP
Primary care physician
- PHCRP
Public Health Critical Race Praxis
- VIF
Variance inflation factor
Appendix:
2017-2018 Brigham and Women’s Hospital Internal Medicine Housestaff include the following contributors: Abel, Samantha; Adams, Ayrenne; Anaya, Joseph; Andrews, Erik H; Atkinson, Benjamin; Avutu, Viswatej; Bachorik, Alexandra; Badri, Omar; Bailey, Mariel; Baird, Katie; Bakshi, Salina; Balaban, Denis; Barshop, Kenneth; Baumrin, Emily; Bayomy, Omar; Beamesderfer, Julia; Becker, Nora; Berg, David D., Berman, Adam N.; Blum, Steven M.; Boardman, Alexander P.; Boden, Kaeleen; Bonacci, Robert A; Brown, Sarah; Campbell, Kirsti; Case, Siobhan; Cetrone, Emily; Charrow, Alexandra; Chiang, David; Clark, Devin; Cohen, Aaron J.; Cooper, Alissa; Cordova, Tomas; Cuneo, C. Nicholas; de Feria Alsina Alejandro; Deffenbacher, Karen; DeFilippis, Ersilia M.; DeGregorio, Geneva; Deutsch, Aaron J.; Diephuis, Bradford; Divakaran, Sanjay; Dorschner, Peter; Downing, Nicholas; Drescher, Caitlin; D'Silva, Kristin M.; Dunbar, Peter; Duong, David; Earp, Sarah; Eckhardt, Christine; Elman, Scott A.; England, Ross; Everett, Kay; Fedotova, Natalie; Feingold-Link, Tamara; Ferreira, Mark; Fisher, Herrick; Foo, Patricia; Foote, Michael; Franco, Idalid; Gilliland, Thomas; Greb, Jacqueline; Greco, Katherine; Grewal, Sungat; Grin, Benjamin; Growdon, Matthew E.; Guercio, Brendan; Hahn, Cynthia K.; Hasselfeld, Brian; Haydu, Erika J; Hermes, Zachary; Hildick-Smith, Gordon; Holcomb, Zachary; Holroyd, Kathryn; Horton, Laura; Huang, George; Jablonski, Stanley; Jacobs, Douglas; Jain, Nina; Japa, Sohan; Joseph, Richard; Kalashnikova, Mariya; Kalwani, Neil; Kang, Daniel; Karan, Abraar; Katz, Joel T; Kellner, Daniel; Kidia, Khameer; Kim, June-Ho; Knowles, Scott M; Kolbe, Laura; Kore, Idil; Koullias, Yiannis; Kuye, Ifedayo; Lang, Joshua; Lawlor, Matthew; Lechner, Melissa G.; Lee, Ken; Lee, Scott; Lee, Zachary; Limaye, Neha; Lin-Beckford, Stephanie; Lipsyc, Marla; Little, Jessica; Loewenthal, Julia; Logaraj, Ranjani; Lopez, Diana M; Loriaux, Daniel; Lu, Yi; Ma, Kevin; Marukian, Nareh; Matias, Wilfredo; Mayers, Jared R.; McConnell, Ian; McLaughlin, Michael; Meade, Christina; Meador, Catherine; Mehta, Anish; Messenger, Elizabeth; Michaelidis, Constantinos; Mirsky, Jacob; Mitten, Emilie; Mueller, Alisa; Mullur, Jyotsna; Munir, Amir; Murphy, Emily; Nagami, Ellen; Natarajan, Abirami; Nsahlai, Michelle; Nze, Chijioke; Okwara, Noreen; Olds, Peter; Paez, Rafael; Pardo, Michael; Patel, Siddharth; Petersen, Alec; Phelan, Laura; Pimenta, Erica; Pipilas, Daniel; Plovanich, Molly; Pong, Denise; Powers, Brian W.; Rao, Anita; Ramirez Batlle Haiyan; Ramsis, Mattheus; Reichardt, Anna; Reiger, Sheridan; Rengarajan, Michelle; Rico, Stephanie; Rome, Benjamin N.; Rosales, Rachael; Rotenstein, Lisa; Roy, Alexis; Royston, Sarah; Rozansky, Hallie; Rudder, Meghan; Ryan, Christine E.; Salgado, Sanjay; Sanchez, Pablo;Schulte, Jennifer; Sekar, Aswin; Semenkovich, Nicholas; Shannon, Evan; Shaw, Neil; Shorten, Andrew Ben; Shrauner, William; Sinnenberg, Lauren; Smithy, James W.; Snyder, Gregory; Sreekrishnan, Anirudh; Stabenau, Hans; Stavrou, Eleni; Stergachis, Andrew; Stern, Robert; Stone, Alexander; Tabrizi, Shervin; Tanyos, Sam; Thomas, Cristina; Thun, Haley; Torres-Lockhart Kristine; Tran, An; Treasure, Carolyn; Tsai, Frederick D.; Tsaur, Stephen; Tschirhart, Evan; Tuwatananurak, Justin; Venkateswaran, Ramkumar V.; Vishnevetsky, Anastasia; Wahl, Lindsay; Wall, April; Wallace, Frances; Walsh, Elisa; Wang, Priscilla; Ward, Heather B.; Warner, Lindsay N.; Weeks, Lachelle D.; Weiskopf, Kipp; Wengrod, Jordan; Williams, Jessica N.; Winkler, Marisa; Wong, Jeffrey L.; Worster, Devin; Wright, Aileen; Wunsch, Caroline; Wynter, Jamila S.; Yarbrough, Chase; Yau, Wai-Ying; Yazdi, Daniel; Yeh, Jennifer; Yialamas, Maria A.; Yozamp, Nicholas; Zambrotta, Marina; Zon, Rebecca.
