Abstract
Pearson syndrome (PS) is a multisystem mitochondrial respiratory chain disorder typically characterized by sideroblastic anemia and exocrine pancreatic insufficiency. PS is caused by a single large scale mitochondrial DNA deletion. PS classically presents in the first year of life and may be fatal in infancy. Children who survive PS may progress to develop Kearns-Sayre syndrome (KSS) later in life. The full phenotypic spectrum and prognosis of the condition continues to evolve.
Here we report five new patients with PS with unique clinical presentations, including four patients with onset later than previously reported in the literature, and one patient with prenatal onset of symptoms. The timing and unique features of these presentations support an expanded phenotypic spectrum of single large scale mitochondrial DNA deletion syndromes (SLSMDS) and reinforce the importance of including SLSMDS in the differential for children with complex multisystem presentations.
Keywords: Pearson syndrome, Kearns-Sayre syndrome, DNA, Mitochondrial, SLSMDS, Single large scale mitochondrial DNA deletion syndromes (SLSMDS)
INTRODUCTION
Single large scale mitochondrial DNA (mtDNA) deletion syndromes (SLSMDS) include three overlapping phenotypes; Pearson syndrome (PS), Kearns-Sayre syndrome (KSS), and Chronic Progressive External Ophthalmoplegia (CPEO). These conditions may evolve in a given individual over time (Goldstein and Falk, 2019). Patients may also present with Leigh syndrome or multisystemic symptoms that do not fit into one of the classically described phenotypes.
Pearson syndrome (Pearson marrow-pancreas syndrome, PS) is a multisystem mitochondrial disorder originally characterized by sideroblastic anemia and variable exocrine pancreatic insufficiency. Rotig et al. first found a deletion of the mitochondrial genome in a patient with PS, supporting the hypothesis that PS is caused by a mitochondrial respiratory enzyme defect (Rotig et al., 1989). It was initially described as fatal in infancy; however, patients who do survive infancy may go on to develop KSS later in life (Pearson et al., 1979, Rotig et al., 1989, Goldstein and Falk, 2019). KSS is defined by the onset before 20 years of age, pigmentary retinopathy, and progressive external ophthalmoplegia (PEO) with presence of at least one of the following: cardiac conduction block, cerebrospinal fluid protein concentration greater than 100 mg/dL, and/or cerebellar ataxia (Kearns and Sayre 1958, Rowland 1983). Other common clinical manifestations of KSS include short stature, hearing loss, dementia, renal tubulopathy, chronic kidney insufficiency, myopathy, diabetes mellitus, hypoparathyroidism, and growth hormone deficiency (Goldstein and Falk 2019, Kearns and Sayre 1958, Rowland 1983). CPEO is characterized by ptosis and ophthalmoplegia. Myopathy and dysphagia may also be present.
PS classically presents in the first year of life with bone marrow failure and exocrine pancreatic dysfunction. Patients have macrocytic sideroblastic anemia that is frequently transfusion-dependent and may be accompanied by thrombocytopenia and neutropenia (Pearson et al., 1979). Pancreatic dysfunction occurs secondary to fibrosis and leads to chronic diarrhea, malabsorption, and failure to thrive (Pearson et al., 1979). Other organ involvement can occur, including renal tubulopathy, liver cholestasis and/or fibrosis, adrenal insufficiency, diabetes mellitus, cardiomegaly and/or cardiac conduction defects (Superti-Furga et al., 1993, Atale et al., 2009, Williams et al., 2012). PS with multi-organ dysfunction can resemble other energy metabolic disorders; however, the exocrine pancreas involvement is relatively specific, although not present in the majority of PS patients (Fellman and Kotarsky, 2011, Honzik et al., 2012). Here we describe five new patients with PS with unique timing and clinical features of presentation. We report four late-onset patients, who were older at diagnosis than any previously reported patients, as well as a patient with evidence of prenatal onset. The age of onset and unique features of these presentations support an expanding phenotypic spectrum of SLSMDS as described by Goldstein and Falk (Goldstein and Falk, 2019) and reinforce the importance of including SLSMDS in the differential for a patient with a suspected energy metabolic defect, even in the absence of a classical presentation.
