Abstract
Protein polarization underlies directional cell growth, cell morphogenesis, cell division, fate specification and differentiation in plant development. Analysis of in vivo protein dynamics reveals differential mobility of polarized proteins in plant cells, which may arise from lateral diffusion, local protein–protein interactions, and is restricted by protein–membrane–cell wall connections. The asymmetric protein dynamics may provide a mechanism for the regulation of asymmetric cell division and cell differentiation. In light of recent evidence for preprophase band (PPB)-independent mechanisms for orienting division planes, polarity proteins and their dynamics might provide regulation on the PPB at the cell cortex to directly influence phragmoplast positioning or alternatively, impinge on cytoplasmic microtubule-organizing centers (MTOCs) for spindle alignment. Differentiation of specialized cell types is often associated with the spatial regulation of cell wall architecture. Here we discuss the mechanisms of polarized signaling underlying regional cell wall biosynthesis, degradation, and modification during the differentiation of root endodermal cells and leaf epidermal guard cells.
Introduction
Cell polarity is fundamental to cell functions in all living organisms. How proteins are asymmetrically distributed in a cell and how polarized proteins regulate a diverse array of cellular events have been fascinating questions for biologists.
In animal systems, many polarized proteins, for example, Ste5 [1] and Bem1 [2] in yeast and PAR3/6 in Caenorhabditis elegans [3], are scaffold proteins that convene multiple components to ensure concerted interaction for signaling specificity and fidelity. Homologs of these conserved polarity proteins are not encoded in plant genomes. The plant-specific protein BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL) represents a polarity factor [4] that scaffolds a mitogen-activated protein kinase (MAPK) signaling cassette in Arabidopsis stomatal lineage cells [5]. How BASL is associated with the plasma membrane (PM) and how BASL polarity is maintained remain unknown.
In plants, protein polarization provides mechanisms for polar cell expansion (thus morphogenesis), asymmetric cell division and functional differentiation. A number of cell types produce daughter cells with unequal sizes and distinct cell fates. Such asymmetric cell divisions are often preceded by protein polarization that presumably leads to the specification of division plane orientation and/or differential cell fates [4,6,7]. What the polarity cue is and how this cue controls subsequent divisional asymmetries (size and fate) are fundamental questions in biology. Using stomatal lineage divisions in maize and Arabidopsis as model systems, we discuss how cell polarity might be instructive to division plane orientation in plants.
In multicellular organisms, cell differentiation typically manifests unique cell morphology and structures for specific physiological roles. One prominent example is that plant cells utilize localized cell wall modification to diversify cell form and function. The differentiation of root endodermal cells and leaf guard cells exemplifies the elaborate spatial regulation of cell wall architecture. Exciting progress has been made towards elucidating the formation of Casparian strip, a lignin band structure in Arabidopsis roots (reviewed in [8]), at a polarized cel signaling level [9]. The differentiation of stomatal guard cells requires spatiotemporally dynamic cell wall deformation and modification that may also require polarized cell signaling.
Protein dynamics and polarity regulation at the plasma membrane
Protein dynamics are critical for their cellular functions. Characterization of protein dynamics and how these dynamics relate to a protein’s physiological roles in vivo is enabled by advanced microscopy techniques, for example, FRAP (fluorescence recovery after photobleaching) and photoactivation [10], which have greatly enlightened our understanding of the mechanisms underlying the regulation of cell polarity.
In the establishment and maintenance of cell polarity in eukaryotic organisms, scaffold proteins were commonly found to assemble various signaling components in the cytoplasm and at the PM to a polar site [11]. For example, Ste5 scaffolds a MAPK cascade in budding yeast to activate the mating-specific MAPK Fus3p [11] (Figure 1a). Yeast Bem1 organizes a feedback loop to generate localized activation of the small GTPase Cdc42 to ensure bud polarity axis establishment [12]. In C. elegans embryos, the PAR3 (partitioning defective 3) and PAR6 scaffolds organize the anterior and posterior polarity complexes to regulate asymmetric cell division [13].
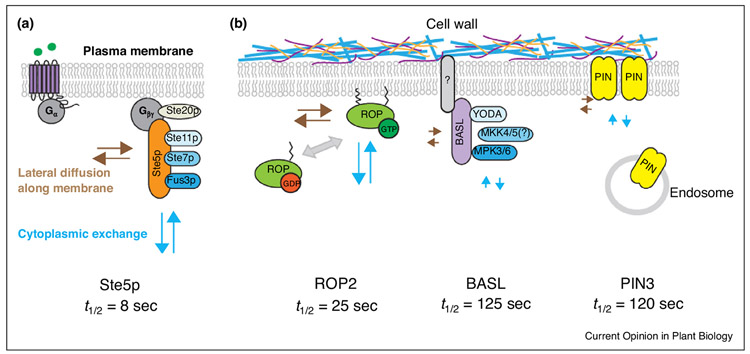
Figure 1.
Differential dynamics of polarity proteins in yeast and plants. (a) Ste5p scaffolds the MAPKKK Ste 11p, MAPKK Ste7p to activate the mating-specific MAPK Fus3p in yeast. Ste5p is highly mobile at the plasma membrane, where it binds to beta-gamma subunits of G-protein to create a lattice for activating the Ste5p–MAPK cascade protein complex. (b) Three polarized proteins in plants: ROP2, a lipid-modified protein for cell polarity; BASL, a scaffold protein for the YODA MAPK cascade; PIN3, a transmembrane auxin transporter. ROP2 rapidly switches between a GTP-bound and GDP-found forms. BASL and PIN3 show similarly slow protein mobility. A hypothetical BASL partner (grey with question mark) was predicted to be membrane-embedded or tightly associated with the plasma membrane. The PIN polarity is mediated by the membranetrafficking system, and plant cell walls exert constraints for lateral diffusion. FRAP measures the recovery rates (t1/2) of the designated PM proteins, which are mainly determined by lateral diffusion along the PM and cytoplasmic exchange (dynamic protein–protein interaction and bulk transport). Brown arrows: long ones show fast lateral diffusion and short ones show slow lateral diffusion. Light blue arrows: long ones show fast cytoplasmic exchange and short ones show slow cytoplasmic exchange. Recovery rates t1/2 of the respective polarity proteins (the time point when half of the final recovered intensity is reached) are specified at the bottom.