Footnotes
Disclosures: None
References
- 1.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Jones CP. Levels of racism: a theoretic framework and a gardener's tale. American journal of public health. 2000;90:1212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead M and Dahlgren G. Concepts and Principles for Tackling Social Inequities in Health: Levelling up, Part 1. 2006. [Google Scholar]
- 4.Wyatt R, Laderman M, Botwinick L, Mate K and Whittington J. Achieving Health Equity: A Guide for Health Care Organizations. IHI White Paper. IHI White Paper. 2016. [Google Scholar]
- 5.Durstenfeld MS, Ogedegbe O, Katz SD, Park H and Blecker S. Racial and Ethnic Differences in Heart Failure Readmissions and Mortality in a Large Municipal Healthcare System. JACC Heart failure. 2016;4:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, Wolfe P, Havranek EP, Ordin DL and Krumholz HM. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. Jama. 2003;289:2517–24. [DOI] [PubMed] [Google Scholar]
- 7.Alexander M, Grumbach K, Remy L, Rowell R and Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. American heart journal. 1999;137:919–27. [DOI] [PubMed] [Google Scholar]
- 8.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ and Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. [DOI] [PubMed] [Google Scholar]
- 9.Qian F, Parzynski CS, Chaudhry SI, Hannan EL, Shaw BA, Spertus JA and Krumholz HM. Racial Differences in Heart Failure Outcomes: Evidence From the Tele-HF Trial (Telemonitoring to Improve Heart Failure Outcomes). JACC Heart failure. 2015;3:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis EF, Claggett B, Shah AM, Liu J, Shah SJ, Anand I, O'Meara E, Sweitzer NK, Rouleau JL, Fang JC, Desai AS, Retta TM, Solomon SD, Heitner JF, Stamos TD, Boineau R, Pitt B and Pfeffer MA. Racial Differences in Characteristics and Outcomes of Patients With Heart Failure and Preserved Ejection Fraction in the Treatment of Preserved Cardiac Function Heart Failure Trial. Circulation Heart failure. 2018;11:e004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha AK, Orav EJ, Li Z and Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Archives of internal medicine. 2007;167:1177–82. [DOI] [PubMed] [Google Scholar]
- 12.Shen JJ, Washington EL, Chung K and Bell R. Factors underlying racial disparities in hospital care of congestive heart failure. Ethn Dis. 2007;17:206–13. [PubMed] [Google Scholar]
- 13.Trivedi AN, Nsa W, Hausmann LR, Lee JS, Ma A, Bratzler DW, Mor MK, Baus K, Larbi F and Fine MJ. Quality and equity of care in U.S. hospitals. N Engl J Med. 2014;371:2298–308. [DOI] [PubMed] [Google Scholar]
- 14.Hasnain-Wynia R, Baker DW, Nerenz D, Feinglass J, Beal AC, Landrum MB, Behal R and Weissman JS. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Archives of internal medicine. 2007;167:1233–9. [DOI] [PubMed] [Google Scholar]
- 15.Hasnain-Wynia R, Kang R, Landrum MB, Vogeli C, Baker DW and Weissman JS. Racial and ethnic disparities within and between hospitals for inpatient quality of care: an examination of patient-level Hospital Quality Alliance measures. Journal of health care for the poor and underserved. 2010;21:629–48. [DOI] [PubMed] [Google Scholar]