MATERIALS AND METHODS
A retrospective chart review of the clinical course and laboratory test results was performed for patients followed in our clinic for PS. Informed consent was provided and all patients were enrolled in the Children’s Hospital of Philadelphia (CHOP) Institutional Review Board (IRB) approved study #08–6177 (Marni J. Falk, PI) that allows for medical record reviews and clinical cohort analyses.
SUBJECT 1
Subject 1 is a 4-year-old male who was born to non-consanguineous African-American parents following an uncomplicated pregnancy. He has a family history notable for benign cyclic neutropenia in his father, migraines and myopia in his mother, a healthy full-sister and a healthy maternal half-sister. He was well until 27 months of age, when routine CBC obtained for a 2-year-old well child visit demonstrated anemia with a Hgb of 6.1 g/dL. Repeat analysis showed pancytopenia and he was admitted with a working diagnosis of viral bone marrow suppression. He was treated with a packed red blood cell transfusion and discharged home. However, he had continued pancytopenia, prompting a bone marrow biopsy that showed 15% sideroblasts and 4% blasts. A sideroblastic anemia gene panel (GeneDx Lab) was sent, which included the following genes: ABCB7, ALAS2, PUS1, SLC19A2, SLC25A38, TRNT1, YARS2 and large mtDNA genome deletion studies. This panel showed a large 2.9 kb pathogenic mtDNA deletion m.11521_14425del2905 at 72% heteroplasmy in blood, confirming a diagnosis of PS.
He has had a clinical course characterized by transfusion-dependent anemia and failure to thrive with intermittent episodes of acute pancreatitis. Given weight loss, an enteral feeding tube was recommended to the family. He has required supplemental pancreatic enzymes for pancreatic insufficiency. He had a transient episode of acute bilateral ptosis in the setting of a viral illness. His transfusion frequency is decreasing from his baseline of every 3–4 weeks to every 6–8 weeks, and episodes of acute pancreatitis have decreased in frequency. His physical, cognitive, social and language development have remained typical for age.
SUBJECT 2
Subject 2 is a 9-year-old female who was born to non-consanguineous Caucasian parents following an uncomplicated pregnancy. Her family history is remarkable for two healthy full-sisters. She was well until 2 years of age, when she presented with poikiloderma, pallor and fatigue. She was found to have anemia with a Hgb of 2 g/dL and required transfusions. A Diamond-Blackfan panel (RPL11, RP5 & RPS10, Ambry Genetics) was negative. Common mtDNA point mutations and deletion panel (Baylor Lab) revealed an 8 kb pathogenic mtDNA deletion by southern blot. Breakpoints were not determined and heteroplasmy was not quantified. She was transfusion-dependent for four years, but achieved hematologic remission by age 6. By age 7, her PS had evolved into KSS with insulin-dependent diabetes mellitus, primary hypoparathyroidism, sensorineural hearing loss, cerebellar ataxia, and renal tubular acidosis. She also has retinopathy, with symptoms of nyctalopia and dysfunction of both rods and cods on electroretinography (ERG). Her sensorineural hearing loss is now profound with need for hearing aids. She does not yet have CPEO. She was treated with EPI743 (Edison Pharmaceuticals, now Bioelectron, NCT02104336) until the study was terminated for inefficacy. However, she may have shown some response as she did not require any further transfusions after starting the drug, but did develop insulin dependent diabetes in that time frame. She has not experienced developmental regression; however, she receives physical therapy, occupational therapy, and vision therapy, and has an individualized education program at school.
SUBJECT 3
Subject 3 is a 5-year-old female who was born to non-consanguineous East Indian parents following an uncomplicated pregnancy. Family history was notable for one healthy full-brother. She was well until age 18 months, when she presented for pallor and fatigue and was found to have a Hgb of 4.1 g/dL. Further work-up revealed pancytopenia. A bone marrow biopsy revealed >15% sideroblasts and 3% blasts. Common mtDNA point mutations and large deletions analysis (GeneDx Lab) revealed a 3.8 kb pathogenic mtDNA deletion m.6141_9908del3767 at 76% heteroplasmy in blood, confirming a diagnosis of PS.