Scaffold proteins, due to their functions in the assembly of protein complexes, were thought to be stable, but emerging evidence suggests that they are often surprisingly dynamic. Based on FRAP data, the recovery rates, thalf or t1/2 (the time point when half of the final recovered intensity is reached), suggested rapid movement of polarity factors: Ste5 t1/2 = 8 s at the mating tip [14], Bem1 t1/2 = 2.37 s at the bud site [15], and cortical PAR6 reached nearly full fluorescence recovery in 1 min [16]. The dynamic features of scaffold proteins suggest that they are under intricate regulation and might confer quick adjustment of the cell polarity machinery in response to external cues and to ensure signaling specificity [17,18].
FRAP analyses were also performed in plant cells to characterize polarity protein dynamics. The available studies examined the membrane-embedded PIN-FORMED (PIN) auxin efflux carriers [19••], ABCG transporters [20], and boron transporters in root cells [21]. In addition, the membrane-associated small GTPase ROPs [22,23] and the MAPK scaffold protein BASL have also been analyzed by FRAP. Interestingly, except for ROP2 (t1/2 = 25 s), all other polarity proteins were found to be relatively immobile, for example, PIN3 (t1/2 = 120 s) and BASL (t1/2 = 125 s), and neither of them reached plateau in 5 min in leaf epidermal cells [24••] (Figure 1b).
The PIN auxin effluxers are fundamental players in the regulation of directional auxin flow. The maintenance of PIN polarity domains mainly depends on three membrane-based mechanisms: polar secretion, endocytosis and endocytic recycling, and lateral diffusion (reviewed in [25,26]). The stabilization of PINs in the PM is regulated by auxin via ROP-dependent inhibition of endocytosis [27,28]. Additionally, the extracellular matrix, cell walls, seemed to play a critical role in restricting protein lateral diffusion at the membrane ([19••] and Figure 1b). Detaching the PM from the cell wall by plasmolysis released the cell wall constraints so that the membrane proteins became more mobile, and interestingly, polarization was also abolished in these cells [20]. These studies provided exemplary models for membrane-centered protein polarization dynamics and highlighted both signaling feedback on trafficking and the unique function of cell walls in polarity maintenance in plant cells.
Rho GTPases from plants (ROPs) breaks cellular symmetry at the PM to regulate many plant developmental processes [29]. The ROP proteins are attached to the PM via lipid modifications [30]. The fast FRAP recovery rates of ROPs were comparable to that of their analog Cdc42 in yeast polarity site [31•], both of which switch between GTP-bound and GDP-bound forms and undergo recycling on and off the PM. The polarity maintenance of these G-proteins involves positive feedback loops via cytoskeleton-dependent cytoplasmic exchange and cytoskeleton-independent lateral diffusion (Figure 1b) [29,32,33].
BASL is another highly polarized protein that is expressed in the stomatal lineage cells to control asymmetric cell division in Arabidopsis [[24••]]. The BASL protein does not contain recognizable functional motifs for membrane localization and was therefore proposed to associate with membranes by interacting with proteins or lipids [34]. Based on in vivo FRAP, BASL showed surprisingly slow mobility at the polarity site, strikingly contrasting to that of the membrane-attached ROPs, but comparable to that of the membrane-embedded PIN3 (Figure 1b) [24••]. Then, why was BASL so immobile at the polarity site? We hypothesize that BASL may bind to a physical partner that is embedded in or tightly associated with the PM (Figure 1b). Because of minimal lateral diffusion detected for BASL [24••], its recovery at the polarity site might mainly arise from cytoplasmic replenishment, either through vesicle delivery of the membranes it tightly binds to or through its diffusion from the cytosol to associate with the membrane partner that is delivered to the PM. This hypothesis is supported by the recent discovery that, after tagged with an artificial transmembrane domain, Cdc42 remains polarized, but is significantly immobilized in yeast cells [31•].
When BASL was engineered with an N-terminal myristoylation site, this covalent modification fixed the protein at the PM but did not disturb the polarity formation [24••]. The FRAP data showed that unequal protein dynamics of myr-BASL along the PM highly resembled that of the membrane-fixed Cdc42 in yeast [24••,31•]. Also, the active forms of both molecules (GTP-bound Cdc42 and a phospho-mimicking form of BASL) exhibited lower mobility [31•]. It was thus proposed that the distinctly lowered mobility of active BASL and Cdc42 at the polar site is due to their association with large protein complexes or membrane microdomains [24••,31•]. Another polarized protein in plants, the AGCVIII D6 protein kinase (D6PK), a PIN activator colocalized with PIN1 at the basal side of root cells [35], directly binds to PtdIns(4,5)P2 and is recruited to the sterol-enriched microdomains at the root hair initiation sites [36]. The D6PK mobility has not been determined by FRAP yet; it would be interesting to investigate how the D6PK dynamics is regulated by its interaction with PIN1 and lipid microdomains.
Altogether, it appears that protein dynamics at the PM is dependent on the modes of their interaction with the membrane, either embedded in the PM, anchored to the PM by lipids, or associated to the PM through protein–protein or protein–lipid interactions. The identification of their binding components at the PM and investigation of their regulatory relationship will shed new light on the mechanisms underlying protein polarity formation and maintenance in plant cells.
