Abstract
The goal of this investigation was to evaluate the physiologic stresses of powered air-purifying respirators (PAPRs) used by workers in many industries (e.g., health care, automobile repair, public safety, building trades, etc.) during rest and three levels of energy expenditure. Twelve men and twelve women wore one tight-fitting and three loose-fitting PAPRs at rest (REST) and while walking for four minutes at oxygen consumption (V̇O2) rates of 1.0 l·min−1(LOW), 2.0 l·min−1 (MODERATE), and 3.0 l·min−1 or maximum (HIGH). Minimum inhaled carbon dioxide concentration (FICO2), maximum inhaled oxygen concentration (FIO2), peak inhalation pressure, and end inhalation temperature were measured continuously breath-by-breath. Repeated measures analysis of variance found that neither the main effect of gender, nor any interactions involving gender were significant. The highest minimum FICO2 among PAPRs occurred for MODERATE and HIGH energy expenditures while wearing the loose-fitting PAPR with the largest dead space. The lowest maximum FIO2 was observed during HIGH intensity energy expenditure also for the loose-fitting PAPR with the largest dead space. Among all PAPR models, peak inhalation pressures were negative at V̇O2 > LOW, suggesting that peak inhalation flow was greater than blower flow. Results using the variables reported here suggest that PAPRs used at various levels of energy expenditure may be tolerated among healthy workers. Further research is needed to determine the source of supplemented air when inhalation flow exceeds blower flow.
Keywords: PAPR, respiratory protection, inhaled gas, peak pressure, overbreathing, inhaled temperature, personal protective equipment
INTRODUCTION
Powered air-purifying respirators (PAPRs) use a battery-powered fan to draw ambient air through a filter and direct the filtered air into the breathing zone. The breathing zone is formed by a loose-fitting hood or helmet, or by a tight-fitting face mask. The airflow provided by a PAPR’s blower can be constant or variable, but must be at least 115 l·min−1 (tight-fitting) or 170 l·min−1 (loose-fitting) to be approved by the National Institute for Occupational Safety and Health (NIOSH) (Approval of Respiratory Protective Devices, 2016b). PAPRs were originally developed in the 1960s to protect various workers from airborne workplace and dermal hazards. These respirators are worn by workers in the agricultural, mining, construction, manufacturing, transportation and public utilities, wholesale trade, retail trade, and services industries (Bureau of Labor Statistics and the National Institute for Occupational Safety & Health, 2003). Although the exact number of workers who wear PAPRs is unknown, it is generally agreed that PAPR use is increasing (Wizner, et al., 2016). A health care market research report estimated that the sales of PAPRs increased from 131,387 in 2011 to 214,171 in 2012 (ASTHO, 2014). Workers in the healthcare industry are trained to use PAPRs while performing aerosol-generating procedures on patients with certain infectious diseases, treating patients posing a risk of airborne infection, and administering certain hazardous aerosolized medications (IOM, 2015).
Reasons for selecting loose-fitting PAPRs include fit testing issues (multiple respirator models in stock to satisfy a workforce of diverse cultures with various face shapes, facial hair, facial jewelry, anatomical deformities and normal variants, scarring, convenience, etc.), personal preference, comfort, and additional time and cost by hospital staff to perform Occupational Safety and Health Administration (OSHA) mandated fit testing of N95 filtering facepiece respirators, or FFRs (IOM, 2015). Every PAPR hood has an integral face shield which provides both respiratory protection and face and eye protection against bodily fluids over separated respirators and face shields. Factors that may favor using N95 FFRs include unfiltered discharged user air into a sterile environment (e.g., during surgery), interference with vulnerable external connections (hoses, blowers, and filters), the inability of the PAPR to remain in place with different work postures, and challenges with disinfecting external parts as workers move from patient to patient (IOM, 2015). Tight-fitting PAPRs require fit testing, thus the user must be clean shaven and pass a quantitative fit testing protocol with a minimum fit factor (Personal Protective Equipment, 2016).
Several occupations require respiratory protection devices (RPDs) in addition to other forms of personal protective equipment. The compatibility of RPDs with hard hats, welding helmets or ear muffs may be challenging (Cuta, 2015). Some PAPR systems have combined respiratory protection with head protection, eye protection, and hearing protection. With improved versatility by these PAPR systems, employers found the opportunities provided by PAPRs improved user comfort and compatibility with lower downtime, less stock to maintain, and improved productivity.
Little research is available on the inhaled breathing gas concentrations while wearing respiratory protection at specific energy expenditures. It is important to understand the ability of PAPRs to sustain safe inhaled oxygen (O2) and carbon dioxide (CO2) concentrations during rest (low tidal volumes) and exercise (larger inhalation pressures) with the PAPRs’ unique designs and sometimes large dead space. Elevated inhaled CO2 concentrations of 1.5% to 3% can cause headaches, increased minute ventilation and respiratory acidosis (NIOSH, 1976). Symptoms of breathing elevated inhaled CO2 may include changes in visual performance (Yang, et al, 1997), modified energy endurance and dyspnea (Raven, et al, 1979). The threshold for the NIOSH Ceiling of CO2 is 3% by volume. The NIOSH Ceiling is used to describe occupational exposures that must not be exceeded through any part of the workday (American Conference of Governmental Industrial Hygienists, 2016). Previous testing of 11 PAPRs (two full-facepiece, two half-mask, four hoods and three helmets) with the Automated Breathing and Metabolic Simulator (ABMS) demonstrated increased inhaled CO2 concentrations that suggest the need for further investigation (Sinkule et al, 2003). Mean results for the four tight-fitting and seven loose-fitting PAPRs, in which oxygen consumption (V̇O2) ranged from 0.5 l·min−1 to 3.0 l·min−1, suggested the minimum inhaled CO2 concentration will increase with incremental energy expenditure (0.10% to 0.99%) with loose-fitting PAPRs and will result in negligible differences among tight-fitting PAPRs (0.03% to 0.04%). Minimum inhaled CO2 reached above 1.0% with V̇O2 > 2.0 l·min−1 among two loose-fitting PAPRs.