Subject 3 had a course characterized by acute pancreatitis requiring inpatient care every 5–6 weeks from 18 months of age until 3 years, complicated by worsening lacticemia (5–6 mmol/L when ill; baseline 2–3 mmol/L). She initially required monthly blood transfusions. At age 5, she is still transfusion-dependent, however her transfusions have been able to be spaced out in frequency since age 4. She has failure to thrive refractory to exogenous pancreatic enzyme supplementation and gastrostomy tube placement. She is on supplemental bicitra for mixed lactic acidosis and renal tubular acidosis.
Subject 3 had normal development until age 18 months, when she experienced a cessation of progress for expressive language. Despite recommendations, she did not have a formal audiology evaluation to rule out a sensorineural hearing impairment. However, her expressive language improved with speech therapy. She will be enrolled in kindergarten in a typical classroom.
SUBJECT 4
Subject 4 is a 2-year-old female who was born to Saudi Arabian parents following an uncomplicated pregnancy. She was well until 7 months of age when she developed frequent stools, failure to thrive, and developmental regression. By 21 months of age, additional manifestations included lactic acidosis, hypoglycemia, hepatomegaly and transaminitis, progressive weakness, and chronic diarrhea. On her first genetics evaluation at 25 months of age, she had severe malnutrition with weight <1% (z-score −6). Bloodwork showed hypophosphatemia and hypokalemia. Brain MRI was notable for diffuse white matter signal changes suggestive of leukodystrophy. Interestingly, there was sparing of the subcortical white matter, which is typically involved in KSS.
She was admitted for stabilization and multidisciplinary team evaluation. Her inpatient stay was complicated by frequent loose stools (5–10/day) with normal fecal fat content but very high fecal elastase, which responded to exogenous pancreatic enzyme supplementation. She had lactic acidosis (lactate 5.9 mmol/L, bicarbonate 15 mmol/L). Her electrolyte abnormalities were corrected with supplemental sodium bicarbonate, potassium chloride and potassium phosphate. Hypoglycemia was controlled with frequent feedings. A gastrostomy tube was placed. Additionally, Diazoxide was started for hypoglycemia. Liver biopsy was performed and showed moderate steatosis, lobular inflammation, and apoptosis with focal bridging and perisinusoidal fibrosis. Although the histologic findings were non-specific, the presence of micro and macrovesicular steatosis, fibrosis, and granular eosinophilic (oncocytic) change of hepatocytes was suggestive of mitochondrial disease. Her hemoglobin nadir was 8.6 g/dL Whole exome sequencing with mtDNA analysis revealed a 5 kb deletion m.8482_13459del4977 at 38% heteroplasmy in blood.
She was discharged after 2 months. Her weight continued to be less than the first percentile; however, her weight z-score had improved from −6 to −3. She has remained hematologically stable. Development was notable for weakness and delayed motor development.
SUBJECT 5
Subject 5 was a 10-month-old male born to non-consanguineous parents of mixed European, Chinese, and Mongolian descent at 39 weeks via repeat low-transverse Cesarean section following an uncomplicated pregnancy. Patient was small for gestational age with a birth weight of 2.62 kilograms. Apgar scores were 7 at 1 minute of life and 8 at 5 minutes of life; however, he was noted to be very pale. At 2 hours of life, he developed grunting and cyanosis. He was transferred to the neonatal intensive care unit (NICU), where he progressed to ventilator-dependent respiratory failure. Bloodwork revealed a hemoglobin of 5.5 g/dL, for which he received a 5 mL/kg transfusion of packed red blood cells without significant improvement. He also had leukopenia and neutropenia. He then received a partial volume exchange transfusion. His initial blood gas showed a base deficit of 18 mmol/L and a lactate of 8 mmol/L. Coagulation studies revealed a prolonged INR, PTT, and hypofibrinogenemia, and LDH was elevated at 2900 (upper limit of normal being 2100). An echocardiogram revealed pulmonary hypertension, for which he was started on inhaled nitric oxide. Ultimately, because of the severity of his multiorgan dysfunction, he was cannulated onto ECMO for 5 days until his pulmonary hypertension improved. Following ECMO decannulation, he reaccumulated lactic acid, with a baseline lactate around 15 mmol/L and a peak greater than the limits of detection (27 mmol/L). He continued to have significant requirements of dextrose (maximum glucose infusion rate of 22 mg/kg/min) and bicarbonate, with a maximum dose of 35 mEq sodium bicarbonate/kg/day. He was treated with 6.25 mg/kg/day of sodium dichloroacetate (TCI America) on a compassionate use basis (his GTSZ haplotype was confirmed to be non-EGT carrier, consistent with being a slow DCA metabolizer). Brain MRI with spectroscopy was overall non-specific, showing an abnormal signal in the deep white matter, scattered foci of chronic blood products; MR spectroscophy showed an elevated lactate doublet in a voxel placed over the basal ganglia, consistent with the patient’s lactatemia. Whole exome sequencing with mtDNA sequencing revealed a 4.2 kb pathogenic deletion m.10776_14945del4170 at 65% heteroplasmy in blood, consistent with the clinical diagnosis of PS.