Cell polarity and division plane orientation: mechanistic connections
During mitosis, the pre-mitotic microtubule (MT) structure, the preprophase band (PPB), marks the future cortical division zone and is accepted as the causal determinant of division plane placement in plant cells [37]. Mutations in key regulators of PPB lead to defects in the formation, orientation and maintenance of the cell division plane (Figure 2, for details, see comprehensive reviews [38-40]).
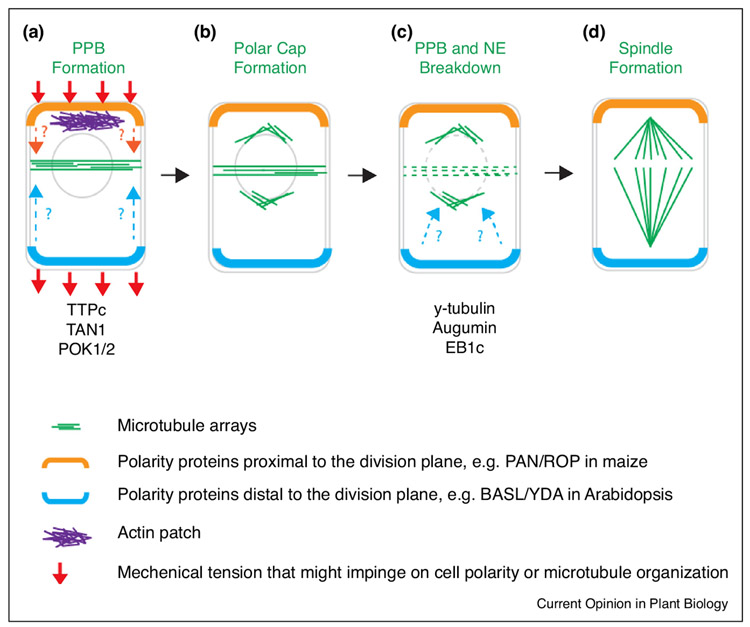
Figure 2.
Division plane orientation in plant asymmetric cell division. Diagrams show four early stages in plant cell division. In an asymmetrically dividing cell, three MT-based structures (PPB, polar cap, and spindle, green lines in a–d) appear successively to regulate division plane orientation. Before the PPB formation, polarized proteins (orange and blue curves) may define divisional asymmetries (a). Polarity maintenance can be regulated by extrinsic mechanical forces (red arrows). Possible functions of polarity proteins on the PPB are indicated by dashed arrows (orange and light blue). The polar caps, representing cytoplasmic microtubules nucleation centers (MTOCs), are transiently formed before the PPB and nuclear envelope (NE) breakdown to guide spindle positioning (b and c). Possible functions of the polarity proteins to the polar caps are marked by dashed arrows (c). At the metaphase, the bipolar spindle axis is perpendicular to the division plane (d).
However, mutants lacking PPBs usually also exhibit significant defects in cortical microtubule organization [41,42], thus hampering an explicit evaluation of the mitotic defects from the interphase misregulation. Mutants lacking apparent PPBs while maintaining mostly normal interphase cortical MT arrays were only reported very recently [43,44••]. Mutations in the MT-associated TONNEAU1a (TON1a) caused the failure of PPB formation in Arabidopsis, but interestingly, different cell types seemed to respond differently: cell divisions in the epidermis were severely misorientated, but normally maintained in the cortical layer, suggesting that the root cortical cell divisions can be controlled by a PPB-independent mechanism [43]. Schaefer et al. identified an important Arabidopsis triple mutant trm6;7;8 that failed to produce discernable PPBs, but displayed nearly normal growth and development. The primary defect of the trm mutant is the reduced precision in division orientation in the roots [44••]. This study reassessed the dominant role of PPB in directing division orientation, and raise the possibility that the PPB might instead provide a correction mechanism. In support of this hypothesis, the moss Physcomitrella patens produces gametophore tissues by cell divisions in the absence of PPBs but relying on the presence of cytoplasmic microtubule-organizing centers (MTOCs) [45••]. In the asymmetrically dividing gametophore initial cells, the discrete cytoplasmic MTOC named ‘gametosomes’ and the MT nucleation factor γ-tubulin were found to control cell division orientation [45••]. Taken together, these new findings support a PPB-independent mechanism that might set the initial spindle axis through cytoplasmic MT nucleation to assist in division plane orientation in seed plants.
These mechanisms, however, are inadequate to explain asymmetric division plane placement. The identification of a few polarly localized proteins at the cell cortex, for example, PAN1/ROP in maize [7,46] and BASL/YDA in Arabidopsis [4,5], provided useful tools towards revealing the regulatory mechanisms connecting cell polarity and division orientation in plants.
In a maize stomatal complex, the asymmetric division of two subsidiary mother cells (SMCs) is controlled by a PAN1 receptor-centered ROP polarity module (see reviews [38,47,48]). The polarized PAN1/ROP signaling was hypothesized to induce the formation of proximal actin patch, thereby nuclear migration, thus asymmetric placement of the division plane [7,46]. Alternatively, polarized PAN1/ROP signaling might influence the PPB positioning indirectly by promoting local cell wall expansion that is achieved by ROP-mediated actin nucleation and vesicle trafficking [38,49]. An uneven mechanical tension in the cell wall imposed by the regional expansion of the SMC may trigger cortical MT reorganization, which guides MT bundling and PPB formation [7,46,50].
