Abstract
Background
Postoperative pain remains a major clinical problem as there are limited analgesic strategies that have been proven to be effective in preventing and relieving this type of pain. Natural products, including flavonoids, have distinct pharmacological properties and play an important role in the discovery of analgesic drugs.
Materials and Methods
In this study, the flavonoid eriocitrin (eriodictyol 7-O-rutinoside), which is the main flavonoid in lemon fruit (Citrus limon), was mechanistically investigated for its prospective antinociceptive effect in a mouse model of postoperative pain. The antinociceptive property was evaluated by utilizing both tonic (acetic acid-induced writhing behavior) and phasic (hot-plate) nociception modalities. The hindpaw incisional surgery was performed and hyperalgesia was assessed using von Frey filaments.
Results
The tested doses of eriocitrin significantly attenuated (P<0.01, P<0.001) the chemically-induced tonic visceral nociception (5, 10, 15, and 30 mg/kg) and acute phasic thermal nociception (10, 15, and 30 mg/kg). A significant dose-dependent reduction in the incisional nociceptive hyperalgesia was exhibited by eriocitrin, with a marked antinociception observed at doses of 15 mg/kg (P<0.05 during 30–60 minutes) and 30 mg/kg (P<0.05, P<0.01 during 30–120 minutes).
Conclusion
The antinociceptive effect of eriocitrin (30 mg/kg) was strongly blocked by the antagonists of the opioid receptor, naltrexone, and GABAA receptor, bicuculline, thereby suggesting the involvement of opioidergic and GABAergic mechanisms in the nociception, reducing proclivity of eriocitrin during transmission of incisional nociception. These results concluded that eriocitrin has a potent antinociceptive effect in postoperative pain conditions, probably mediated through opioid and GABAA receptors.
Keywords: flavanones, surgical pain, analgesia, incisional pain, natural products
Introduction
Postoperative pain occurs following burn excision and/or grafting procedures and is most commonly the result of increased pain from newly-created wounds at the skin graft harvesting site.1 Postoperative pain may occur all over the body, including joints and muscles, head, and limbs, and is accompanied by restlessness, insomnia, sweating or lack of sweating, fatigue, poor appetite, or even dysfunction of the limbs.2 Various mechanisms have been identified for mediation of postoperative pain, which include the role of certain receptors, mediators, and neurotransmitters involved in the peripheral and central sensitization after incision.3 Poorly managed postoperative pain can lead to complications and prolonged rehabilitation. Uncontrolled acute pain is associated with the development of chronic pain, with a reduction in quality-of-life.4
Postoperative pain remains a major clinical problem and limited preventive strategies or analgesic agents have been proven effective in preventing and relieving this type of pain.4,5 The goal for postoperative pain management is to reduce or eliminate pain and discomfort with minimal side-effects.6 This is often best accomplished with a multimodal pain management approach.1,6 Various agents (opioid vs non-opioid), routes (oral, intravenous, neuraxial, regional) and modes (patient controlled vs as-needed) have been tested for the treatment of postoperative pain.3,4,7 However, the clinical utility of these approaches and analgesics is greatly limited by decreased clinical effectiveness and the occurrence of drug-specific side-effects among other factors.8,9
Natural products have been shown to play an important role in the discovery of analgesic drugs.10,14 Different chemical moieties having potent antinociceptive effects have been obtained from natural sources, resulting in novel lead compound classes for designing of analgesic drugs.15,16 Among natural compounds, flavonoids are considered important phytochemical classes that displayed distinct pharmacological properties.17–19 The current necessity for additional safe analgesic drugs having lessened side-effects can be efficiently covered by investigating both natural and synthetic flavonoids for their pain reducing properties.20,21 The activity of flavonoids does not depend on abolishing a single mechanism, but rather reducing varied mechanisms and therefore impacting multiple targets in the neurological processes underlying the expression of pain.22 Flavonoids are multi-target molecules, and increasing attention has been given to these molecules due to their anti-inflammatory and analgesic properties.21,23 Flavonoids have shown potent antinociceptive properties in different animals models of nociception.24–26
Citrus limon (family: Rutaceae) has been widely studied as an important source of natural flavonoids. The fruit, peel, essential oil, and isolated compounds from Citrus limon possess strong antioxidant and antinociceptive,27 anti-inflammatory,28 antibacterial,29,30 antifungal,29 antiviral,31 anticancer,32 antidiabetic and hypolipidemic,33 hepatoprotective,34 antiproliferative,35 nephroprotective,36 and neuroprotective37,38 properties. Eriocitrin (eriodictyol 7-O-rutinoside) is the major flavonoid found in lemon fruit. Experimental studies have shown that eriocitrin possesses strong antioxidant properties,39 ameliorates diet-induced hepatic steatosis and activates mitochondrial biogenesis,40 suppresses oxidative damage in the liver caused by acute exercise-induced oxidative stress,41 reduces the level of lipids by causing a decrease in low density lipoproteins and very low density lipoproteins,42 inhibits the activities of lipoxygenases,43 suppresses the proliferation of human hepatocellular carcinoma cells by inducing apoptosis and arresting cell cycle,44 and decreases the levels of pro-inflammatory cytokines TNF-α and IL-1β.45 In the present study, eriocitrin was investigated for antinociceptive propensity in a mouse model of postoperative pain, and the mediation of its antinociception was evaluated by using specific opioid and GABAA receptor antagonists.
Materials and Methods
Chemicals
Eriocitrin (Sigma-Aldrich, St. Louis, MO), naltrexone hydrochloride (Sigma-Aldrich), bicuculline methiodide (Sigma-Aldrich), acetic acid (PanReac AppliChem GmbH, Darmstadt, Germany), diclofenac (Voren® injection 75 mg/3.0 mL, Asian Continental (Pvt) Ltd., Karachi, Pakistan), morphine (Morfscot® injection, 10 mg/mL), ketamine (Ketarol®, 50 mg/mL, Global Pharmaceutical, Islamabad, Pakistan), and xylazine (Xylaz®, 20 mg/mL, Bladel, Netherlands). Diclofenac, morphine, naltrexone and bicuculline were dissolved in 0.9% normal saline. Eriocitrin was dissolved in a vehicle comprising of 1% Tween 80 (Scharlau, Barcelona, Spain), 5% dimethyl sulfoxide (Unichem, Greenville, USA), and 94% normal saline. All the drugs were administered intraperitoneally in a volume of 10 mL/kg.