He was supported in the NICU for the first two months of life. He was weaned off of supplemental respiratory support by about 3 weeks of life, was weaned off of his dextrose infusion, and was eventually transitioned to enteral bicarbonate of 34 mEq/kg/day with a stable lactate baseline of 9 mmol/L. He was stabilized on nasogastric bolus feeding. He was also started on pancreatic supplementation for pancreatic insufficiency. His course was complicated by transfusion-dependence for both thrombocytopenia and anemia. He required packed red blood cell and platelet transfusions every 1–4 weeks. He required a total of 8 re-admissions for vomiting, complicated by severe hypochloremia. At 10 months of age, his neutropenia and thrombocytopenia worsened and he was readmitted for evaluation. He developed skin lesions and was diagnosed with Sweet’s syndrome on skin biopsy. He then developed pseudomonas bacteremia with ecthyma gangrenosum, multifocal pneumonia, pressor-refractory septic shock, anasarca, and multisystem organ failure. His lactic acidosis worsened to a peak of 25 mmol/L and he ultimately passed away.
RESULTS AND DISCUSSION
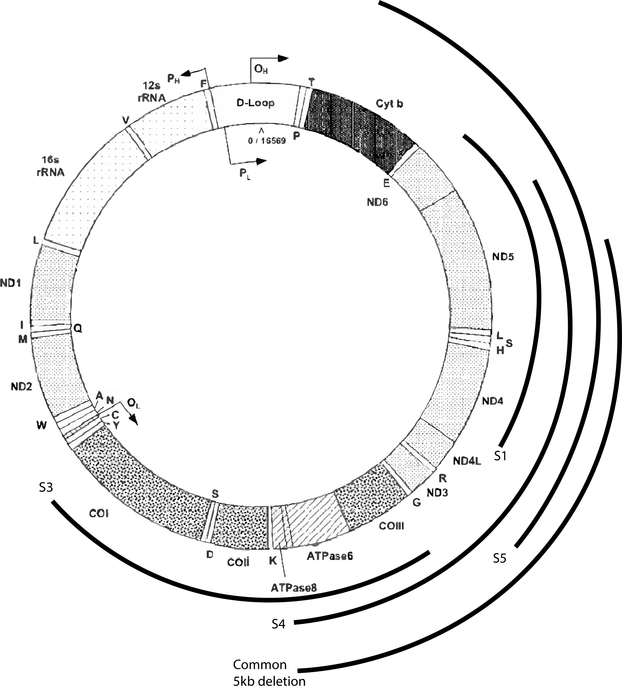
Here we present 5 subjects with unique clinical presentations of PS (Table 1). These subjects emphasize the variability of PS in onset, with 4/5 presenting between 18 months and 3 years of age, and 1 patient presenting with prenatal bone marrow failure. In contrast to the transfusion-dependent infantile anemia of classic PS, one of our subjects presented without anemia, and three more presented with an indolent anemia. In this cohort, episodes of pancreatitis and failure to thrive were severe recurrent features. For most subjects, episodes of pancreatitis presented with abdominal pain and anorexia. All 5 patients were also found to have a lactic acidosis. Their mtDNA deletions (Figure 1) include both the most common 5kb deletion, which encompasses 4,977 base pairs and removes mtDNA genes between the ATP8 and ND5 (Mita et al., 1990), as well as both larger and smaller deletions. The genetic basis of inheritance is sporadic. Although there is a theoretic risk of maternal inheritance, no cases have ever been reported, so maternal testing was not routinely performed on this cohort. However, for subject 5, maternal testing was performed and was negative.