In Arabidopsis, the polarity protein BASL controls asymmetric division of the stomatal precursor cells [4]. The orientation of BASL crescent was found to be guided by tissue-level mechanical forces as well as local peptide-receptor chemical signaling [51]. Before the mitotic division, the BASL polarity pole predicts the division plane to be placed distally (Figure 2) [4], yet the underlying mechanism has not been elucidated. In plant cells, MAPKs were found to regulate MT dynamics and organization, for example, Arabidopsis MPK4 phosphorylates the MAP65 bundling factors to promote MT turnover of the phragmoplast during cytokinesis [52]. With a MAPK-scaffolding function, BASL might regulate PPB positioning through the established function of MAPKs on the MT regulatory proteins, such as MAP65s [53]. Alternatively, at one end of the cell, high MAPK signaling may counteract the PP2A complex-mediated de-phosphorylation events, which promote MT ordering and bundling [54-56], to prevent proximal PPB formation.
Could these polarity complexes at the cell cortex impinge on the cytoplasmic MTOC-mediated spindle positioning system? At least for BASL, its polarity persists throughout the cell cycle [[45••]], allowing possible regulations on spindle positioning. Then, how could cytoplasmic MT nucleation contribute to the bipolar spindle establishment in plants? In the PPB-expressing angiosperms, ‘polar caps’ were suggested to be analogous structures of the cytoplasmic MTOCs [57], as ‘gametosomes’ in Physcomitrella patens [45••]. The formation of polar caps involves aggregation of MT nucleation at the nuclear periphery, and the two polar caps are segregated along an axis that is perpendicular to the PPB and decisive to the spindle orientation (Figure 2) [58]. In plant cells, microtubule nucleation is mainly mediated by an evolutionarily conserved γ-tubulin-containing ring complex (gTuRC) [59] that can be regulated by the augmin complex [60]. Recent studies showed that activated MPK6 localizes to MTs, physically binds to γ-tubulin and phosphorylates the MT plus end protein EB1c [61]. Defective MAPK signaling and lowered expression of EB1c in Arabidopsis resulted in mitotic abnormalities in chromosomal segregation and spindle orientation [61]. These findings hint a potential role of asymmetric MAPK signaling (e.g., induced by the BASL/YDA polarity complex) in the regulation of cytoplasmic MT nucleation, spindle orientation, thus division plane orientation in plants.
Plant cell differentiation: a link between cell polarity and localized cell wall modifications
Cell walls not only provide strength and mechanical support, but they also play diverse functions in plant growth, development, cell–cell communication, and cell morphogenesis. Specialized cell functions can be easily revealed by cell shape and structure. One prominent example is the production of a water-impermeable Casparian strip in the root endodermis.
The Casparian strip is a central ring-like lignin structure embedded in the root radial and transverse walls to physically prevent apoplastic diffusion of nutrients from the stele. The identification of key regulators and mechanisms for constructing the Casparian strip in Arabidopsis roots suggested that the formation of this central ring structure is tightly controlled by cell polarity signaling [9,62-64,65••]. An asymmetric signal triggered by the sulfated CIF peptides is perceived by the receptor-like kinase SCHENGEN3 (SGN3) [63,64], whose expression partially overlaps with a polarly distributed receptor kinase SGN1 (Figure 3a) [9]. The CIF1/2-SGN3/SGN1 signaling module defines the Casparian strip positioning. Lignin deposition at the Casparian strip depends on the EXO70A1 exocyst-mediated targeted delivery of the PM-localized CASPARIAN STRIP MEMBRANE DOMAIN PROTEINS (CASP1–5) that scaffold the enzymes for lignin biosynthesis [62,65••]. EXO70A1 appears to specifically function in directed targeting of CASPs to the PM, but not as a general regulator for secretion [65••]. The distribution of PtdIns(4,5)P2 coincides with the exocyst accumulation, suggesting that phospholipid signaling may be important for exocyst-based CASP localization [65••]. The studies on the Casparian strip formation disclosed an elegant integration of signaling events in cell–cell communication and spatially organized membrane trafficking that ensure precise cell wall modification during plant cell differentiation.
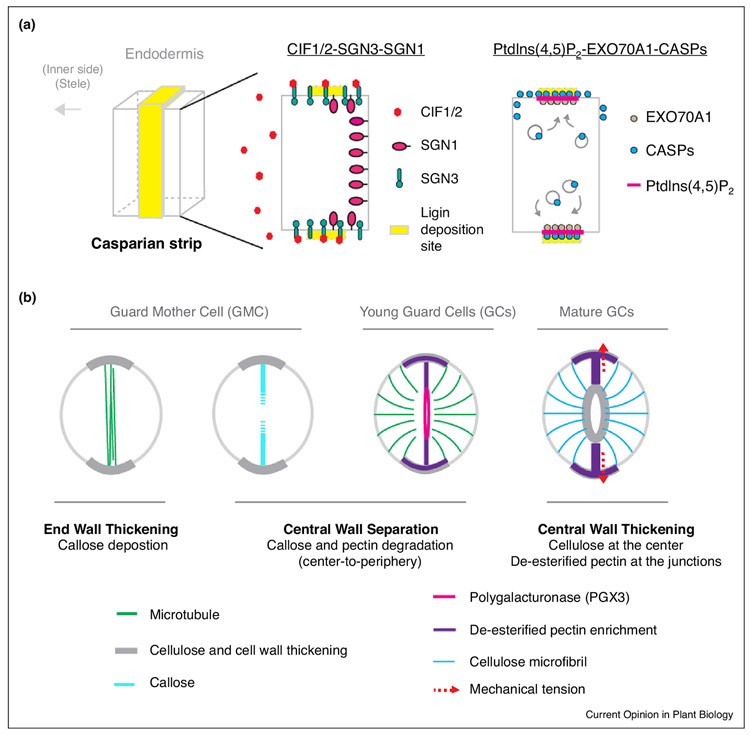
Figure 3.