In addition to inhaled gas concentrations, inhaled pressures at the mouth and inhaled gas temperatures are of interest when PAPRs are worn during activity. PAPR blowers should provide an airflow that is greater than the peak flow of the wearer. This is not always the case and negative pressures at the mouth have been reported. Mackey et al. (2005) tested a loose-fitting PAPR on subjects exercising at 80–85% maximum oxygen consumption and found that all 16 subjects’ peak inhalation flows exceeded that provided by the PAPR blower. From the NIOSH study using the ABMS, every loose-fitting PAPR resulted in negative peak inhalation pressures at V̇O2 ≥ 2 l·min−1, whereas every tight-fitting PAPR resulted in negative peak inhalation pressures at V̇O2 ≥ 1.5 l·min−1 (Sinkule et al, 2003). Caretti and Gardner (2003) investigated the heat stress effects of wearing a tight-fitting mask PAPR in a warm environment while walking on a treadmill. No differences in any physiological variables (average core temperature, heart rate, mean skin temperature, sweat rate, and heat storage rate) with the PAPR compared to no PAPR were found. However, comfort ratings were lower (less comfortable) with the PAPR versus without. Others have reported the preference of PAPR use over N95 FFRs based on subjective perceptions of comfort (Khoo K-L, et al, 2005).
There are currently no NIOSH human subject certification test standards for PAPRs that evaluate the effect of the respirator on the user. A draft concept paper for human subject testing was published (http://www.cdc.gov/niosh/docket/archive/docket008.html, 2003). Recent human subject data on PAPR wearers is needed.
The purpose of this laboratory-based research project was to evaluate the physiological stresses experienced by both men and women wearing commercially available NIOSH-approved PAPRs at rest and during three exercise intensities. An objective of this research project was to provide the findings of this study to the Conformity Verification and Standards Development Branch of the National Personal Protective Technology Laboratory (NPPTL), for incorporation into the final revision of the module for the certification testing of PAPRs. The null hypothesis tested was that exercising while using any type of PAPR would have no effect on any of the dependent variables (minimum FICO2, maximum FIO2, peak inhaled pressure, and end inhalation temperature at the mouth).
METHODS
Healthcare and other workers between the ages of 18 and 45 with work experience and/or training involving wearing respirators, who were low-risk for cardiovascular disease, and who passed the medical screening procedure were recruited for the study. Upon arrival at the laboratory, subjects provided informed consent which was approved by the NIOSH Institutional Review Board, and completed a medical history. Nude body weight was measured at the beginning and end of the first day of testing. Subjects donned short-sleeved coveralls (65% polyester/35%cotton) fitted for their height and somatotype. Exercise evaluations were used to determine the treadmill speed (walking only) and elevation for the absolute oxygen consumption (V̇O2) of 1 l·min−1, 2 l·min−1, and 3 l·min−1, labeled LOW, MODERATE, and HIGH, respectively. Each exercise evaluation began with a warm-up period lasting four to six minutes using a level treadmill at two miles per hour (mph). One minute at each target energy expenditure (1 ± 0.1 l·min−1, 2 ± 0.1 l·min−1, and 3 ± 0.1 l·min−1) was the criteria used to confirm the acceptable treadmill speed and elevation. The evaluations were performed with a metabolic measurement system (Vmax Encore, Care Fusion Inc., Yorba Linda, CA) in mixing chamber mode using a two-way non-rebreathing valve (Hans Rudolph, Inc., Shawnee, KS) and nose clip. If a subject could not achieve the V̇O2 = 3 l·min−1 intensity while walking, the subject could qualify to participate if s/he achieved at least two additional minutes at a speed and/or elevation higher than V̇O2 = 2 l·min−1 before reaching termination criteria or volitional fatigue. Criteria for test termination included the following: heart rate reaching 90% of age-predicted maximum, lightheadedness, dizziness, chest pain, nausea, the subject asked to stop, or equipment failure. After the exercise evaluation, subjects were seated and encouraged to consume water or another beverage (Gatorade Co., Chicago, IL).
Following the exercise evaluation, subjects were administered a quantitative fit test for the tight-fitting PAPR model by a trained fit test technician. A fit test was used to ensure that the proper size and model of a tight-fitting PAPR used by the subject offered an adequate seal at the respirator and face interface. The protocol utilized the OSHA fit test protocol (OSHA, 2016). A minimum fit factor pass level of at least 100 was necessary for the respirator. The inability to pass the fit test prevented a subject from participating in the study.
Subjects then wore a tight-fitting PAPR (PAPR-T) and three different loose-fitting PAPRs (PAPR-L1, PAPR-L2, and PAPR-L3) from different manufacturers while standing for four minutes (REST) and while walking for four minutes at each intensity of LOW, MODERATE, and HIGH treadmill exercise. To avoid complications from fatigue after the exercise evaluation, the four respirator experiments were randomized and split between two visits separated by at least two days. All respirators were NIOSH-approved. Manufacturer instructions were used to prepare each PAPR and battery for testing, in addition to proper size selection of the loose-fitting hood or tight-fitting face mask. The full face mask for PAPR-T was ported for sample lines using airtight connections. An instrument mask with a nose clip was used for sampling from the loose-fitting PAPRs. The instrument mask (Hans Rudolph, Shawnee, KS) was a silicone rubber oral-nasal nose cup developed to contain sample lines within one centimeter in front of the upper lip, and added minimal dead space to the breathing zone immediately around the mouth and nose. A mask adapter contained sampling ports for inhaled gases by fast-response CO2 and O2 gas analyzers (CD-3A carbon dioxide analyzer and S-3A/I oxygen analyzer, AEI Technologies, Chicago, IL), breathing pressures (Datum 2000 digital manometer, Setra Systems, Inc., Boxborough, MA), and breathing temperatures (Type T copper-constantan fast response thermocouple, Omega Engineering, Stamford, CT; and high-speed temperature monitor, Thermalert Model TH-8, Physitemp Instruments, Inc., Clifton, NJ) measured continuously breath-by-breath (60 Hz) using a data acquisition system. The instrument mask, available in small, medium, and large sizes, was fitted to each participant’s face. Ambient laboratory conditions were as follows (average ± std dev): barometric pressure = 737 ± 5 mmHg; room temperature = 20 ± 2°C; and relative humidity = 29 ± 15%. Prior to each test, all instruments were calibrated, and response and transport times were measured for the gas analyzers. Heart rate and oxygen saturation were measured continuously with a pulse oximeter (Model RADICAL-7, Massimo Americas, Inc., Los Angeles, CA). Heart rate responses plateaued within two minutes at each target energy expenditure (except during the HIGH period by the participants that could not achieve the V̇O2 = 3 l·min−1). Between experiments, subjects were seated to rest (approximately 10 minutes) while they consumed water or another beverage, ad libitum, followed by 5 to 10 minutes of standing rest. Participant preparation for each trial occurred during the standing rest period. The PAPR blower flows were measured and confirmed (Chain-Compensated Gasometer (a.k.a. Tissot tank), Warren O. Collins, Inc., Braintree, MA) prior to daily experiments and after each experiment (with blower still powered on) while the participants rested.