Animals
Adult male BALB/c mice weighing 25–30 g were used. They were kept in a 12 h/12 h light/dark cycle (lights on at 08:00, lights off at 20:00) and were acclimatized 1 week before the start of experiments. The animals were randomly assigned to the various treatment groups and were used only once. The experiments were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986, and the protocols were approved by the Institutional Animals Care Committee (13/EC-15/Pharm, dated: April 10, 2015) and the institutional approval reference number AMSEC19-15/4/2019 of Laboratory Medicine Department, Umm Al-Qura University, KSA. The experimental procedures were also in compliance with the guidelines of the International Association for the Study of Pain. All efforts were taken in order to minimize suffering to animals. The number of animals used was the minimum number consistent with obtaining significant data.
Acetic Acid-Induced Abdominal Constrictions Test
For the assessment of tonic nociception, the acetic acid-induced abdominal constrictions test was utilized.11,46 The animals were randomly divided into various groups, with each group consisting of six mice. Group 1 was administered with the vehicle and served as the negative control. Group 2 was administered the standard diclofenac at a dose of 50 mg/kg (i.p.).13 Groups 3–6 were injected with the tested doses of eriocitrin (5, 10, 15, and 30 mg/kg), respectively. After an interval of 20 minutes, all the animals were intraperitoneally injected with a 1% acetic acid solution and the number of abdominal constrictions were counted after 10 minutes of acetic acid injection and continued for an additional 20 minutes. The number of writhes in all the groups were noted and was converted into the percentage antinociception using the following equation:
 |
Hot-Plate Test
The hot-plate test was used to assess the antinociceptive effect of eriocitrin against acute phasic thermal nociception, as reported previously.47 The animals were randomly divided into various groups with each group consisting of six mice. The hot-plate was maintained at a temperature of 50ºC±1ºC. The latency time was determined until escape behavior in the form of jumping, licking, and shaking of hindpaws was observed. A cut-off time of 90 seconds was imposed in order to avoid tissue injury to the paws. Group 1 was administered with the vehicle. Group 2 was injected with the standard drug, morphine at a dose of 6 mg/kg.48 Groups 3–6 were given with eriocitrin at the tested doses of 5, 10, 15, and 30 mg/kg, respectively. The hot-plate latency time was determined 30 minutes after the various drugs treatments. The latency times were converted into percentage antinociception using the following equation:
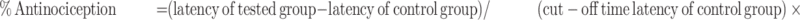 |
Induction of Surgical Nociception and Its Assessment
The antinociceptive propensity of eriocitrin in postoperative pain conditions was evaluated using the mouse model of hindpaw incisional surgery, as previously reported.49 The randomly allocated mice in the various groups were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (8 mg/kg). On the plantar surface of hindpaw, a 5 mm longitudinal incision was made with a surgical blade, starting 2 mm from the proximal edge of the heel and extending towards the toes. The plantaris muscle was elevated and incised longitudinally. At the end, a single 6/0 nylon suture was placed through the midpoint of the incision. The animals were allowed to recover in their respective home-cages. The nociception induced by the incisional surgical procedure was assessed using a set of calibrated von Frey monofilaments. The mice were placed on an elevated wire mesh table and the mechanical hyperalgesia was assessed using the up–down method with bending forces of 0.008, 0.04, 0.02, 0.07, 0.4, 0.6, 1, 1.4, 2, 4 g.50 The collected data were interpreted and converted into the 50% withdrawal threshold.51
Experimental Protocol
The antinociceptive doses of eriocitrin for assessment in the postoperative nociception experiment were determined from the results obtained in the preliminary antinociceptive activities as mentioned above. The animals were tested postoperatively for nociception expression and only those animals that displayed marked hyperalgesia were selected. The expression of nociception was determined after 2 hours of incisional surgery and again on day 1 and day 2, to ascertain the maintenance of hyperalgesia induced after the incision. On day 2 of the experiment, the selected animals were randomly assigned to various experimental groups. Group 1 was non-operated and was only administered with the vehicle and served as the negative control. The animals in group 2 were subjected to incisional surgery and were administered the vehicle. The incisional surgical procedure was also performed on animals in groups 3–5 and were then administered with the tested doses of eriocitrin at 10, 15, and 30 mg/kg, respectively, through the intraperitoneal route. The possible effect of eriocitrin on the nociception expression was assessed on post-treatment time-periods of 30, 60, and 120 minutes. On these time-periods, the animals were assessed for nociception on the wire mesh table using calibrated von Frey mono filaments, as mentioned above. The antagonism study was conducted in another cohort in which the incision induced animals were administered with the opioid receptor antagonist, naltrexone (3 mg/kg), and GABAA receptor antagonist, bicuculline (3 mg/kg), 10 minutes prior to eriocitrin administration. The different groups were then tested for mechanical hyperalgesia using von Frey mono filaments at a predetermined time-period of 30–120 minutes. The data collected from the drug-treated cohorts were then compared with the vehicle-treated incisional animals group. The anti-hyperalgesia was quantified as the percentage maximum possible effect (MPE) from the paw withdrawal threshold data by using the following equation:
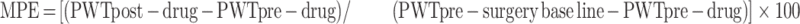 |
Statistical Analysis
The data were represented as mean±standard error of the mean (SEM). When two groups were compared, the Wilcoxon matched-pairs signed ranked test was used. For multiple comparison the data were analyzed using either one-way or two-way analysis of variance (ANOVA) followed by appropriate post-hoc tests. The statistical analyses were performed using GraphPad Prism Version 8.0.2 (GraphPad Software, Inc., USA). A value of P less than 0.05 was considered as statistically significant.