Polarized cell wall specification in plant cell differentiation. (a) Diagrams show the formation of the Casparian strip in root endodermal development. The lignin deposition site is precisely defined by a CIF1/2-SGN3-SGN1 signaling module (left). The CASP proteins, which mark the Casparian strip site, are delivered to the PM by the exocysts containing EXO70A1 to the membrane sites coincides with the PtdIns(4,5)P2 enrichment (right). (b) Diagrams show asymmetric cell wall deformation, accumulation, and modification in stomatal differentiation. The end wall thickening of GMC predicts where the PPB (green) is placed. Central wall separation involves polarized callose and pectin degradation at the pore initiation site. A functional stomatal complex requires cell wall thickening at the central pore region and modified pectins (rigid de-esterified forms) at the two poles. The pectin hardening at the polar sites imposes cell wall stiffening and mechanical force (red arrows) for functional stomatal movement. Microtubules (green lines) guide the deposition of cellulose microfibrils (blue lines) in maturing GCs.
Stomata are highly specialized functional units for gaseous exchange between plants and the atmosphere. Stomatal guard cell walls must be both strong, to retain changes in turgor pressure, and flexible, to allow the stomatal apertures adjustable to environmental changes. The formation of a pair of kidney-shaped stomatal guard cells in dicot plants involves an one-time symmetric division of the guard mother cell (GMC), followed by stomatal pore formation and guard cell morphogenesis; a process that heavily requires asymmetric cell wall modification (Figure 3b and specified below).
In fact, prior to the symmetric cell division, the ovalshaped GMCs are already polarized, manifested by end-wall thickening at the two poles that predict the division plane orientation (Figure 3b) [66,67], but the underlying molecular mechanisms remain obscure. In ferns, after the GMC division, stomatal pore initiation coincides with the formation of the anticlinal MT bundles along the midregion of the ventral wall. This is soon followed by mutual separation of the adjacent plasmalemmata and stomatal pore broadening [68]. In this process, callose and pectin degradation takes place at the pore initiation sites.
Callose is produced by specialized cells in specific stages in the development of plant cell walls as well as in defense of environmental attacks [69]. During the formation of a stomatal pore, for example, in ferns and mosses, callose is deposited into the newly-synthesized cell wall, and soon degrades in a precisely controlled central-to-peripheral manner (Figure 3b) [70•,71]. An optimal level of callose in the differentiating stomatal wall seems to play an essential role in creating the central pore because chemical treatments that disturb callose synthesis or degradation inhibited normal pore formation in the fern Asplenium nidus [70•].
Active pectin degradation was also found in the process of stomatal pore formation. The pectin-degrading enzyme PGX3 (POLYGALACTURONASE INVOLVED IN EXPANSION3) accumulates polarly at the stomatal pore initiation sites (Figure 3b) [72••]. The loss-of-function mutant pgx3 produces shorter and smaller stomatal pores, suggesting that PGX3-mediated asymmetric pectin degradation is important for central wall separation [72••]. How callose and pectin are degraded spatiotemporally and how cell wall modifying enzymes, for example, PGX3, are precisely targeted to the pore initiation sites remain enigmatic. Future work is anticipated to reveal polarity signaling events underlying these procedures.
The maturation of guard cells is featured by striking wall thickening at two positions: (1) the inner walls at the pore site and (2) the end walls at the two poles (Figure 3b) [66]. The pore thickening was thought to promote stomatal opening and assist stomatal bending when guard cells inflate [73]. The new technical advances in atomic force microscopy (AFM) that measures mechanical properties of the cell surface showed that functional guard cells in Arabidopsis have a surprisingly high cell wall stiffening at the GC polar poles, coinciding with the polar enrichment of de-esterified (hard) pectins [74••]. Digestion of pectins with polygalacturonase led to dramatic changes in the stiffness pattern across the GCs, as well as defective stomatal function [74••]. The GC polar stiffening and polar enrichment of de-esterified pectin likely require an as yet unknown polar secretion of pectin methylesterases, for example, PME6 [75•]. Again, the final stage of guard cell differentiation requires the regulation of cell polarity, but the mechanisms for this regulation remain mysterious.
Concluding remarks
Research in recent years has greatly expanded our understanding of the molecular mechanisms underlying protein polarization, which unifies and cross-links polar cell expansion, asymmetric cell division, and cell differentiation in plants. This mechanistic understanding has been greatly aided by the analysis of in vivo protein dynamics using the FRAP microscopy technique. FRAP has the potential to uncover new principles of protein dynamics when applied to other polarity proteins, for example, D6PK that interacts with the PM through electrostatic interactions [76] and BIK1/MARK that localizes to the PM by interacting with the transmembrane receptor FLS2 [77].
It is clear that polarity signaling plays critical roles in the regulation of division orientation and cell differentiation in plants, and some relevant polarity signaling components and pathways have been identified in recent years. Yet many challenges remain towards elucidating the mechanisms behind protein polarization, the dynamics of polarity signaling machinery, and in understanding how polarity signaling controls cell division orientation and cell differentiation. In the regulation of cell division orientation, polarity signaling may distantly impinge on MT organization, either at the cell cortex or in the cytoplasm. Elucidating the composition and regulation of the polarity signaling complexes holds the promise to functionally connect the polarity module to the cell division machinery. In differentiating cells, how cell signaling pathways lead the way to ultimately achieve specific cell shape and specialized function remains a fascinating and fundamental question in biology. An emerging theme in this area is the importance of polar deposition, deformation and modification of cell wall components, such as lignin, suberin, pectin, and callose, and polar secretion of factors modifying them, as shown in root endodermal cells and aerial guard cells. The new advances raise exciting challenges in understanding the polarity signaling machinery that orchestrates multiple polarization processes and the mechanisms that coordinate these processes with cellulose microfibril deposition, which is spatially controlled by cortical MTs.