- 16.Jha AK, Orav EJ, Zheng J and Epstein AM. The characteristics and performance of hospitals that care for elderly Hispanic Americans. Health affairs (Project Hope). 2008;27:528–37. [DOI] [PubMed] [Google Scholar]
- 17.Downing NS, Wang C, Gupta A and et al. Association of racial and socioeconomic disparities with outcomes among patients hospitalized with acute myocardial infarction, heart failure, and pneumonia: An analysis of within- and between-hospital variation. JAMA Network Open. 2018;1:e182044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters J, Morton G, Anton I, Szymanski J, Greenwood E, Grogono J, Flett AS, Cleland JG and Cowburn PJ. Specialist intervention is associated with improved patient outcomes in patients with decompensated heart failure: evaluation of the impact of a multidisciplinary inpatient heart failure team. Open heart. 2017;4:e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jong P, Gong Y, Liu PP, Austin PC, Lee DS and Tu JV. Care and outcomes of patients newly hospitalized for heart failure in the community treated by cardiologists compared with other specialists. Circulation. 2003;108:184–91. [DOI] [PubMed] [Google Scholar]
- 20.Foody JM, Rathore SS, Wang Y, Herrin J, Masoudi FA, Havranek EP and Krumholz HM. Physician specialty and mortality among elderly patients hospitalized with heart failure. The American journal of medicine. 2005;118:1120–5. [DOI] [PubMed] [Google Scholar]
- 21.Salata BM, Sterling MR, Beecy AN, Ullal AV, Jones EC, Horn EM and Goyal P. Discharge Processes and 30-Day Readmission Rates of Patients Hospitalized for Heart Failure on General Medicine and Cardiology Services. The American journal of cardiology. 2018;121:1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uthamalingam S, Kandala J, Selvaraj V, Martin W, Daley M, Patvardhan E, Capodilupo R, Moore S and Januzzi JL Jr., Outcomes of patients with acute decompensated heart failure managed by cardiologists versus noncardiologists. The American journal of cardiology. 2015;115:466–71. [DOI] [PubMed] [Google Scholar]
- 23.Selim AM, Mazurek JA, Iqbal M, Wang D, Negassa A and Zolty R. Mortality and readmission rates in patients hospitalized for acute decompensated heart failure: a comparison between cardiology and general-medicine service outcomes in an underserved population. Clinical cardiology. 2015;38:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS and Muntner P. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crenshaw K Mapping the Margins: Intersectionality, Identity Politics, and Violence against Women of Color. Stanford Law Review. 1991;43:1241–1299. [Google Scholar]
- 26.Ford CL and Airhihenbuwa CO. Commentary: Just What is Critical Race Theory and What's it Doing in a Progressive Field like Public Health? Ethn Dis. 2018;28:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford CL and Airhihenbuwa CO. Critical Race Theory, race equity, and public health: toward antiracism praxis. American journal of public health. 2010;100 Suppl 1:S30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGuzmán M Latinx: ¡Estamos aquí!, or Being “Latinx” at UNC-Chapel Hill. Cultural CanDynamics. 2017;29:214–230. [Google Scholar]
- 29.University of Wisconsin School of Medicine and Public Health. Area Deprivation Index. URL: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed 5/1/2018.