Results
Effect of Eriocitrin in Acetic Acid-Induced Nociception
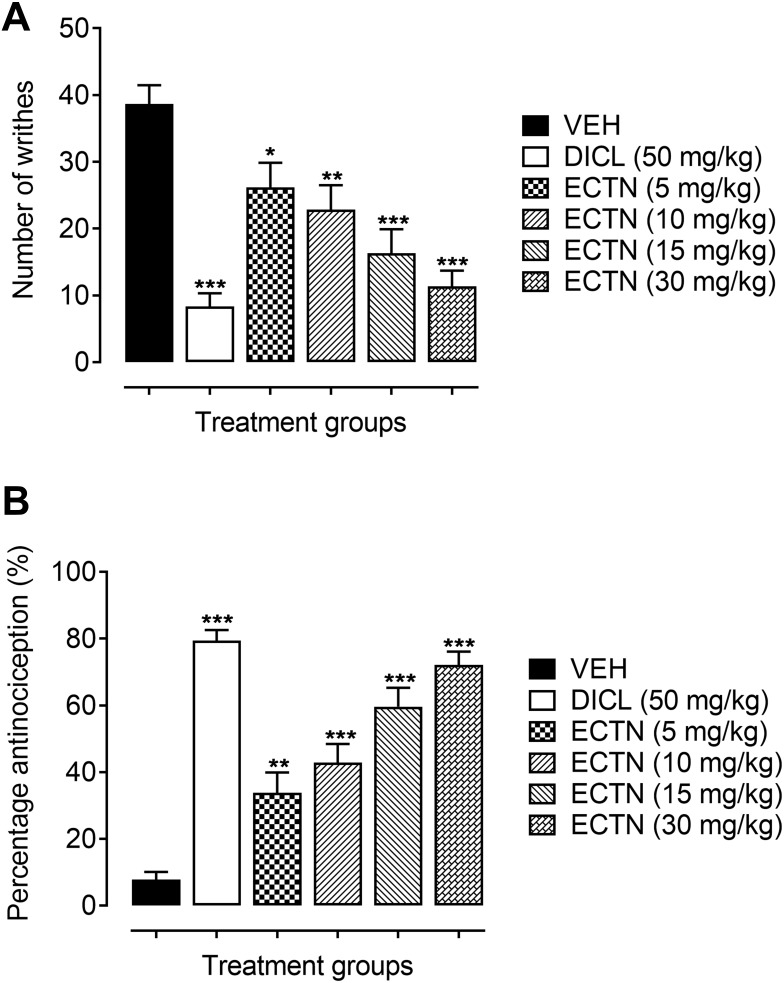
A marked increase in the number of abdominal constrictions were observed in the group of animals administered only with the vehicle (38.67±2.82). This increase in the number of writhes was significantly attenuated [F(5, 30)=12.89, P<0.001] by the tested doses of eriocitrin and the standard, diclofenac. The post hoc analysis revealed a significant decrease in the acetic acid-induced abdominal constrictions at a dose of 5 mg/kg (26.17±3.70, P<0.05), as compared to the vehicle administered group. Similarly, the higher doses of eriocitrin also significantly lessened the acetic acid-induced nociception as revealed from the significant decrease in the number of writhes at doses of 10 mg/kg (22.83±3.67, P<0.01), 15 mg/kg (16.33±3.57, P<0.001), and 30 mg/kg (11.33±2.36, P<0.001), as compared to the vehicle administered control group (Figure 1A). The percentage antinociception further confirmed the potent antinociceptive property of eriocitrin [F(5, 30)=31.91, P<0.001]. It was observed that a significant increase in the percentage protection was afforded by eriocitrin at the tested doses of 5 mg/kg (33.79%, P<0.01), 10 mg/kg (42.84%, P<0.001), 15 mg/kg (59.85%, P<0.001), and 30 mg/kg (72.13%, P<0.001), as compared to the vehicle treated animals group (7.75%). The administration of the standard diclofenac at a dose of 50 mg/kg also produced a robust nociception decreasing property as revealed from the significant decrease in the number of acetic acid-induced writhes (8.33±2.82, P<0.001) and a significant increase in the percentage of antinociception (79.36%, P<0.001), when compared to the vehicle administered control group (Figure 1B). The ED50 (50% effective dose) for eriocitrin in the acetic acid test was calculated as 11.7 mg/kg.
Figure 1.
Antinociceptive effect of ERCN and DICL in the acetic acid-induced abdominal constriction test. Effect of eriocitrin on number of abdominal constrictions (A), and percentage nociception inhibition (B). Each column represents the mean number of writhes or percentage antinociception±SEM. *P<0.05, **P<0.01, ***P<0.001 as compared to the VEH administered group, one-way ANOVA followed by Dunnett’s post hoc test, n=6 mice per group.
Abbreviations: DICL, diclofenac; ERCN, eriocitrin; VEH, vehicle.
Effect of Eriocitrin in Hot-Plate Test
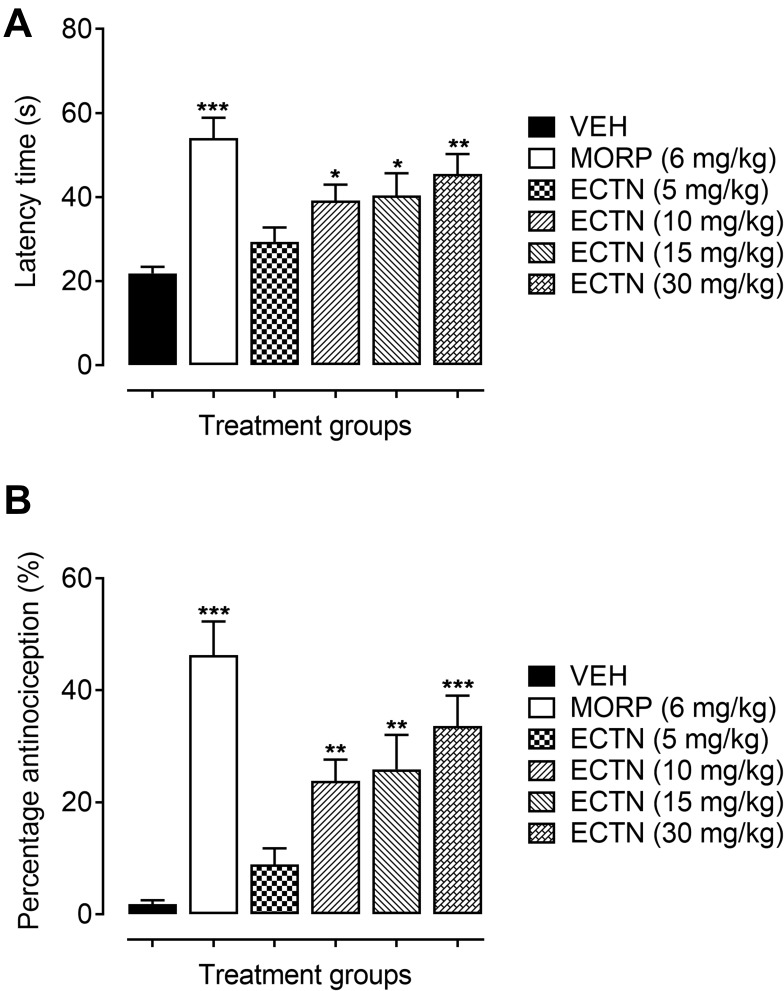
In the hot-plate test, the tested doses of eriocitrin and the standard, morphine, has a significant effect on the thermal-induced phasic nociception [F(5, 30)=7.643, P<0.001]. The post hoc analysis revealed that the tested dose of eriocitrin at 10 mg/kg produced a significant increase (39.17±3.79 seconds, P<0.05) in the withdrawal latency as compared to the vehicle administered control group (21.93±1.53 seconds). Similarly, the 15 mg/kg dose of eriocitrin also exhibited an attenuating effect on acute phasic thermal nociception, as the latency time was significantly increased (40.33±5.32 seconds, P<0.05) as compared to the vehicle treated group. The 30 mg/kg dose of eriocitrin produced a potent antinociceptive effect against the heat-induced phasic nociception, which was observed from the significant increase (45.50±4.72 seconds, P<0.01) in the thermal latency time as compared to the vehicle control group (Figure 2A). The phasic nociception attenuating effect of eriocitrin was further corroborated from the significant percentage antinociception [F(5, 30)=12.45, P<0.001]. A significant increase in the percentage protection against thermal-induced nociception was produced by eriocitrin at the tested doses of 10 mg/kg (23.81%, P<0.01), 15 mg/kg (25.89%, P<0.01), with a strong protection affinity exemplified by the 30 mg/kg dose (33.61%, P<0.001), as compared to the vehicle treated control group. The antinociceptive effect of eriocitrin was comparable to that produced by the standard, morphine, which at a dose of 6 mg/kg significantly increased (54±4.86 seconds, P<0.001) the latency time and bestowed a significant percentage of antinociception (46.30%, P<0.001) (Figure 2B). The ED50 for eriocitrin in the hot-plate test was calculated as 28.3 mg/kg.