Data were processed into individual breaths using LabVIEW™8.2 software (National Instruments Corp., Austin, TX). Minimal inhaled CO2 concentration, maximal inhaled O2 concentration, peak inhalation pressures, and inhalation temperatures were the dependent variables for analyses. An average of the data from the last 60 seconds of minute four for each period (REST, LOW, MODERATE, and HIGH) was used for analysis. Repeated measures analysis of variance (ANOVA) was used to evaluate the main effects of gender, PAPR model, and intensity level on the four dependent variables, as well as to evaluate all interaction effects: gender by PAPR model, intensity level by PAPR model and gender by intensity by PAPR model. Because Mauchly’s test of sphericity indicated that the assumption of sphericity was violated for all four dependent variables, the multivariate form of repeated measures was used. Analyses were conducted using PROC GLM in SAS 9.3 (SAS Institute, Inc., Cary, NC).
Respirator Selection
The PAPRs were selected for this investigation after consulting with respirator manufacturers and healthcare providers attending an industrial hygiene conference. The recommended PAPRs were a full facepiece tight-fitting PAPR (PAPR-T; 2.23 kg); a loose-fitting PAPR (PAPR-L1; 1.86 kg) where the hood covered the top of the head and only the face; a loose-fitting PAPR (PAPR-L2; 1.37 kg) where the hood covered the head with elastic that pulled the hood material under the neck and contained a double shroud/bib; and, a loose-fitting PAPR (PAPR-L3; 2.04 kg) where the hood covered the entire head to the shoulders with a double shroud/bib. Visually, PAPR-L1 had the smallest dead space, and PAPR-L3 had the largest dead space among the loose-fitting PAPR hoods. The inner shroud/bib was tucked inside the coveralls worn by subjects during the experiments, per the user instructions. Hoods were available in three sizes for PAPR-L1, in two sizes for PAPR-L2, and one universal size for PAPR-L3. The loose-fitting PAPRs used single-use hoods. PAPR-T was available in three sizes and the fit test was used to determine the appropriate size. The Hans-Rudolph instrument mask and the PAPR-T facepiece were cleaned with a disinfectant (CaviCide™, Metrex Research, Orange, CA) between participants’ experiments. All PAPRs used single-use filters rated for high-efficiency particulate air (HEPA).
RESULTS
Twelve men and twelve women completed the study. Subject characteristics and data from the exercise evaluations are reported in Table I. The nude body weights reported in Table I were measured at the beginning of the first day. At the end of that day, the nude body weights for the men were not significantly different (92.45 ± 9.93 kg, P > 0.05), whereas the nude body weights for women were significantly different (73.11 ± 7.81 kg, P = 0.01).
Table I.
Subject Characteristics and Exercise Metabolic Data (Mean ± SD)
| Variable | Men (n=12) | Women (n=12) | P value |
|---|---|---|---|
| Age (years) | 27.3 ± 6.4 | 22.3 ± 2.8 | 0.03 |
| Body weight (kg) | 92.15 ± 10.17 | 72.89 ± 7.71 | <0.001 |
| Height (cm) | 184.2 ± 4.3 | 167.5 ± 7.1 | <0.001 |
| V̇O2 (l·min−1, STPD) | |||
| REST | 0.37 ± 0.07 | 0.27 ± 0.04 | <0.001 |
| LOW | 1.08 ± 0.07 | 1.07 ± 0.10 | ns |
| MODERATE | 2.05 ± 0.10 | 2.00 ± 0.07 | ns |
| HIGH | 3.04 ± 0.22 | 2.66 ± 0.24 | <0.001 |
| V̇E (l·min−1, BTPS) | |||
| REST | 11.8 ± 1.4 | 11.1 ± 3.8 | ns |
| LOW | 25.0 ± 2.7 | 25.1 ± 3.5 | ns |
| MODERATE | 47.0 ± 6.5 | 51.5 ± 5.8 | ns |
| HIGH | 89.2 ± 22.8 | 86.0 ± 14.6 | ns |
| Heart rate (beats·min−1) | |||
| REST | 75 ± 10 | 83 ± 15 | ns |
| LOW | 89 ± 10 | 103 ± 10 | 0.003 |
| MODERATE | 119 ± 12 | 149 ± 17 | <0.001 |
| HIGH | 160 ± 16 | 178 ± 7 | 0.002 |
ns = not significant
Results from the multivariate analysis of variance (MANOVA) performed on the dependent variables are reported in Table II. The PAPR model by intensity interaction was significant for minimum inhaled CO2 and maximum inhaled O2 (P < 0.001), and for peak inhalation pressure (P < 0.01). The main effect of PAPR model was also significant (P < 0.001) for the first two dependent variables shown in Table II; the main effect of intensity was significant for all dependent variables (P < 0.001). Neither the main effect of gender, nor any interactions involving gender, were significant. For that reason, data for men and women were combined when examining the effect of intensity for each PAPR model. The significant PAPR model by intensity interaction was followed by tests of simple effects to compare PAPR models at each intensity level. A significant effect for PAPR model was found for all intensity levels and all dependent variables with one exception – the MODERATE intensity level for end inhalation temperature. Pairwise comparisons of PAPR models were then performed using the Bonferroni correction and the results are summarized in Table III.
Table II.
Multivariate Analysis of Variance (MANOVA) Table
|
|
|||||||
|---|---|---|---|---|---|---|---|
| SOURCE | |||||||
|
|
|||||||
| Variable | PAPR | PAPR X Gender |
Intensity | Intensity X Gender |
PAPR X Intensity |
PAPR X Intensity X Gender |
|
| Minimum Inhaled CO2 | Wilks' Lambda | 0.060 | 0.854 | 0.134 | 0.895 | 0.027 | 0.389 |
| F | 104.0 | 1.137 | 43.240 | 0.782 | 55.643 | 2.441 | |
| P | < .001 | 0.358 | < .001 | 0.518 | < .001 | 0.065 | |
|
| |||||||
| Maximum Inhaled O2 | Wilks' Lambda | 0.046 | 0.913 | 0.285 | 0.863 | 0.039 | 0.480 |
| F | 139.6 | 0.637 | 16.696 | 0.865 | 38.169 | 1.682 | |
| P | < .001 | 0.600 | < .001 | 0.466 | < .001 | 0.185 | |
|
| |||||||
| Peak Inhalation Pressure | Wilks' Lambda | 0.917 | 0.856 | 0.055 | 0.697 | 0.228 | 0.423 |
| F | 0.602 | 1.123 | 115.229 | 2.901 | 5.258 | 2.119 | |
| P | 0.621 | 0.363 | < .001 | 0.060 | 0.003 | 0.100 | |
|
| |||||||
| End Inhalation Temperature | Wilks' Lambda | 0.640 | 0.874 | 0.184 | 0.924 | 0.542 | 0.670 |
| F | 3.757 | 0.965 | 29.568 | 0.548 | 1.313 | 0.766 | |
| P | 0.027 | 0.429 | <0.001 | 0.655 | 0.313 | 0.649 | |
Table III.