TABLE 1:
Patient Characteristics At Diagnosis
| Subject | Age of Onset | Transfusion dependent (Y/N) | Pancreas Involvement (Y/N) | Presenting feature | Development | Race | Hgb g/dL | Lactate Mmol/L | Bicarb Mmol/L | Deletion | Bone Marrow |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 months | Y | Y | Asymptomatic anemia | Typical | African American | 6.1 | 5.4 | 20 | 2.9 kb pathogenic mtDNA deletion m.11521_14425del2905 with 72% heteroplasmy in blood | 15% sideroblasts and 4% blasts |
| 2 | 2 years | Y | N | Poikiloderma | Typical | White | 2.0 | 3.0 | 15 | 8 kb pathogenic mtDNA deletion in blood, heteroplasmy not performed | N/A |
| 3 | 18 months | Y | Y | Anemia with fatigue and pallor | Speech Delay | East Indian | 4.1 | 6.8 | 18 | 3.8 kb pathogenic mtDNA deletion m.6141_9908 with 76% heteroplasmy in blood | Macrocytic sideroblastic anemia >15% sideroblasts, 3% blasts |
| 4 | 21 months | N | Y | Failure to thrive, weakness, diarrhea | Motor Delay | Saudi Arabian | 8.6 | 5.9 | 15 | 5 kb deletion pathogenic mtDNA m.8482_13459del4977 with 38% heteroplasmy in blood | N/A |
| 5 | 0 days | Y | Y | Anemia, respiratory failure | Gross motor delay | White | 5.5 | 8 | 15 | 4.2 kb pathogenic mtDNA deletion m.10776_14945del4170 with 65% heteroplasmy in blood | N/A |
FIGURE 1: mtDNA Deletion Map.
Deletions found in the 5 reported patients labeled as Subject 1 (S1) to Subject 5 (S5) as well as the common 5kb deletion described above. The deletion size of S2 is unknown. mtDNA genome from Wallace et al., 1995.
Subject 5 had a very atypical and severe presentation on day of life 0, with apparently prenatal onset of bone marrow failure. He had severe primary lactic acidosis, accompanied by pulmonary hypertension. While mitochondrial dysfunction was suspected on the basis of his biochemical testing, the picture was complicated by his severe multisystem presentation. The etiology of his severe hypoglycemia was not well understood, but may have been secondary to overall inefficient ATP production. While his high GIR requirements did likely aggravate his underlying lacticemia, he likely had poor residual oxidative phosphorylation and was highly reliant on anaerobic respiration. In this case, there was no a priori suspicion for PS. There is only one other previously reported case of prenatal onset of PS (Rotig et al, 1990). In that case, the infant was also term, but weighed 3.3 kg at birth compared to our patient who weighed only 2.62 kg. The previously reported patient also had a severe anemia, as well as hydrops fetalis, neutropenia and thrombocytopenia, significant metabolic acidosis, diarrhea, hepatomegaly, and failure to thrive.
Some studies have suggested that the disease severity of SLSMDS is highly correlated with the size of the deletion and/or the level of heteroplasmy. Studies have also suggested that deletion of the mitochondrial cytochrome b (MT-CYB) gene is significantly associated with a severe KSS phenotype (Lopex-Gallardo et al., 2009, Grady et al., 2014). Among our patient sample, only subject 5 had a deletion including cytochrome b. This is intriguing as he did have an unusually severe phenotype. There was otherwise not a strong correlation between phenotype and deletion size, location, or heteroplasmy in our sample. To the contrary, subject 4 had a relatively severe phenotype despite heteroplasmy of only 38%.