Figure 2.
Antinociceptive effect of ERCN and MORPH in the hot-plate test. Effect of eriocitrin on latency time (A), and percentage nociception inhibition (B). Each column represents mean latency time in seconds or percentage antinociception±SEM. *P<0.05, **P<0.01, ***P<0.001 as compared to the VEH administered group, one-way ANOVA followed by Dunnett’s post hoc test, n=6 mice per group.
Abbreviations: ERCN, eriocitrin; MORPH, morphine; VEH, vehicle.
Effect of Eriocitrin in Post-Operative Nociception
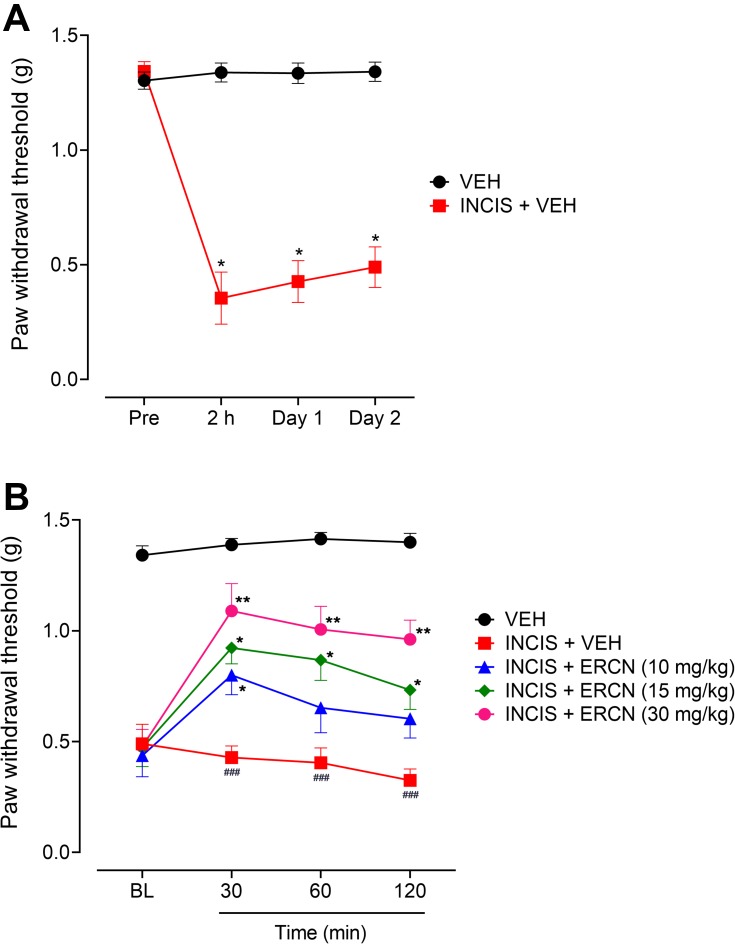
The incisional surgical procedure induced a considerable nociception in the paw that was observed from the decrease in the threshold to withdrawal the paw after application of a static stimulus and was assessed from the significant decrease in the paw withdrawal threshold. The expression of this was noted after 2 hours of incision (P<0.05) and remained significant with similar intensity when evaluated after day 1 (P<0.05) and day 2 (P<0.05) of incision, as compared to the paw withdrawal thresholds of the vehicle-administered non-surgical control animals group (Figure 3A).
Figure 3.
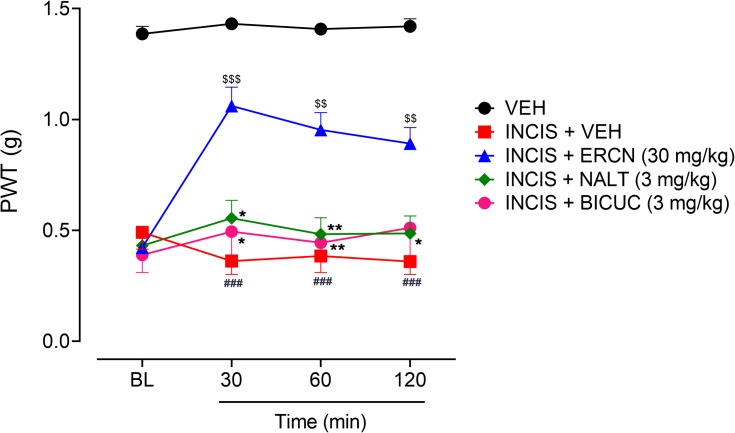
Antinociceptive effect of eriocitrin in the postoperative nociception. (A) Expression of nociception after INCIS surgery. Each symbol represents mean paw withdrawal threshold in g±SEM. *P<0.05 as compared to the VEH administered group, Wilcoxon matched-pairs signed ranked test, n=6 mice per group. (B) Effect of ERCN on postoperative nociception expression. Each symbol represents mean paw withdrawal threshold in g±SEM. ###P<0.05 as compared to the VEH administered group. *P<0.05, **P<0.01 as compared to the VEH administered incisional control group, two-way repeated measures ANOVA followed by Tukey’s post hoc test, n=6 mice per group.