Between PAPR Post Hoc Comparisons for Each Dependent Variable at Each Level of Energy Expenditure
| Energy Expenditure |
Minimum FICO2 | Maximum FIO2 | Peak Inhalation Pressure |
End Inhalation Temperature |
|---|---|---|---|---|
| REST | L1, L2, L3 > T | L2, L3 < T | L1, L2, L3 < T L3 | L2 > T |
| L3 > L1 | < L2 | |||
|
| ||||
| LOW | L1, L2, L3 > T | L1, L2, L3 < T | L1, L2, L3 < T | L2 > T |
| L2, L3 > L1 | L2, L3 < L1 | |||
|
| ||||
| MODERATE | L2, L3 > T | L2, L3 < T | L1, L2, L3 < T | no significant differences |
| L2, L3 > L1 | L2, L3 < L1 | |||
| L3 > L2 | L3 < L2 | |||
|
| ||||
| HIGH | L2, L3 > T | L2, L3 < T | L1, L2, L3 > T | L2 > L1 |
| L2, L3 < L1 | L2, L3 < L1 | |||
| L3 > L2 | L3 < L2 | |||
L1 = PAPR-L1; L2 = PAPR-L2; L3 = PAPR-L3; T = PAPR-T
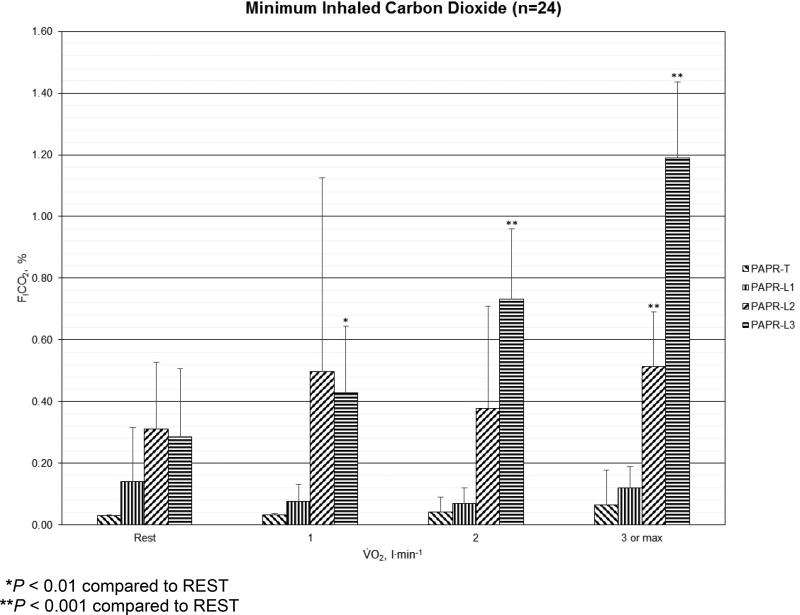
For minimum inhaled CO2 concentrations (FICO2, %), the results for PAPR-T, PAPR-L1, PAPR-L2, and PAPR-L3 at REST, LOW, MODERATE, and HIGH are shown in Figure 1. Minimum FICO2 peaked at 1.4% among men and 1.0% among women, both during HIGH for PAPR-L3. In PAPR-T, the minimum FICO2 differences between work rates were small. In PAPR-L1, the highest minimum FICO2 was at REST and the lowest minimum FICO2 was at LOW, then progressively increased to MODERATE and HIGH. In PAPR-L2, the lowest minimum FICO2 was at REST followed by a large increase at LOW, a decrease at MODERATE, then rose again at HIGH, where the results were significantly different from REST (P < 0.001). The highest minimum inhaled CO2 results occurred in PAPR-L2 at REST and LOW. The highest minimum FICO2 among PAPRs occurred for MODERATE and HIGH work rates with PAPR-L3, which also were significantly higher than REST (P < 0.001).
Figure 1.
Mean (error bars represent standard deviations) minimum inhaled carbon dioxide concentration (%) for one tight-fitting (PAPR-T) and three loose-fitting powered air-purifying respirators (PAPR-L1, PAPR-L2, and PAPR-L3) among men (n=12) and women (n=12) at REST, LOW (V̇O2 = 1 l·min−1), MODERATE (V̇O2 = 2 l·min−1), and HIGH (V̇O2 = 3 l·min−1 or maximum) energy expenditures.
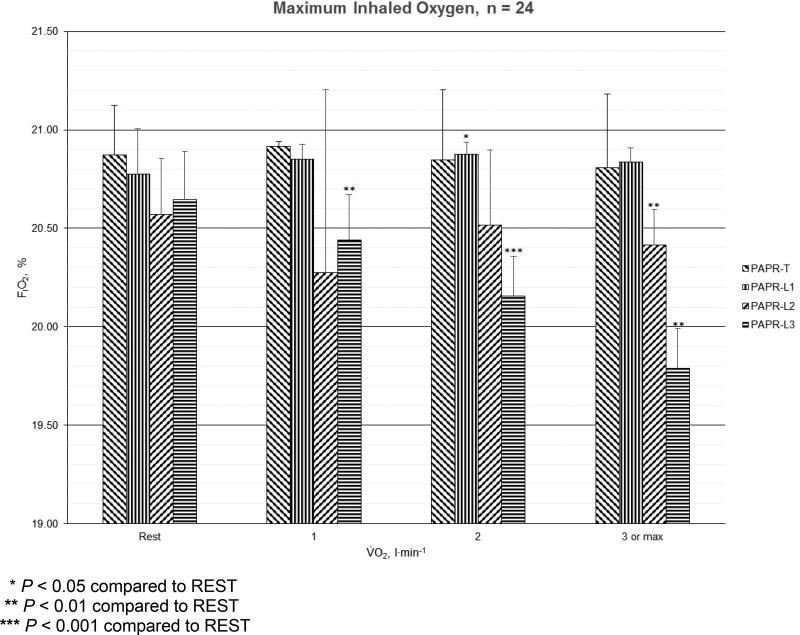
For maximum inhaled O2 concentrations (FIO2, %), the results for PAPR-T, PAPR-L1, PAPR-L2, and PAPR-L3 at REST, LOW, MODERATE, and HIGH are shown in Figure 2. Maximum FIO2 was lowest at 19.7% among men and 19.9% among women, both during HIGH for PAPR-L3. For PAPR-T, the maximum FIO2 differences between work rates were small. For PAPR-L1, the lowest maximum FIO2 was observed during REST among men and women. With PAPR-L1, the only significant difference from REST was during MODERATE exercise (P < 0.05). For PAPR-L2, the lowest maximum FIO2 was observed at LOW followed by HIGH, and changes at HIGH exercise were significantly different from REST (P < 0.01). For PAPR-L3, the lowest FIO2 occurred in HIGH followed by MODERATE and LOW, and each were significantly different from REST (LOW, P < 0.01; MODERATE, P < 0.001; and, HIGH, P < 0.01). Among the respirators, the lowest maximum FIO2 occurred in PAPR-L3 during MODERATE and HIGH, whereas the lowest maximum FIO2 occurred in PAPR-L2 during REST and LOW.