While many mitochondrial disorders present in the neonatal period, they remain difficult to diagnose because of the absence of specific features. In a large review of 129 patients with neonatal onset of mitochondrial disease, prematurity, intrauterine growth retardation, hypotonia, ventilatory requirement, cardiomyopathy, neonatal seizures, Leigh syndrome, lactic acidosis, and hyperammonemia were among some of the presenting features (Honzik et al., 2012). Many of these features can be present in critically ill neonates regardless of the underlying disease, it is therefore important to keep mitochondrial disease in the differential for critically ill neonates such as Subject 5.
PS is a rare metabolic disease, with very few previously reported cases. Table 2 outlines the age of onset and features of 17 previously reported cases of PS in the literature. Unifying characteristics shared among this cohort include transfusion-dependence and exocrine pancreatic insufficiency. Several patients experienced recurrent infections and liver failure, which were the most common causes of death.
TABLE 2:
Patient Characteristics of Previously Reported Cases of Pearson Syndrome
| Citation | Age of onset | Transfusion dependent (Y/N) | Pancreas Involvement (Y/N) | Lactate Mmol/L | Other Features | Development | Deletion | Bone Marrow |
|---|---|---|---|---|---|---|---|---|
| McShane et al | 8 days | Y | Y | N/A | Patchy vitiligo and chronic candida infections | Typical until age 5, then no progression of reading skills | 4.9 kb at 63% heteroplasmy in blood and 95% in skeletal muscle | Abundant sideroblasts |
| Santorelli et al | 7 mos | Y | Y | N/A | Short statue, ataxia, ophthalmoparesis, bilateral lesions of pons, medulla, cerebellar white matter | Psychomotor delay | 3.6 kb m.8515_12120del3605 | N/A |
| Blaw et al | 1 mos | Y | Y | 3–4 | Short statue, ataxia | Typical, but with impaired executive function in school | 6 kb | N/A |
| Rotig et al - Subject 1 | 2 mos | Y | Y | 11.4 | Liver failure with nodular cirrhosis, death at 14 mos from acute metabolic acidosis with liver failure | N/A | 11 bp direct repeat flanking 4.192 kb deletion | Normal cellularity, vacuolization, 18% sideroblasts |
| Rotig et al - Subject 2 | 1 month | Y | Y | 6.7 | Major liver enlargement with progressive hepatic failure leading to death at 30 mos | N/A | 9 bp direct repeat flanking 2.748 kb deletion | Normal cellularity, vacuolization, 26% sideroblasts |
| Rotig et al - Subject 3 | 0 days | Y | Y | 11.9 | Severe anemia with hydrops fetalis requiring blood exchange in first few hours of life, died from liver failure at 25 months of age | N/A | 13 bp direct repeat flanking 4.977 kb deletion | Normal, cellularity, vacuolization |
| Rotig et al - Subject 4 | 7 mos | Y | Y | 7–9.8 | Progressive liver failure with tubulopathy, punctuate keratitis, died at 30 mo from intractable metabolic acidosis | N/A | 13 bp direct repeat flanking 4.977 kb deletion | Increased cellularity, vacuolization, 45% sideroblasts |
| Rotig et al - Subject 5 | 9 mos | Y | Y | 3.5 | Enlarged liver with hepatocellular dysfunction and cytolysis. Died from severe bacterial infection at 2 years | N/A | 13 bp direct repeat flanking 4.977 bp deletion | Increased cellularity, vacuolization, 10% sideroblasts |
| Muraki et al-Subject 1 | 18 days | Y | Y | N/A | Convulsions at 1 year due to hypoglycemia | Gross motor delay | 3.6 kb m.9337_12974del3637 | Hypocellularity and vacuolated precursors |