Abbreviations: ERCN, eriocitrin; INCIS, incisional; Pre, pre-incisional surgery paw withdrawal threshold; BL, baseline paw withdrawal threshold before eriocitrin administration; VEH, vehicle.
A considerable nociception attenuating effect was observed after intraperitoneal administration of the tested doses of eriocitrin [time=(F(3, 75)=11.14, P<0.0001), treatment=(F(4, 25)=96.67, P<0.0001), interaction=(F(12, 75)=2.63, P=0.006)]. The 10 mg/kg dose of eriocitrin was able to significantly modify the surgery-induced reduction in the nociceptive threshold and was observed as a significant increase (P<0.05) in the paw withdrawal threshold after 30 minutes of post-treatment as compared to the vehicle-treated incisional control group. However, the increase in PWT was non-significant when the nociception was assessed after 60 and 120 minutes of dose administration. The 15 mg/kg dose of eriocitrin also produced a significant increase in the static pressure induced reduced withdrawal threshold in the paw incision animals after 30 minutes of injection and the effect remained significant for the subsequent study duration of 60 minutes (P<0.05) and 120 minutes (P<0.05). The surgery inflicted reduction in the nociceptive threshold was potently modified by the higher dose of eriocitrin (30 mg/kg), which significantly elevated the reduced von Frey filaments applied static nociceptive threshold. The nociception expression was significantly altered at the tested time-period of 30 minutes (P<0.01), 60 minutes (P<0.01), and 120 minutes (P<0.01), as compared to the vehicle administered incisional control animals’ group (Figure 3B).
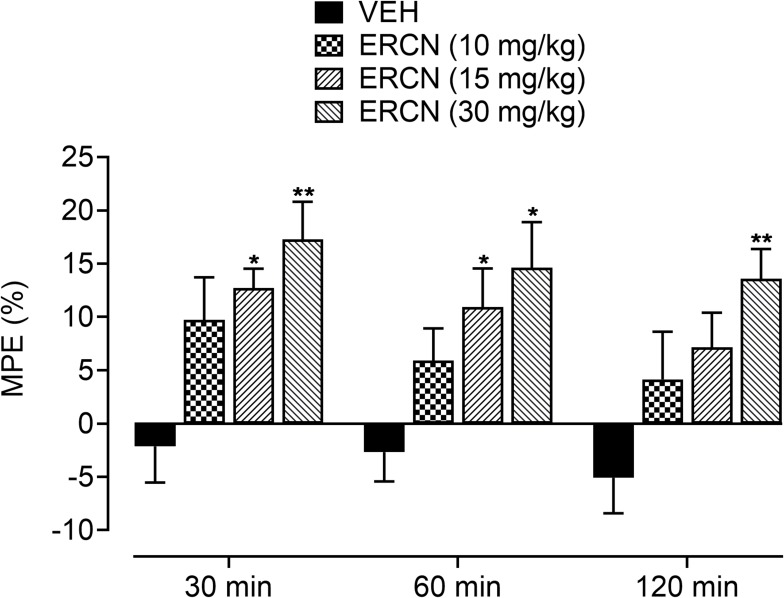
The intrinsic antinociceptive property against postoperative nociception was further affirmed from the maximum possible effect, which was considerably increased for the tested dose of eriocitrin [time=(F(2, 40)=3.03, P=0.07), treatment=(F(3, 20)=8.29, P<0.001), interaction=(F(6, 40)=0.13, P=0.99)]. The vehicle administered animals showed a marked reduction in the percentage protection from the surgery induced nociception proclivity. A non-significant increase in the percentage of antinociception was apparent for the 10 mg/kg dose of eriocitrin. However, the increase in the maximum protection afforded was significant (P<0.01) for the 15 mg/kg dose of eriocitrin after 30 minutes (12.7%) and 60 minutes (10.9%) of study duration, when compared to the vehicle-treated incisional control animals. A long lasting increment in the percentage maximum possible effect was yielded by eriocitrin at 30 mg/kg, the antinociceptive effect of which remained significant throughout the paradigm testing duration of 30 minutes (17.2%, P<0.01), 60 minutes (14.6%, P<0.05) and 120 minutes (13.5%, P<0.01), as compared to that yielded by vehicle treatment on the respective time-period (Figure 4).
Figure 4.
Percentage antinociceptive effect of ERCN in postoperative nociception. Each column represents the mean maximum possible effect±SEM. *P<0.05, **P<0.01 as compared to the VEH administered incisional control group, two-way repeated measures ANOVA followed by Dunnett’s post hoc test, n=6 mice per group.
Abbreviations: ERCN, eriocitrin; VEH, vehicle.
Antinociceptive Mechanism of Eriocitrin in Postoperative Nociception
The mechanisms underlying the antinociceptive property in postoperative nociception were investigated by administration of specific antagonists including naltrexone for the involvement of opioidergic mechanism and bicuculline for probable mediation of GABAergic transmission. The administration of antagonists significantly altered the antinociceptive effect of eriocitrin [time=(F(3, 75)=4.40, P=0.008), treatment=(F(4, 25)=118.41, P<0.0001), interaction=(F(12, 75)=3.35, P<0.001)]. As previously evaluated, the 30 mg/kg dose of eriocitrin produced a marked antinociceptive effect, as observed from the significant increase in the monofilament applied paw pressure associated reduction in the nociceptive threshold in the surgical incision animals (P<0.001 after 30 minutes, P<0.01 during 60–120 minutes). The marked antinociceptive effect of eriocitrin was strongly blocked when the naltrexone administered animal’s paws were stimulated by the static pressure application. The antagonism produced by the opioid antagonist, naltrexone remained in place throughout the study duration (30–120 minutes) as the paw withdrawal threshold observed was not significantly different from the vehicle administered incision animals group. Similarly, administration of GABAA receptor antagonist, bicuculline, also completely antagonized the antinociceptive effect produced by eriocitrin (30 mg/kg) as the paw withdrawal threshold was non-significant for all the testing duration in comparison to the vehicle treated incision control animals’ group. However, in comparison to the eriocitrin alone treated group, the naltrexone injected animals group showed a significant decrease in the paw withdrawal thresholds at 30 minutes (P<0.05), 60 minutes (P<0.01), and 120 minutes (P<0.05), while for the bicuculline injected group, the decrease was noted at 30 minutes (P<0.05) and 60 minutes (P<0.01) of study duration (Figure 5). These effects implied the mediation of opioidergic and GABAergic neurotransmission in the antinociceptive effect associated with eriocitrin in postoperative pain conditions.
Figure 5.