Figure 2.
Mean (error bars represent standard deviations) maximum inhaled oxygen concentration (%) for one tight-fitting (PAPR-T) and three loose-fitting powered air-purifying respirators (PAPR-L1, PAPR-L2, and PAPR-L3) among men (n=12) and women (n=12) at REST, LOW (V̇O2 = 1 l·min−1), MODERATE (V̇O2 = 2 l·min−1), and HIGH (V̇O2 = 3 l·min−1 or maximum) energy expenditures.
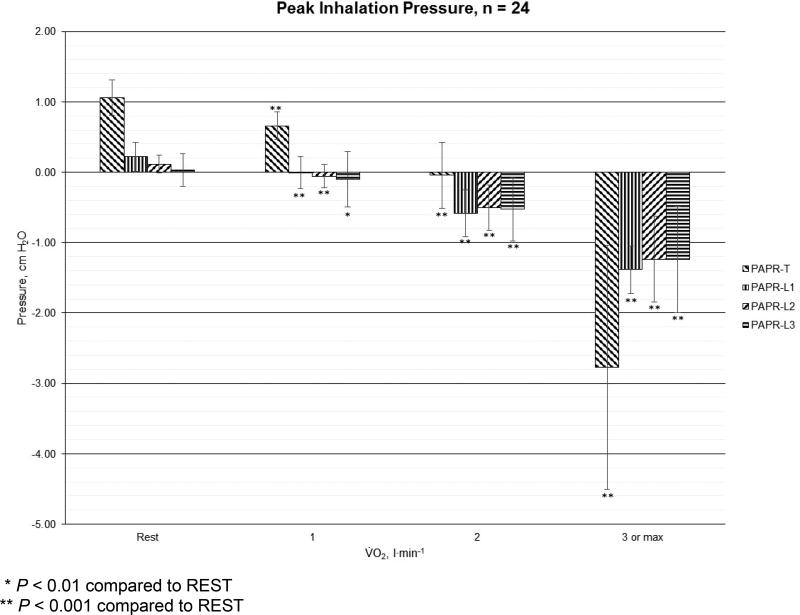
For peak inhaled pressure (cm H2O), the results for PAPR-T, PAPR-L1, PAPR-L2, and PAPR-L3 at REST, LOW, MODERATE, and HIGH are shown in Figure 3. Among the loose-fitting PAPRs, the peak inhalation pressures were equal to zero (PAPR-L1 at LOW intensity), or became negative at the mouth when V̇O2 > REST. The peak inhalation pressure became negative at the mouth with the tight-fitting PAPR when V̇O2 > LOW. The peak inhaled pressures at the mouth with all PAPRs at each activity level were significantly different from REST (P < 0.001, except PAPR-L3 for LOW intensity where P < 0.01).
Figure 3.
Mean (error bars represent standard deviations) peak inhalation pressure (cm H2O) for one tight-fitting (PAPR-T) and three loose-fitting powered air-purifying respirators (PAPR-L1, PAPR-L2, and PAPR-L3) among men (n=12) and women (n=12) at REST, LOW (V̇O2 = 1 l·min−1), MODERATE (V̇O2 = 2 l·min−1), and HIGH (V̇O2 = 3 l·min−1 or maximum) energy expenditures.
The end inhalation temperature for men (n=12) and women (n=12) combined are presented in Table IV. The range of temperatures was 24.4 – 26.0°C.
Table IV.
End Inhalation Temperature (°C) in Each PAPR Model for Both Men and Women (Mean ± SD)
| PAPR | REST | LOW | MODERATE | HIGH |
|---|---|---|---|---|
| PAPR-T | 24.4±1.1 | 24.7±1.3 | 24.9±1.1 | 25.5±1.0 |
| PAPR-L1 | 24.9±1.5 | 25.0±1.4 | 24.9±1.4 | 25.3±1.3 |
| PAPR-L2 | 25.3±1.1 | 25.6±1.2 | 25.4±1.1 | 26.0±1.1 |
| PAPR-L3 | 25.0±1.1 | 25.1±1.2 | 25.1±1.1 | 25.7±1.1 |
Unexpected Adverse Results from PAPR-L2
PAPR-L2 caused sudden unexpected changes during the LOW period for one man and two women. The changes made it necessary to stop the experiment due to maximum inhaled O2 concentrations below the termination point (< 19.0%), and minimum inhaled CO2 concentrations above the termination point (>2.0%). Subjects reported fogging inside the hood face shield and one subject stated “I cannot breathe.” The PAPRs were removed from each participant immediately. Blower flow was measured using the manufacturer-supplied flow meter, in addition to the Tissot tank. In each case, the flows were confirmed as adequate for PAPR use. The PAPRs were re-donned, adequate flow inside the hood confirmed, and the experiments resumed. One of the two women that experienced the unexpected changes did not attend the second day of testing, and the data from this woman was not included in the analyses due to her incomplete data set.
PAPR-L2 used a filter that was inserted from behind the blower or the side of the blower compartment against the participant’s lumbar region. The filter cover required removal to replace the filter. It was observed that the filter cover contained the intake vents for the PAPR blower and the vents could be blocked by the participant’s clothing. It was unclear if the blocked vents caused cumulative damage to the blower. After the adverse episode occurred to the third participant, a backup PAPR of the same model was used to replace the first model, and a flexible plastic cutting mat (Walmart, Inc.) was cut to fit around the PAPR belt separating the blower vents and the participant’s clothing. No additional adverse events from PAPR-L2 occurred after replacement with a back-up PAPR-L2 model.