| Muraki et al-Subject 2 | 3 days | Y | Y | N/A | Severe metabolic acidosis, hypoglycemia, anemia by day of life 3 | N/A | 3.2 kb m.12203_15355del3151 | Vacuolization |
| Shanske et al | 4 mos | Y | Y | N/A | Recurrent episodes pneumonia, UTI, gastroenteritis, died at 1 year from septic shock | Global delay | 5.4 kb m.10004_15359del5355 at 40% heteroplasmy | Hypocellular marrow with decreased erythroid precurosors |
| Lee et al | 16 days | Y | Y | N/A | Phagocyte dysfunction, ptosis, ataxia | Gross motor delay | 6 kb m.7950_13993del6043 | Hypocellularity, vacuolization |
| Lacbawan et al | N/A | Y | N | N/A | Short stature, recurrent bacterial and fungal infections, flaky skin, transient alopecia | N/A | 4.4 kb m.10560_14980del4420 | N/A |
| Crippa et al-Subject 1 | 2 mos | Y | Y | 10–14 | Progressive liver failure with cholestasis, death at 23 mos from septic shock with multiorgan failure | Gross motor delay | N/A | Hypocellularity, vacuolization |
| Crippa et al-Subject 2 | 6 mos | Y | Y | 3–4 | Seizures, recurrent dental abscesses, head and hand tremors, progressive weakness | Mild motor and speech delays | N/A | Hypocellularity |
| Crippa et al-Subject 3 | 6 mos | Y | Y | 7 | Diabetes mellitus type 1 | Typical | 4.6 kb | Hypocellularity, vacuolization, sideroblasts |
| Crippa et al-Subject 4 | 6 mos | Y | Y | N/A | Cardiomegaly | Typical | 5 kb | Vacuolization, sideroblasts |
| Total number of subjects: # | Age (mos.) Median (range-range) | % showing | % showing | Median (range-range) | Median (range-range) | |||
| 17 | 3 (birth-9 mos) | 100% | 94% | 7 (3–14) | 5 kb (2.75–6) | |||
| Historic subjects + current cohort: # | Age (mos.) Median (range-range) | % showing | % showing | Median (range-range) | Median (range-range) | |||
| 22 | 6 (birth-27 mos) | 95% | 91% | 7 (3–14) | 4.2 kb (2.75–8) |
Although PS is classically thought of as a homogenous entity with bone marrow failure and/or exocrine pancreatic dysfunction in the first year of life, these subjects demonstrate the true diversity of this condition. Indeed, although 2 of our subjects had bone marrow biopsies showing a sideroblastic anemia, none of our patients had a clinical diagnosis of PS prior to the discovery of their mtDNA deletions ranging from 2.9–8 kb. Given improved testing techniques, it is also important to note that all of these mtDNA deletions were detected via blood samples and not muscle biopsies as was done in prior decades. Therefore, it is especially important to consider PS in the differential diagnosis of any patient with overlapping characteristics such as anemia and lactic acidosis, even in the absence of the full clinical syndrome. While most children who survive PS are thought to develop KSS, most of these patients are not followed longitudinally. Based on the patients presented here, PS is also likely underdiagnosed in children who may present later in life or with non-traditional presentations. This cohort reinforces that the natural history of PS may be poorly understood due to underdiagnosis of PS.
Overall, the timing and unique features of these 5 subjects support an expanding phenotype of single large scale mtDNA deletion syndromes (SLSMDS) as described by Goldstein et al. (Goldstein and Falk, 2019). The spectrum of disease in SLSMDS is broader than the conventionally described PS, KSS, and CPEO and it is important to recognize the variable presentations in the differential for mitochondrial disease.
Acknowledgments
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
The authors listed above have no conflicts of interest to disclose.