Acknowledgment
This work is supported by National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM109080.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Elion EA: The Ste5p scaffold. J Cell Sci 2001, 114:3967–3978. [DOI] [PubMed] [Google Scholar]
- 2.Irazoqui JE, Gladfelter AS, Lew DJ: Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol 2003, 5:1062–1070. [DOI] [PubMed] [Google Scholar]
- 3.Kemphues K: PARsing embryonic polarity. Cell 2000, 101:345–348. [DOI] [PubMed] [Google Scholar]
- 4.Dong J, MacAlister CA, Bergmann DC: BASL controls asymmetric cell division in Arabidopsis. Cell 2009, 137:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang P, Shao W, Zhu JK, Dong J: The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev Cell 2015, 33:136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao W, Dong J: Polarity in plant asymmetric cell division: division orientation and cell fate differentiation. Dev Biol 2016, 419:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartwright HN, Humphries JA, Smith LG: PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science 2009, 323:649–651. [DOI] [PubMed] [Google Scholar]
- 8.Doblas VG, Geldner N, Barberon M: The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 2017, 39:136–143. [DOI] [PubMed] [Google Scholar]
- 9.Alassimone J, Fujita S, Doblas VG, van Dop M, Barberon M, Kalmbach L, Vermeer JE, Rojas-Murcia N, Santuari L, Hardtke CS et al. : Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat Plants 2016, 2:16113. [DOI] [PubMed] [Google Scholar]
- 10.Lippincott-Schwartz J, Altan-Bonnet N, Patterson GH: Photobleaching and photoactivation: following protein dynamics in living cells. Nat Cell Biol 2003, Suppl:S7–14. [PubMed] [Google Scholar]
- 11.Henzler-Wildman K, Kern D: Dynamic personalities of proteins. Nature 2007, 450:964–972. [DOI] [PubMed] [Google Scholar]
- 12.Yeaman C, Grindstaff KK, Hansen MD, Nelson WJ: Cell polarity: versatile scaffolds keep things in place. Curr Biol 1999, 9:R515–517. [DOI] [PubMed] [Google Scholar]
- 13.Munro E, Bowerman B: Cellular symmetry breaking during Caenorhabditis elegans development. Cold Spring Harb Perspect Biol 2009, 1:a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garbett D, Bretscher A: The surprising dynamics of scaffolding proteins. Mol Biol Cell 2014, 25:2315–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Drogen F, Stucke VM, Jorritsma G, Peter M: MAP kinase dynamics in response to pheromones in budding yeast. Nat Cell Biol 2001, 3:1051–1059. [DOI] [PubMed] [Google Scholar]
- 16.Goehring NW, Hoege C, Grill SW, Hyman AA: PAR proteins diffuse freely across the anterior–posterior boundary in polarized C. elegans embryos. J Cell Biol 2011, 193:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B: C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol 2004, 14:851–862. [DOI] [PubMed] [Google Scholar]
- 18.Goehring NW: PAR polarity: from complexity to design principles. Exp Cell Res 2014, 328:258–266. [DOI] [PubMed] [Google Scholar]
- 19. ••.Martiniere A, Lavagi I, Nageswaran G, Rolfe DJ, Maneta-Peyret L, Luu DT, Botchway SW, Webb SE, Mongrand S, Maurel C et al. : Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc Natl Acad Sci U S A 2012, 109:12805–12810.This paper used fluorescence recovery after photobleaching (FRAP) to estimate the mobility of a set of membrane proteins. Retarded lateral diffusion of plasma membrane proteins in plants was reasoned from cell wall constraints, but not the cytoskeleton or membrane microdomains.
- 20.Langowski L, Wabnik K, Li H, Vanneste S, Naramoto S, Tanaka H, Friml J: Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells. Cell Discov 2016, 2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T: Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci U S A 2010, 107:5220–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan A, Yang Z: FRAP-based analysis of Rho GTPase-dependent polar exocytosis in pollen tubes. Methods Mol Biol 2012, 827:393–401. [DOI] [PubMed] [Google Scholar]
- 23.Sorek N, Segev O, Gutman O, Bar E, Richter S, Poraty L, Hirsch JA, Henis YI, Lewinsohn E, Jurgens G et al. : An S-acylation switch of conserved G domain cysteines is required for polarity signaling by ROP GTPases. Curr Biol 2010, 20:914–920. [DOI] [PubMed] [Google Scholar]
- 24. ••.Zhang Y, Guo X, Dong J: Phosphorylation of the polarity protein BASL differentiates asymmetric cell fate through MAPKs and SPCH. Curr Biol 2016, 26:2957–2965.The paper used FRAP to demonstrate low dynamics and minimal lateral diffusion of the membrane-associated polarity protein BASL in the stomatal lineage cells. Phosphorylated and active BASL showed further retarded mobility.
- 25.Friml J: Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur J Cell Biol 2010, 89:231–235. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald W, Friml J: The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J 2010, 29:2700–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, Yang Z: ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 2012, 10:e1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin D, Nagawa S, Chen J, Cao L, Chen X, Xu T, Li H, Dhonukshe P, Yamamuro C, Friml J et al. : A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol 2012, 22:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Lavagi I: Spatial control of plasma membrane domains: ROP GTPase-based symmetry breaking. Curr Opin Plant Biol 2012, 15:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalovsky S: Protein lipid modifications and the regulation of ROP GTPase function. J Exp Bot 2015, 66:1617–1624. [DOI] [PubMed] [Google Scholar]
- 31. •.Bendezu FO, Vincenzetti V, Vavylonis D, Wyss R, Vogel H, Martin SG: Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking. PLoS Biol 2015, 13:e1002097.In this study, FRAP analyses of functional fluorescently tagged Cdc42 protein revealed active lateral diffusion of Cdc42 at the plasma membrane. Engineered Cdc42 alleles with a transmembrane domain significantly slowed down the recoveries of membrane-fixed Cdc42.