DISCUSSION
The purpose of this investigation was to evaluate the physiologic effects from using four different PAPRs on the inhaled gas concentrations, peak inhaled pressures, and end inhalation temperatures at rest and three levels of energy expenditure. The effects from various flow characteristics, elevated minimal inhaled CO2 concentrations, and reciprocally depressed maximal inhaled O2 concentrations have been studied extensively by respirator scientists. This study, however, is only the second study that compared the results from different PAPR models. In the first study, Sinkule et al. (2003) investigated five types of respiratory protection including eleven models of PAPRs using the ABMS. Using the same three levels of energy expenditure, the grouped mean results of PAPRs (without stratification by type (tight-fitting or loose-fitting) or construction (helmet, hood, cap, full facepiece, or half-mask)) produced the lowest levels of minimal inhaled CO2 concentrations and the highest maximal inhaled O2 concentrations when compared to the grouped mean results from eleven models of N95 FFRs, 27 models of elastomeric air-purifying respirators, six models of gas masks, and 20 models of air-supplied respirators. Furthermore, similar to the results reported in this investigation, the grouped peak inhalation pressures also were negative when V̇O2 ≥ 1 l·min−1. The current investigation was able to demonstrate that all dependent variables (inhaled gas concentrations, peak inhaled pressure, and end inhalation temperature) were affected by the intensity of energy expenditure.
The PAPR by intensity interaction was significant for all dependent variables except temperature, indicating that each PAPR responded to intensity in a different way. The post hoc comparisons reported in Table 3 were conducted to help understand this interaction. Except for end inhalation temperature, the benefits of using a tight-fitting PAPR over each of the loose-fitting PAPRs are seen among the inhaled breathing gases, as well as the peak inhalation pressures. The cluster of results at the MODERATE and HIGH energy expenditures on the inhaled breathing gases are from the physical effects of respirator dead space. As the amount of respirator dead space increased from PAPR-T to PAPR-L1, from PAPR-L1 to PAPR-L2, and PAPR-L2 to PAPR-L3, the concentrations of inhaled CO2 increased in like fashion. The respirator dead space management was most effective in the tight-fitting PAPR for two reasons: the lowest amount of dead space, and the presence of a nose cup which helped to reduce the dead space further. The effect of a nose cup was studied by Harber et al (1991) in the investigation of the physiological effects of an elastomeric full facepiece air-purifying respirator (APR) with and without a nose cup. The APR with a nose cup was associated with a lower respiratory rate, lower average inhaled flow, and lower minute ventilation during the same activities compared to the APR without a nose cup. The effect of a nose cup with three different open-circuit positive pressure self-contained breathing apparatus (SCBA) versus the same respirators without a nose cup was investigated by Turner et al (1996). In that study, the respirator facepiece with a nose cup increased the time to alarm, reduced the minimum inhaled CO2, and decreased the inhaled minute ventilation at the high intensity work rate.
Respirators provide a microenvironment for the exposure pathway of inhaled CO2. Of the PAPRs in this investigation, most minimum inhaled CO2 concentrations were below 0.8%. For the PAPR with the largest amount of dead space visually (PAPR-L3), the minimum inhaled CO2 increased as intensity increased until 1.8% inhaled CO2 was measured at the HIGH work rate. The respiratory rate, tidal volume, and alveolar CO2 will rise with inhaled CO2 concentration above ambient (Schneider and Truesdale, 1922; Consolazio et al 1947; Patterson et al 1955). The abnormal diffusion of CO2 from the blood due to a decrease in the ratio of alveolar to capillary CO2 are compensated by these responses (Schulte, 1964). Increased cardiac output, respiratory rate, and breathing depth will compensate for additional CO2 (Schulte, 1964). PAPR-T and PAPR-L1 responded in a similar fashion to intensity, which was a relatively low amount of change. The response by PAPR-L2 was inconsistent, perhaps due to the behavior of unknown etiology by the respirator prior to replacing it with a backup model from the same manufacturer.
Changes seen in maximum inhaled O2 among the respirators were reciprocal to the changes observed in minimum inhaled CO2. The changes in the maximum inhaled O2 concentration closely followed the reciprocal displacement by the minimum inhaled CO2. This occurred when the inhaled O2 concentration increased in conditions where inhaled CO2 decreased, and in reverse. Relative displacement of the gases in air is one reason for the changes seen in the inhaled O2 concentrations relative to the changes in the inhaled CO2 concentrations. Except for PAPR-L3 at the HIGH work rate, the maximum inhaled O2 concentrations were above 20.0% among the PAPRs at each level of energy expenditure. From the respiratory protection standard promulgated by federal regulation (Approval of Respiratory Protection Devices, 2016a), a hazardous atmosphere includes any oxygen-deficient atmosphere of less than a partial pressure of 148 mmHg, or 19.5%, O2 concentration.
The peak inhaled pressures behaved in a similar fashion and produced similar results among the loose-fitting PAPRs. When the energy expenditure was V̇O2 > 2.0 l·min−1 while using the tight-fitting PAPR, it was not apparent where inhaled breathing air was supplemented when inhalation pressures were negative. This phenomenon sometimes is referred to as overbreathing. Overbreathing occurs when the users’ inhaled flow exceeds the flow produced by the PAPR blower, which may result in the inhalation of filtered air from the blower filter or the inhalation of contaminated air by pathways other than the blower filter (Mackey, 2005; Quinn, 2015). The increased pressure may cause a decrease in respiratory rate (Harber et al., 1982; Louhevaara, 1984) and tidal volume (Harber et al., 1982). Among older individuals, respiratory rate may not change, and tidal volume may decrease with increased inspiratory resistance (Louhevaara, 1984).
The intensity effect for end inhalation temperature may have been statistically significant, but the difference may not be practically significant. To evaluate the thermal sensory difference among the end inhalation temperatures, Meh and Denišlič (1994) compared the thermal specific thresholds among various body points throughout a wide range of ages in men (10–73 years old) and women (10–69 years old). On the face, men could sense a temperature difference of 0.94°C, whereas women could sense a temperature difference of 0.80°C. Between the PAPRs at each intensity and at these temperature thresholds, it can be seen that the temperature differences may be noticeable at the REST and LOW energy expenditures (temperature within the PAPR-T was 0.9°C less than the PAPR-L2 at both levels) and unnoticeable at the MODERATE and HIGH levels. This difference may be explained by the alteration described for the PAPR-L2 among three participants in the Results. Others have provided information to determine significant additional changes (Stevens and Choo, 1998). Aging affects the sense of temperature differences at different rates around the body, and the face is the most sensitive. Using the temperature changes observed in this investigation (Table 4), each PAPR responded to intensity in a different way, and most differences between intensity and between PAPRs may not be sensed by the participants.