Effect of NALT and BICUC on ERCN antinociceptive effect in postoperative nociception. Each symbol represents the mean PWT in g±SEM. ###P<0.05 as compared to the vehicle administered control group. $$P<0.01, $$$P<0.001 as compared to the VEH administered incisional control group, *P<0.05, **P<0.01 as compared to the ERCN alone administered incisional group, two-way repeated measures ANOVA followed by Tukey’s post hoc test, n=6 mice per group.
Abbreviation: BICUC, bicuculline; BL, baseline paw withdrawal threshold before administration of eriocitrin and antagonists; ERCN, eriocitrin; NALT, naltrexone; PWT, paw withdrawal threshold; VEH, vehivle.
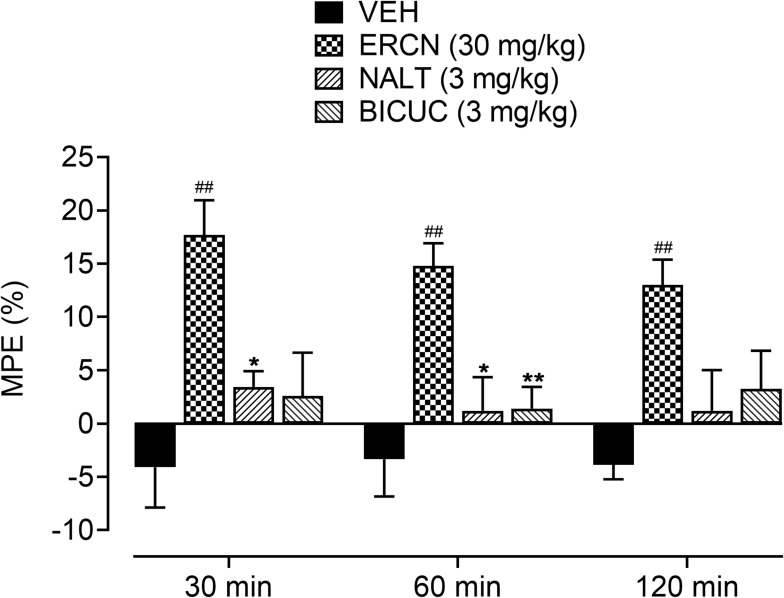
The maximum possible effect in the presence of antagonists (naltrexone and bicuculline) confirmed the involvement of opioid and GABAA receptors in the antinociceptive effect of eriocitrin [time=(F(2, 40)=0.56, P=0.56), treatment=(F (3, 20)=11.05, P<0.001), interaction=(F(6, 40)=0.36, P=0.90)]. A significant increase (P<0.01) in the percentage protective effect was produced by the 30 mg/kg dose of eriocitrin at the testing time-period of 30–120 minutes (17.6–13.1%). Administration of naltrexone and bicuculline to groups of animals pretreated with eriocitrin at 30 mg/kg significantly antagonized the maximum protection afforded by eriocitrin, as no significant difference was observed when compared to the vehicle-administered control group. However, in comparison to the eriocitrin (30 mg/kg) treated group, the naltrexone-administered group showed a significant decrease in the percentage of antinociception after 30 minutes (3.43%, P<0.05) and 60 minutes (1.19%, P<0.01). For the bicuculline-administered eriocitrin pretreated group, a significant decrease in the maximum possible effect was only observed at a time-period of 60 minutes (1.40%, P<0.01) of study duration, as compared to the eriocitrin-alone treated group (Figure 6).
Figure 6.
Effect of NALT and BICUC on ERCN percentage antinociceptive effect in postoperative nociception. Each column represents mean MPE±SEM. ##P<0.01 as compared to the VEHadministered incisional control group. *P<0.05, **P<0.01 as compared to the ERCN alone administered incisional group, two-way repeated measures ANOVA followed by Tukey’s post hoc test, n=6 mice per group.
Abbreviations: BICUC, bicuculline; ERCN, eriocitrin; MPE, mean possible effect; NALT, naltrexone; VEH, vehicle.
Discussion
In this study, eriocitrin showed potential antinociceptive properties against both acetic acid and thermal induced nociception. The acetic acid-induced abdominal constriction test is widely used for assessing the peripheral antinociceptive activity.46,52 The nociception in this test is caused by the release of endogenous substances including prostaglandins through the activation of cyclooxygenases that stimulates the sensory pathways in the peritoneum and thus incites a viscero-somatic reflex (writhing).53 The sensory afferents in the peritoneum contains α1/2-adrenoceptors, β-adrenoceptors, and opioid receptors on their terminals and, when activated by appropriate agonists, these receptors suppress the generation of pain impulses.54,55 The tested doses of eriocitrin significantly attenuated the acetic acid-induced abdominal constrictions and produced an increase in the percentage of antinociception. The effect was equipotent to the standard, diclofenac, implying the inhibition of local peritoneal receptors or cyclooxygenases. The central antinociceptive effect of eriocitrin was investigated in the hot-plate test, which measures the response to acute nociceptive or non-inflammatory inputs and is considered as a suitable model for the determination of central antinociceptive mechanism.56,58 The tested doses of eriocitrin significantly increased the withdrawal latency time and the increase in percentage antinociception was comparable to the standard, morphine. Studies have shown that both the acetic acid and hot-plate tests can detect dose-dependent opioid mediated effects and GABA agonist antinociception in mice.59,60
In the present study, the citrus flavonoid, eriocitrin, produced a strong antinociceptive effect in the mouse model of postoperative pain. Natural products have been tested for their potential to reduce mechanical/heat hyperalgesia and/or non-evoked pain after incision. It has been observed that the flavonoid, vitexin, binds to GABAA and opioid receptors and produced a potent antinociceptive effect in incisional pain model.49 Moreover, curcumin, a phenolic constituent of turmeric, reduces inflammation, nociceptive hypersensitivity, spontaneous pain, and the effect has been mediated by increasing the tissue level of TGF-β.61 A randomized controlled study has shown that micronized flavonoid fractions decreased the severity of pain and reduced the requirement of intramuscular analgesic treatment after hemorrhoidectomy.20 The flavonoids, hesperidin alone and in combination with diosmin, produced strong anti-hyperalgesia, and the effect has been shown to be mediated by dopaminergic D2, GABAA, and opioidergic mechanisms.62 The flavonoid quercetin has been shown to attenuate muscular pain by inhibiting peripheral and spinal cord nociceptive mechanisms.63 Moreover, quercetin also inhibits Ehrlich tumor-induced cancer pain by targeting peripheral and spinal cord mechanisms and involve a reduction in the hyperalgesic cytokines by inhibiting oxidative stress and reducing the production of hyperalgesic cytokines, infiltration of neutrophils, and inhibition of oxidative stress as well as activation of opioid-dependent analgesic pathway and potentiation of morphine analgesia.64