- 32.Chiou JG, Balasubramanian MK, Lew DJ: Cell polarity in yeast. Annu Rev Cell Dev Biol 2017, 33:77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Z: Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol 2008, 24:551–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Bergmann DC, Dong J: Fine-scale dissection of the subdomains of polarity protein BASL in stomatal asymmetric cell division. J Exp Bot 2016, 67:5093–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbosa IC, Zourelidou M, Willige BC, Weller B, Schwechheimer C: D6 PROTEIN KINASE activates auxin transport-dependent growth and PIN-FORMED phosphorylation at the plasma membrane. Dev Cell 2014, 29:674–685. [DOI] [PubMed] [Google Scholar]
- 36.Stanislas T, Huser A, Barbosa IC, Kiefer CS, Brackmann K, Pietra S, Gustavsson A, Zourelidou M, Schwechheimer C, Grebe M: Arabidopsis D6PK is a lipid domain-dependent mediator of root epidermal planar polarity. Nat Plants 2015, 1:15162. [DOI] [PubMed] [Google Scholar]
- 37.Smertenko A, Assaad F, Baluska F, Bezanilla M, Buschmann H, Drakakaki G, Hauser MT, Janson M, Mineyuki Y, Moore I et al. : Plant cytokinesis: terminology for structures and processes. Trends Cell Biol 2017, 27:885–894. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen CG, Humphries JA, Smith LG: Determination of symmetric and asymmetric division planes in plant cells. Annu Rev Plant Biol 2011, 62:387–409. [DOI] [PubMed] [Google Scholar]
- 39.Lipka E, Herrmann A, Mueller S: Mechanisms of plant cell division. Wiley Interdiscip Rev Dev Biol 2015, 4:391–405. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen CG, Wright AJ, Muller S: The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J 2013, 75:258–269. [DOI] [PubMed] [Google Scholar]
- 41.Muller S, Han S, Smith LG: Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr Biolo 2006, 16:888–894. [DOI] [PubMed] [Google Scholar]
- 42.Azimzadeh J, Nacry P, Christodoulidou A, Drevensek S, Camilleri C, Amiour N, Parcy F, Pastuglia M, Bouchez D: Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 2008, 20:2146–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Iakovidis M, Costa S: Control of patterns of symmetric cell division in the epidermal and cortical tissues of the Arabidopsis root. Development 2016, 143:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. ••.Schaefer E, Belcram K, Uyttewaal M, Duroc Y, Goussot M, Legland D, Laruelle E, de Tauzia-Moreau ML, Pastuglia M, Bouchez D: The preprophase band of microtubules controls the robustness of division orientation in plants. Science 2017, 356:186–189.The authors identified an Arabidopsis mutanttrm6,7,8 in which the PPB formation is impaired, but the interphase microtubules are mostly normal. The PPB absence impairing the robustness of cell division orientation suggested the role of PPB acting as a stabilizer rather than a primary determinant for division plane orientation in plants.
- 45. ••.Kosetsu K, Murata T, Yamada M, Nishina M, Boruc J, Hasebe M, Van Damme D, Goshima G: Cytoplasmic MTOCs control spindle orientation for asymmetric cell division in plants. Proc Natl Acad Sci U S A 2017, 114:e8847–e8854.This paper studied how asymmetric cell division plane is determined in the PPB-less gametophore of the moss Physcomitrella patens. The authors identified discrete cytoplasmic microtubule organizing centers (MTOCs) that are dispensable for spindle formation but required for spindle orientation, thus division plane orientation.
- 46.Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG: ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 2011, 23:2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Facette MR, Smith LG: Division polarity in developing stomata. Curr Opin Plant Biol 2012, 15:585–592. [DOI] [PubMed] [Google Scholar]
- 48.Apostolakos P, Livanos P, Giannoutsou E, Panteris E, Galatis B: The intracellular and intercellular cross-talk during subsidiary cell formation in Zea mays: existing and novel components orchestrating cell polarization and asymmetric division. Ann Bot 2018, 00:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panteris E, Apostolakos P, Galatis B: Cytoskeletal asymmetry in Zea mays subsidiary cell mother cells: a monopolar prophase microtubule half-spindle anchors the nucleus to its polar position. Cell Motil Cytoskelet 2006, 63:696–709. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Facette M, Humphries JA, Shen Z, Park Y, Sutimantanapi D, Sylvester AW, Briggs SP, Smith LG: Identification of PAN2 by quantitative proteomics as a leucine-rich repeat-receptor-like kinase acting upstream of PAN1 to polarize cell division in maize. Plant Cell 2012, 24:4577–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bringmann M, Bergmann DC: Tissue-wide mechanical forces influence the polarity of stomatal stem cells in Arabidopsis. Curr Biol 2017, 27:877–883. [DOI] [PubMed] [Google Scholar]
- 52.Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y: Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev 2006, 20:1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komis G, Illes P, Beck M, Samaj J: Microtubules and mitogen-activated protein kinase signalling. Curr Opin Plant Biol 2011, 14:650–657. [DOI] [PubMed] [Google Scholar]
- 54.Camilleri C, Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D: The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 2002, 14:833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spinner L, Gadeyne A, Belcram K, Goussot M, Moison M, Duroc Y, Eeckhout D, De Winne N, Schaefer E, Van De Slijke E et al. : A protein phosphatase 2A complex spatially controls plant cell division. Nat Commun 2013, 4:1863. [DOI] [PubMed] [Google Scholar]