- 30.US Census Bureau. 2010. Census. URL: https://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed 5/1/2018.
- 31.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 32.Mickey RM and Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989;129:125–37. [DOI] [PubMed] [Google Scholar]
- 33.Parsons S Reducing Bias in a Propensity Score Matched-Pair Sample Using Greedy Matching Techniques. The Twenty-Sixth Annual SAS Users Group International Conference 2001:214–26. [Google Scholar]
- 34.Drescher CS, Britton KA, Stevenson LW and Desai AS. Clinical Outcomes during Generalist Vs. Subspecialty Care of Inpatients with Heart Failure and Preserved Ejection Fraction. Journal of Cardiac Failure. 2017;23:S23. [Google Scholar]
- 35.Breathett K, Liu WG, Allen LA, Daugherty SL, Blair IV, Jones J, Grunwald GK, Moss M, Kiser TH, Burnham E, Vandivier RW, Clark BJ, Lewis EF, Mazimba S, Battaglia C, Ho PM and Peterson PN. African Americans Are Less Likely to Receive Care by a Cardiologist During an Intensive Care Unit Admission for Heart Failure. JACC Heart failure. 2018;6:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NL, Ayanian JZ, Orav EJ and Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119:2463–70. [DOI] [PubMed] [Google Scholar]
- 37.Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, Eng E, Day SH and Coyne-Beasley T. Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. American journal of public health. 2015;105:e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehon E, Weiss N, Jones J, Faulconer W, Hinton E and Sterling S. A Systematic Review of the Impact of Physician Implicit Racial Bias on Clinical Decision Making. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2017;24:895–904. [DOI] [PubMed] [Google Scholar]
- 39.Finucane TE and Carrese JA. Racial bias in presentation of cases. J Gen Intern Med. 1990;5:120–1. [DOI] [PubMed] [Google Scholar]
- 40.Schrader CD and Lewis LM. Racial disparity in emergency department triage. The Journal of emergency medicine. 2013;44:511–8. [DOI] [PubMed] [Google Scholar]
- 41.van Ryn M and Burke J. The effect of patient race and socio-economic status on physicians' perceptions of patients. Social science & medicine (1982). 2000;50:813–28. [DOI] [PubMed] [Google Scholar]
- 42.Wiltshire J, Cronin K, Sarto GE and Brown R. Self-advocacy during the medical encounter: use of health information and racial/ethnic differences. Medical care. 2006;44:100–9. [DOI] [PubMed] [Google Scholar]
- 43.Johnson TJ, Hickey RW, Switzer GE, Miller E, Winger DG, Nguyen M, Saladino RA and Hausmann LR. The Impact of Cognitive Stressors in the Emergency Department on Physician Implicit Racial Bias. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2016;23:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daugherty SL, Blair IV, Havranek EP, Furniss A, Dickinson LM, Karimkhani E, Main DS and Masoudi FA. Implicit Gender Bias and the Use of Cardiovascular Tests Among Cardiologists. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichtman JH, Leifheit EC, Safdar B, Bao H, Krumholz HM, Lorenze NP, Daneshvar M, Spertus JA and D'Onofrio G. Sex Differences in the Presentation and Perception of Symptoms Among Young Patients With Myocardial Infarction: Evidence from the VIRGO Study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients). Circulation. 2018;137:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