Study Limitations
The most significant limitation for this investigation was the unanticipated results when PAPR-L2 was used by three participants. For these participants, the minimum inhaled CO2 climbed and the maximum inhaled O2 decreased within seconds during the LOW work rate, to the point of stopping the experiment due to achieving termination criteria. Two theories for the abnormal results seem plausible: participant’s clothing blocked the intake vents which shut down blower flow, or the PAPR was defective. Two attempts were made to contact the manufacturer for discussion of a solution. The replacement of the PAPR with a back-up model and the placement of a barrier between the PAPR and participants were attempts to satisfy both theories.
The experimental trials were of sufficient duration to characterize the respiratory responses while using different PAPRs at increasing levels of energy expenditure. The 4-minute duration was selected in order to capture the responses while avoiding participant fatigue. One limitation is the possible results that would have been different had the participants worn the PAPRs during various periods of a full shift, e.g. one hour, two hours, four hours, etc. Another limitation was the relatively small number of PAPR samples. In the NIOSH Certified Equipment List (CEL) (NIOSH, 2016), 224 PAPRs have received NIOSH-approved status. A larger sample size would have provided an improved representative sample. However, a survey of health care providers reported two PAPRs used in this investigation were among the PAPRs most common in U.S. health care facilities for 2014 and one PAPR was among the most common for 2015 (Wizner, et al, 2016).
Future research could increase the body of knowledge by incorporating subjective information with the physiologic information. Examples of the subjective information could include effects of vision and hearing, effects from the weight of each PAPR, the level of difficulty to don and/or doff each device as well as operating the apparatus, and the effects on perceived exertion. Another opportunity to evaluate each PAPR is the possible comparison between devices using the ABMS. The ABMS has been used in the past to evaluate N95 FFR, air-supplied respirators, elastomeric respirators, gas masks, N95 FFR with surgical mask covers, escape hood respirators for applications of chemical, biological, radiological, and nuclear (CBRN) protection, and closed-circuit escape respirators (CCERs) (CDC, 2008; Sinkule, 2003; Sinkule, 2004; Sinkule, 2013). The advantages of the ABMS evaluation would include the elimination of human variability and human responses that mask conditions produced by the respirators, reduction of risk to human participants, and near identical experimental conditions. Another consideration for future research is the effect of overbreathing on supplemental air pathways. These considerations might include the properties of the loose-fitting hood as a reservoir of supplemented air, and the pathways of supplemented air when a tight-fitting PAPR is used.
CONCLUSIONS
The conclusions from this investigation are as follows:
When men and women used tight- and loose-fitting PAPRs at the same absolute energy expenditure, there is an insignificant gender effect.
Using data from the minimum inhaled CO2 concentrations, maximum inhaled O2 concentrations, peak inhalation pressures, and end inhalation temperatures, the PAPRs from this investigation may be safely worn by healthy participants. Longer durations of PAPR use would be an important area of future research.
The source of supplemented air is unknown when inhalation pressures are negative. This is another area for future research.
Consideration for user conditions (e.g., how the PAPR blower is worn relative to the user’s clothing) is important especially when these devices are considered for use by healthcare personnel during aerosol-generating procedures.
Acknowledgments
The authors wish to express their gratitude to Nina L Turner, PhD (CDC/NIOSH/OD, Office of Extramural Projects) for sharing this idea; Kathleen M. Stabryla, MLS (CDC Public Health Library and Information Center/Information Reference, Research, and Education Services Team (Pittsburgh Branch)) and her staff of professional librarians; Maria Ressler (CDC/NIOSH/NPPTL) for her technical and editorial assistance during the preparation of this manuscript; Judi Coyne, MBA, MA (CDC/NIOSH/NPPTL), Deborah Novak, PhD, RN (CDC/NIOSH/NPPTL), Jeffrey Palcic, BS (CDC/NIOSH/NPPTL), Lewis Radonovich, MD (CDC/NIOSH/NPPTL), Markee Shamblin, BA (CDC/NIOSH/NPPTL), W Jonathan Williams, PhD, MS (CDC/NIOSH/NPPTL), and Kerri Wizner, MPH (CDC/NIOSH/NPPTL) for their thorough and thoughtful reviews and suggestions. Finally, our gratitude is extended to the many study participants for which the many hours of data collection would not have been possible without their participation, patience, and sweat.
Footnotes
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the National Personal Protective Technology Laboratory, the Centers for Disease Control and Prevention, or the National Institute for Occupational Safety and Health. Mention of commercial products or trade names does not constitute endorsement. The authors do not have any financial interests in the present research.
References
- American Conference of Governmental Industrial Hygienists. Guide to occupational exposure values. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2016. Occupational exposure values. [Google Scholar]
- Approval of Respiratory Protection Devices. 42 CFR § 84.2(y) Definitions. 2016a Accessed at http://www.ecfr.gov/cgi-bin/textidx?type=simple;c=ecfr;cc=ecfr;sid=c1d86cdda642af92fd35976e5ce03a24;region=DIV1;q1=Respiratory%20Protective%20Devices;rgn=div5;view=text;idno=42;node=42%3A1.0.1.7.67 on 8 August 2016.
- Approval of Respiratory Protection Devices. 42 CFR § 84. Subpart KK—Dust, Fume, and Mist; Pesticide; Paint Spray; Powered Air-Purifying High Efficiency Respirators and Combination Gas Masks. 2016b Accessed at http://www.ecfr.gov/cgibin/retrieveECFR?gp=29&SID=c3d8b7fafad48415fb76014a278699b1&ty=HTML&h=L&mc=true&n=pt42.1.84&r=PART#sp42.1.84.kk 25 October 2016.