Postoperative pain involves activation and sensitization of peripheral nociceptors, and spinal dorsal horn neurons. These include the role of voltage/ligand gated ion channels (calcium channels, NMDA receptor, AMPA, serotonin, GABAA, TRPV1, TRPA1), neurotransmitter-transporter (glutamate transporter), G-protein-coupled receptors (serotonin, histamine H1, GABAB, somatostatin receptor type 2, µ-opioid receptor, cannabinoid receptor, α2B-adrenoceptor), and ion channels modulated by G-protein-coupled receptors including calcium and potassium channels, neurotrophic factors/peptides/mediators (brain-derived neurotrophic factor, nerve growth factor, calcitonin gene-related peptide, complement component C5, endothelin-1, neuropeptide Y, nuclear factor kappa-light-chain-enhancer of activated B cells), cytokines (tumor necrosis factor α, interleukin-1α, IL-6, IL-10, TLR4, leukemia inhibitory factor), and cells including neutrophil granulocytes, mast cells, microglia, and astrocytes.3
In the present study, the antinociceptive effect of eriocitrin produced against incisional nociception was strongly antagonized by the opioid-receptor antagonist, naltrexone, thus showing the involvement of opioidergic transmission. It has been considered that opioids are the mainstay treatment for the management of postoperative pain.4 Opioids bind to receptors in the central nervous system and peripheral tissues and modulate the effect of the nociceptors. The μ-opioid receptors are widely distributed in different parts of the body; while, δ-opioid receptor and κ-opioid receptor are distributed in different systems such as the immune system, the neuroendocrine system, the peripheral nervous system, and the central nervous system, where they are involved in the modulation of pain and inflammation.65 Opioid agonists produce analgesia either by indirectly increasing neuronal traffic through the descending pathway at the nucleus raphe magnus and periaqueductal grey, or by directly inhibiting nociceptive afferents in the periphery. Opioid agonists act at the nucleus reticularisparagigantocellularis to indirectly inhibit spinal pain transmission and, in addition, reduce spinal nociception. Binding of an opioid agonist to a G-protein-coupled opioid receptor results in inhibition of adenylyl cyclase with a reduction in intracellular cyclic adenosine monophosphate (cAMP) levels and produce activation of potassium conductance and an inhibition of calcium conductance.66 Activation of peripheral κ-opioid receptors directly inhibits inflammatory hyperalgesia through triggering of the phosphoinositide 3-kinase gamma (PI3Kg)/AKT, which stimulates the neuronal nitric oxide (nNOS/NO) signaling pathway.67 Moreover, peripheral μ-opioid receptors activation ameliorates acute inflammation by activating antiapoptotic molecule Bcl-xL and suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB).68 Peripherally active opioids can reduce release of proinflammatory neuropeptides, cytokines, plasma extravasation, vasodilation, immune mediators, expression of adhesion molecules, and tissue destruction.69 Flavonoids are potential opioid receptor ligands, and systemic administration of flavonoid has been shown to elicits a dose-dependent inhibition of the nociceptive behavioral response, and the action is related to the activation of the µ-opioid system.70,71
In addition to opioidergic transmission, the antinociceptive effect of eriocitrin was also antagonized by a GABAA receptor antagonist, bicuculline, suggesting the role of GABAergic transmission in the nociception attenuating effect of eriocitrin. GABAergic neurons and GABAA receptors play an essential role in pain perception, and GABAA receptors can serve as targets for developing novel analgesics in pathological pain.72 GABAergic neurons are widely distributed throughout the central nervous system, including spinal cord dorsal horn, which is important for the transmission of pain signals into the higher centers of the brain. GABA neurons and receptors are also found in the supraspinal regions that coordinate the perception and response to painful stimuli and thus are important in regulating and controlling sensory information processing in the spinal cord.73 A loss of GABAA receptor-mediated inhibitory neurotransmission within central pain circuits is one of a number of key mechanisms contributing to behavioral allodynia and hyperalgesia in animal pain models.74,75 The antinociceptive effects of both GABAA and GABAB receptor agonists are known to involve activation or inhibition of other neurotransmitter or neuromodulator pathways76 and it is evident that central GABAergic systems are involved in opioid-mediated analgesia.77 Thus, it is possible that administration of GABA receptor agonists in combination with other agents may yield GABA receptor-related therapies for the treatment of acute and chronic pain.76 Specific modulators of GABAA receptor function possess advantages that, in addition to possessing analgesic properties, can mediate anxiolytic-like actions by virtue of their ability to modulate α2 and possibly α3 GABAA receptors.78 Flavonoids impact multiple targets in their analgesic effects and involve the participation of a 5HT receptor, opioid receptor, and GABAA/benzodiazepine or adenosine receptors. Medicinal plants present a promising source for the development of new analgesic drugs, and flavonoids are promising candidates for the management of painful conditions and deserve particular attention in further research and development.21
Conclusion
The citrus flavonoid, eriocitrin, has a strong antinociceptive property against both tonic and phasic nociception modalities. Eriocitrin effectively counteracted the incisional surgical nociception, and this effect was potently antagonized by naltrexone and bicuculline, suggesting the possible involvement of opioid and GABAA receptors in analgesia. Natural products including eriocitrin by virtue of their multi-targeted actions can impact multiple areas in the nociception transmission and processing systems and may therefore possess clinical therapeutic utility in various pain syndromes, including postoperative pain conditions.
Acknowledgments
The author is grateful to Dr Muhammad Umar Khayam Sahibzada for the help and support during the project.
Disclosure
No conflict of interest to declare.