- 56.Kirik A, Ehrhardt DW, Kirik V: TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells. Plant Cell 2012, 24:1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marc J: Microtubule-organizing centres in plants. Trends Plant Sci 1997, 2:223–230. [Google Scholar]
- 58.Ambrose JC, Cyr R: Mitotic spindle organization by the preprophase band. Mol Plant 2008, 1:950–960. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto T: A ring for all: gamma-tubulin-containing nucleation complexes in acentrosomal plant microtubule arrays. Curr Opin Plant Biol 2013, 16:698–703. [DOI] [PubMed] [Google Scholar]
- 60.Liu T, Tian J, Wang G, Yu Y, Wang C, Ma Y, Zhang X, Xia G, Liu B, Kong Z: Augmin triggers microtubule-dependent microtubule nucleation in interphase plant cells. Curr Biol 2014, 24:2708–2713. [DOI] [PubMed] [Google Scholar]
- 61.Kohoutova L, Kourova H, Nagy SK, Volc J, Halada P, Meszaros T, Meskiene I, Bogre L, Binarova P: The Arabidopsis mitogen-activated protein kinase 6 is associated with gamma-tubulin on microtubules, phosphorylates EB1c and maintains spindle orientation under nitrosative stress. New Phytol 2015, 207:1061–1074. [DOI] [PubMed] [Google Scholar]
- 62.Roppolo D, De Rybel B, Denervaud Tendon V, Pfister A, Alassimone J, Vermeer JE, Yamazaki M, Stierhof YD, Beeckman T, Geldner N: A novel protein family mediates Casparian strip formation in the endodermis. Nature 2011, 473:380–383. [DOI] [PubMed] [Google Scholar]
- 63.Doblas VG, Smakowska-Luzan E, Fujita S, Alassimone J, Barberon M, Madalinski M, Belkhadir Y, Geldner N: Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 2017, 355:280–284. [DOI] [PubMed] [Google Scholar]
- 64.Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa-Ohnishi M, Matsubayashi Y: A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 2017, 355:284–286. [DOI] [PubMed] [Google Scholar]
- 65. ••.Kalmbach L, Hematy K, De Bellis D, Barberon M, Fujita S, Ursache R, Daraspe J, Geldner N: Transient cell-specific EXO70A1 activity in the CASP domain and Casparian strip localization. Nat Plants 2017, 3:17058.EXO70A1 encoding a subunit of the exocyst complex was identified from a genetic screen for its function in localizing the CASP scaffold proteins during the formation of the Casparian strip. The exo70a mutations did not alter general protein secretion, suggesting its specific role in targeted protein localization.
- 66.Lucas JR, Nadeau JA, Sack FD: Microtubule arrays and Arabidopsis stomatal development. J Exp Bot 2006, 57:71–79. [DOI] [PubMed] [Google Scholar]
- 67.Zhao L, Sack FD: Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Am J Bot 1999, 86:929–939. [PubMed] [Google Scholar]
- 68.Apostolakos P, Galatis B: Probable involvement of cytoskeleton in stomatal–pore formation in Asplenium nidus L. Protoplasma 1998, 203:48–57. [Google Scholar]
- 69.Pirelová B, Matuíková I: Callose: the plant cell wall polysaccharide with multiple biological functions. Acta Physiol Plant 2013, 35:635–644. [Google Scholar]
- 70. •.Apostolakos P, Livanos P, Nikolakopoulou TL, Galatis B: The role of callose in guard-cell wall differentiation and stomatal pore formation in the fern Asplenium nidus. Ann Bot 2009, 104:1373–1387.This paper presented dynamic callose deposition and degradation during stomatal pore formation. Chemical treatments that inhibited or prolonged callose accumulation affected stomatal pore formation in the ferns.
- 71.Merced A, Renzaglia K: Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Ann Bot 2014, 114:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. ••.Rui Y, Xiao C, Yi H, Kandemir B, Wang JZ, Puri VM, Anderson CT: POLYGALACTURONASE INVOLVED IN EXPANSION3 functions in seedling development, rosette growth, and stomatal dynamics in Arabidopsis thaliana. Plant Cell 2017, 29:2413–2432.The paper described detailed analysis of the polygalacturonase PGX3-mediated pectin degradation in stomatal pore formation and functional opening and closure. PGX3-GFP localizes to the cell wall and is enriched at the sites of stomatal pore initiation.
- 73.Keerthisinghe S, Nadeau JA, Lucas JR, Nakagawa T, Sack FD: The Arabidopsis leucine-rich repeat receptor-like kinase MUSTACHES enforces stomatal bilateral symmetry in Arabidopsis. Plant J 2015, 81:684–694. [DOI] [PubMed] [Google Scholar]
- 74. ••.Carter R, Woolfenden H, Baillie A, Amsbury S, Carroll S, Healicon E, Sovatzoglou S, Braybrook S, Gray JE, Hobbs J et al. : stomatal opening involves polar, not radial, stiffening of guard cells. Curr Biol 2017, 27:2974–2983 e2972.Using atomic force microscopy, the authors showed that mature guard cells display a radial gradient of stiffness. More importantly, an unexpected stiffening at the polar regions of the stomatal complexes was revealed for improved response sensitivity of stomatal aperture movement.
- 75. •.Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE: Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol 2016, 26:2899–2906.The paper showed that the enrichment of unesterified pectin in GC cell walls promotes stomata movement. A pectin methylesterase gene PME6 is required for pectin de-methyl-esterification and stomatal function.
- 76.Barbosa IC, Shikata H, Zourelidou M, Heilmann M, Heilmann I, Schwechheimer C: Phospholipid composition and a polybasic motif determine D6 PROTEIN KINASE polar association with the plasma membrane and tropic responses. Development 2016, 143:4687–4700. [DOI] [PubMed] [Google Scholar]
- 77.Lu D, Wu S, Gao X, Zhang Y, Shan L, He P: A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci U S A 2010, 107:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]