REFERENCES
- Atale A, Bonneau-Amati P, Rotig A, Fischer A, Perez-Martin S, de Lonlay P, Niaudet P, De Parscau L, Mousson C, Thauvin-Robinet C, Munnich A, Huet F, Faivre L. 2009. Tubulopathy and pancytopaenia with normal pancreatic function: A variant of Pearson syndrome. Eur J Med Genet 52:23–26. [DOI] [PubMed] [Google Scholar]
- Blaw ME, Mize CE. Juvenile Pearson syndrome. J Child Neurol. 1990;5(3):187–90. [PubMed] [Google Scholar]
- Crippa BL, Leon E, Calhoun A, Lowichik A, Pasquali M, Longo N. Biochemical abnormalities in Pearson syndrome. Am J Med Genet A. 2015;167A(3):621–8. [DOI] [PubMed] [Google Scholar]
- Fellman V, Kotarsky H. 2011. Mitochondrial hepatopathies in the newborn period. Semin Fetal Neonatal Med 16:222–228. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Falk M. 2019. Mitochondrial DNA deletion syndromes. Seattle (WA): GeneReviews. [Google Scholar]
- Honzik T, Tesarova M, Magner M, Mayr J, Jesina P, Vesela K, Wenchich L, Szentivanyi K, Hansikova H, Sperl W, Zeman J. 2012. Neonatal onset of mitochondrial disorders in 129 patients: Clinical and laboratory characteristics and a new approach to diagnosis. J Inherit Metab Dis 35: 749–759. [DOI] [PubMed] [Google Scholar]
- Kearns TP, Sayre GP. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch Ophthalmol. 1958;60:280–9. [PubMed] [Google Scholar]
- Lacbawan F, Tifft CJ, Luban NL, et al. Clinical heterogeneity in mitochondrial DNA deletion disorders: a diagnostic challenge of Pearson syndrome. Am J Med Genet. 2000;95(3):266–8. [PubMed] [Google Scholar]
- Lee HF, Lee HJ, Chi CS, Tsai CR, Chang TK, Wang CJ. The neurological evolution of Pearson syndrome: case report and literature review. Eur J Paediatr Neurol. 2007;11(4):208–14. [DOI] [PubMed] [Google Scholar]
- Mcshane MA, Hammans SR, Sweeney M, et al. Pearson syndrome and mitochondrial encephalomyopathy in a patient with a deletion of mtDNA. Am J Hum Genet. 1991;48(1):39–42. [PMC free article] [PubMed] [Google Scholar]
- Mita S, Rizzuto R, Moraes CT, Shanske S, Arnaudo E, Fabrizi GM, Koga Y, DiMauro S, Schon EA. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990;18:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HA, Lobel JS, Kocoshis SA, Naiman JL, Windmiller J, Lammi AT, Hoffman R, Marsh JC. 1979. A new syndrome of refractory sideroblastic anemia with vacuolization of marrow precursors and exocrine pancreatic dysfunction. J Pediatr 95:976–984. [DOI] [PubMed] [Google Scholar]
- Rötig A, Cormier V, Blanche S, Bonnefont JP, Ledeist F, Romero N, Schmitz J, Rustin P, Fischer A, Saudubray JM. Pearson’s marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J Clin Invest. 1990;86:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotig A, Colonna M, Bonnefont P, Blance S, Fischer A, Saudubray JM, Munnich A. Mitochondrial DNA Deletion in Pearson’s Marrow/Pancreas Syndrome. Lancet. 1989; 333, 8643: 902–903. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Hays AP, DiMauro S, De Vivo DC, Behrens M. Diverse clinical disorders associated with morphological abnormalities in mitochondria In: Scarlato G, Cerri C, eds. Mitochondrial Pathology in Muscle Diseases. Padua, Italy: Piccin; 1983:141–58. [Google Scholar]
- Santorelli FM, Barmada MA, Pons R, Zhang LL, Dimauro S. Leigh-type neuropathology in Pearson syndrome associated with impaired ATP production and a novel mtDNA deletion. Neurology. 1996;47(5):1320–3. [DOI] [PubMed] [Google Scholar]
- Shanske S, Tang Y, Hirano M, et al. Identical mitochondrial DNA deletion in a woman with ocular myopathy and in her son with pearson syndrome. Am J Hum Genet. 2002;71(3):679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A, Schoenle E, Tuchschmid P, Caduff R, Sabato V, DeMattia D, Gitzelmann R, Steinmann B. 1993. Pearson bone mar- row-pancreas syndrome with insulin-dependent diabetes, progressive renal tubulopathy, organic aciduria and elevated fetal haemoglobin caused by deletion and duplication of mitochondrial DNA. Eur J Pediatr 152:44–50. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Brown MD, Huoponen K, Torroni A 1995. Report of the committee on human mitochondrial DNA In Cuticchia AJ (ed) Human gene mapping 1995: a compendium. Johns Hopkins University Press, Baltimore, pp 910–954. [Google Scholar]