- Association of State and Territorial Health Officials. Assessment of Respiratory Personal Protective Equipment in U.S. Acute Care Hospitals–2012. Rep. Vol. Oct 24, 2014. 2014. [Google Scholar]
- Bureau of Labor Statistics and the National Institute for Occupational Safety & Health. BLS/NIOSH [2003], “Respirator Usage in Private Sector Firms, 2001”. U.S. Department of Labor, Bureau of Labor Statistics/U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (A8-10); 2003. Accessed at http://www.cdc.gov/niosh/docs/respsurv/ on 18 February 2016. [Google Scholar]
- Caretti DM, Garner PD. Quantifying the Heat Stress Attributable to Respirator Wear. J Int Soc Resp Pro. 2003 Fall-Winter;20:110–124. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Tenth phase results. US Department of Health and Human Services, National Institute for Occupational Safety and Health; Washington, DC: 2008. RI 9675 Report of Investigations/2008: Self-contained self-rescuer long term field evaluation. DHHS (NIOSH) Publication Number 2008-138. [Google Scholar]
- Consolazio WV, Fisher MB, Pace N, Pecora LJ, Pitts GC, Behnke AR. Effects on man of high concentrations of carbon dioxide in relation to various oxygen pressures during exposures as long as 72 hours. American Journal of Physiology. 1947;151:479–503. doi: 10.1152/ajplegacy.1947.151.2.479. [DOI] [PubMed] [Google Scholar]
- Cuta K. Powered Air Purifying Respirators: Versatility Beyond Respiratory Protection. Occupational Health & Safety. 2015;84(11):20–22. [PubMed] [Google Scholar]
- Harber P, Tamimie RJ, Bhattacharya A, Barber M. Physiologic effects of respirator dead space and resistance loading. Journal of Occupational Medicine. 1982;24:681–689. doi: 10.1097/00043764-198209000-00015. [DOI] [PubMed] [Google Scholar]
- Harber P, Beck J, Brown C, Luo J. Physiologic and Subjective Effects of Respirator Mask Type. American Industrial Hygiene Journal. 1991;52(9):357–362. doi: 10.1080/15298669191364875. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies (IOM) The Use and Effectiveness of Powered Air-Purifying Respirators in Health Care: Workshop Summary. The National Academies Press; Washington, DC: 2015. [PubMed] [Google Scholar]
- Khoo K-L, Leng P-H, Ibrahim IB, Lim TK. The changing face of healthcare worker perceptions on powered air-purifying respirators during the SARS outbreak. Respirology. 2005;10:107–110. doi: 10.1111/j.1440-1843.2005.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman CT, Domnitz SB, McCoy MA. The Use and Effectiveness of Powered Air Purifying Respirators in Health Care: Workshop Summary. National Academies Press; Washington, DC: 2015. Accessed at http://www.nap.edu/catalog.php?record_id=18990 on 18 February 2016. [PubMed] [Google Scholar]
- Louhevaara VA. Physiological effects associated with the use of respiratory protective devices – a review. Scandinavian Journal of Work and Environmental Health. 1984;10:275–281. doi: 10.5271/sjweh.2327. [DOI] [PubMed] [Google Scholar]
- Mackey KRM, Johnson AT, Scott WH, Koh FC. Over Breathing a Loose-Fitting PAPR. J Int Soc Resp Pro. 2005 Spring-Summer;22:1–10. [Google Scholar]
- Meh D, Denišlič M. Quantitative assessment of thermal and pain sensitivity. Journal of Neurological Sciences. 1994;127:164–169. doi: 10.1016/0022-510x(94)90069-8. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health. Criteria for a Recommended Standard. Occupational Exposure to Carbon Dioxide. Washington, D.C.: United States Department of Health and Human Services, Centers for Disease Control and Prevention; 1976. [Google Scholar]
- National Institute for Occupational Safety and Health. Certified Equipment List. 2016 Accessed at https://wwwn.cdc.gov/NIOSHCEL/Results?pageSize=50&includeObsolete=false&tcApproval=&schedule=21C&AppFromDate=&AppToDate=&facepieceType=All&facepiecePower=Powered&sarType=All&scbaType=All&scbaUse=All on 4 August 2016.
- Occupational Safety & Health Administration (OSHA) Fit Testing Procedures. Washington, DC: United States Department of Labor; Accessed at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9780 on 2 August 2016. [Google Scholar]
- Patterson JL, Heyman A, Battey LL, Ferguson RW. Threshold of Response of the Cerebral Vessels of Man to Increase in Blood Carbon Dioxide. Journal of Clinical Investigation. 1955;34:1857–1864. doi: 10.1172/JCI103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personal Protective Equipment. Respiratory protection. 29 CFR § 1910.134. (f)(7) Fit testing. 2016 Accessed at http://www.ecfr.gov/cgi-bin/textidx?SID=950e53dcdda567422be041806193c6d3&mc=true&node=se29.5.1910_1134&rgn=div8 on 14 November 2016.
- Quinn T, Sinkule EJ, Goss F. Breathing Pressures and Inhaled Gases of Tight- and Loose-Fitting Powered Air-Purifying Respirators. Medicine & Science in Sports & Exercise. 2015;47(5 Suppl):S653. [Google Scholar]
- Raven PB, Dodson AT, Davis TO. The physiological consequences of wearing industrial respirators: a review. Am Ind Hyg Assoc J. 1979;40:517–34. doi: 10.1080/15298667991429912. [DOI] [PubMed] [Google Scholar]
- Schneider EC, Truesdale D. The effects on the circulation and respiration of an increase in the carbon dioxide content of the blood in man. American Journal of Physiology. 1922;63:155–175. [Google Scholar]
- Schulte JH. Sealed environments in relation to health and disease. Archives in Environmental Health. 1964;8:438–452. doi: 10.1080/00039896.1964.10663693. [DOI] [PubMed] [Google Scholar]
- Sinkule E, Turner N, Hota S. Abstracts of the 2003 American Industrial Hygiene Conference and Exposition, #227. Dallas, TX: 2003. Automated Breathing and Metabolic Simulator (ABMS) CO2 test for powered and non-powered air-purifying respirators, airline respirators, and gas masks; p. 54. [Google Scholar]
- Sinkule E, Turner N. Inhaled Carbon Dioxide and Oxygen Concentrations in Three Escape Hood Respirators During Rest and Exercise. Medicine & Science in Sports & Exercise. 2004;36(5 Suppl):S245. [Google Scholar]
- Sinkule EJ, Powell JB, Goss FL. Evaluation of N95 respirator use with a surgical mask cover: Effects on breathing resistance and inhaled carbon dioxide. Annals of Occupational Hygiene. 2013;57(3):384–398. doi: 10.1093/annhyg/mes068. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Choo KK. Temperature sensitivity of the body surface over the life span. Somatosensory & Motor Research. 1998;15(1):13–28. doi: 10.1080/08990229870925. [DOI] [PubMed] [Google Scholar]
- Turner N, Beeckman D, Hodous T. Effects of a Nosecup on Inspired Carbon Dioxide Concentration and Service Time in Selected Open-Circuit Self-Contained Breathing Apparatus. Journal of the International Society for Respiratory Protection. 1996 Spring;14(1):5–17. [Google Scholar]
- Wizner K, Stradtman L, Novak D, Shaffer R. Prevalence of Respiratory Protective Devices in U.S. Health Care Facilities. Workplace Health & Safety. 2016;64(855):359–368. doi: 10.1177/2165079916657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sun C, Sun M. The effect of moderately increased CO2 concentration on perception of coherent motion. Aviat Space Environ Med. 1997;68:187–91. [PubMed] [Google Scholar]