References
- 1.Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83(1):11–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 3.Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain—from mechanisms to treatment. Pain Rep. 2017;2(2):e588. doi: 10.1097/PR9.0000000000000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(03):191–196. doi: 10.1055/s-0033-1351138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477–487. doi: 10.1111/papr.12108 [DOI] [PubMed] [Google Scholar]
- 6.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anesthesio. 2009;22(5):588–593. [DOI] [PubMed] [Google Scholar]
- 7.Corke P. Postoperative pain management. Aust Prescr. 2013;36(6):202–205. doi: 10.18773/austprescr.2013.079 [DOI] [Google Scholar]
- 8.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep. 2007;59(3):247–258. [PubMed] [Google Scholar]
- 9.LaxmaiahManchikanti M, Bert Fellows M, HaryAilinani M. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician. 2010;13:401–435. [PubMed] [Google Scholar]
- 10.Islam NU, Amin R, Shahid M, Amin M. Gummy gold and silver nanoparticles of apricot (Prunus armeniaca) confer high stability and biological activity. Arab J Chem. 2019;12(8):3977–3992. doi: 10.1016/j.arabjc.2016.02.017 [DOI] [Google Scholar]
- 11.Islam NU, Amin R, Shahid M, Amin M, Zaib S, Iqbal J. A multi-target therapeutic potential of Prunus domestica gum stabilized nanoparticles exhibited prospective anticancer, antibacterial, urease-inhibition, anti-inflammatory and analgesic properties. BMC Complement Altern Med. 2017;17(1):276. doi: 10.1186/s12906-017-1791-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam NU, Jalil K, Shahid M, Muhammad N, Rauf A. Pistaciaintegerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab J Chem. 2019;12(8):2310–2319. doi: 10.1016/j.arabjc.2015.02.014 [DOI] [Google Scholar]
- 13.Islam NU, Jalil K, Shahid M, et al. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab J Chem. 2019;12(8):2914–2925. doi: 10.1016/j.arabjc.2015.06.025 [DOI] [Google Scholar]
- 14.Islam NU, Khan I, Rauf A, Muhammad N, Shahid M, Shah MR. Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii. BMC Complement Altern Med. 2015;15(1):160. doi: 10.1186/s12906-015-0691-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Rahman RF. Natural products as a source for novel analgesic compounds In: Maldonado C, editor. Pain Relief - from Analgesics to Alternative Therapies. IntechOpen; 2017:277–299. doi: 10.5772/66770 [DOI] [Google Scholar]
- 16.Khan A, Naz S, Farooq U, et al. Bioactive chromone constituents from Vitex negundo alleviate pain and inflammation. J Pain Res. 2018;11:95–102. doi: 10.2147/JPR.S145551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ognibene E, Bovicelli P, Adriani W, Saso L, Laviola G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6, 8-dibromoflavanone as anxiolytic compounds. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):128–134. doi: 10.1016/j.pnpbp.2007.07.023 [DOI] [PubMed] [Google Scholar]
- 18.Cho N, Lee KY, Huh J, et al. Cognitive-enhancing effects of Rhusverniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food ChemToxicol. 2013;58:355–361. doi: 10.1016/j.fct.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 19.Johnston GA. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem Int. 2015;89:120–125. doi: 10.1016/j.neuint.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 20.Colak T, Akca T, Dirlik M, Kanik A, Dag A, Aydin S. Micronized flavonoids in pain control after hemorrhoidectomy: a prospective randomized controlled study. Surg Today. 2003;33(11):828–832. doi: 10.1007/s00595-003-2604-5 [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Wang X, Gui X, Chen L, Huang B. Natural flavonoids as promising analgesic candidates: a systematic review. Chem Biodivers. 2016;13(11):1427–1440. doi: 10.1002/cbdv.201600060 [DOI] [PubMed] [Google Scholar]
- 22.Ferraz CR, Carvalho TT, Manchope MF, et al. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. 2020;25(3):762. doi: 10.3390/molecules25030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maleki SJ, Crespo JF, Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124 [DOI] [PubMed] [Google Scholar]
- 24.Azevedo MI, Pereira AF, Nogueira RB, et al. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013;9(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandhare AD, Raygude KS, Kumar VS, et al. Ameliorative effects quercetin against impaired motor nerve function, inflammatory mediators and apoptosis in neonatal streptozotocin-induced diabetic neuropathy in rats. Biomed Aging Pathol. 2012;2(4):173–186. doi: 10.1016/j.biomag.2012.10.002 [DOI] [Google Scholar]
- 26.Çivi S, Emmez G, Dere ÜA, Börcek AÖ, Emmez H. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir (Wien). 2016;158(5):959–965. doi: 10.1007/s00701-016-2761-0 [DOI] [PubMed] [Google Scholar]
- 27.LML C, AAC DA, RLM DF, et al. Antioxidant and antinociceptive effects of citrus limon essential oil in mice. Biomed Res Int. 2011;2011:678673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amorim JL, Simas DLR, Pinheiro MMG, et al. Anti-inflammatory properties and chemical characterization of the essential oils of four citrus species. PLoS One. 2016;11(4):e0153643. doi: 10.1371/journal.pone.0153643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali J, Abbas S, Khan F, et al. Biochemical and antimicrobial properties of citrus peel waste. Pharmacologyonline. 2016;3:98–103. [Google Scholar]
- 30.Saeb S, Amin M, Gooybari RS, Aghel N. Evaluation of antibacterial activities of citrus limon, citrus reticulata, and citrus grandis against pathogenic bacteria. Int J Enteric Pathog. 2016;4(4):3–37103. doi: 10.15171/ijep.2016.13 [DOI] [Google Scholar]
- 31.Battistini R, Rossini I, Ercolini C, et al. antiviral activity of essential oils against hepatitis A virus in soft fruits. Food Environ Virol. 2019;11(1):90–95. doi: 10.1007/s12560-019-09367-3 [DOI] [PubMed] [Google Scholar]
- 32.Osman A, Moon JY, Hyun HB, Kang HR, Cho SK. The chloroform fraction of citrus limon leaves inhibits human gastric cancer cell proliferation via induction of apoptosis. J Appl Biol Chem. 2016;59(3):207–213. doi: 10.3839/jabc.2016.036 [DOI] [Google Scholar]
- 33.Basli A, Younici S, Benkerrou Z, Khettal B, Madani K. Evaluation of in-vitro antidiabetic and hypolipidaemic activities of extracts citrus lemon fruit. J Environ Sci Eng A. 2016;5:612–618. [Google Scholar]
- 34.Bhavsar SK, Joshi P, Shah MB, Santani DD. Investigation into hepatoprotective activity of citrus limon. Pharm Biol. 2007;45(4):303–311. doi: 10.1080/13880200701214995 [DOI] [Google Scholar]
- 35.Spadaro F, Circosta C, Costa R, Pizzimenti F, Palumbo DR, Occhiuto F. Volatile fraction composition and biological activity of lemon oil (Citrus limon L. Burm.): comparative study of oils extracted from conventionally grown and biological fruits. J Essent Oil Res. 2012;24(2):187–193. doi: 10.1080/10412905.2012.659518 [DOI] [Google Scholar]
- 36.Sridharan B, Michael ST, Arya R, Mohana Roopan S, Ganesh RN, Viswanathan P. Beneficial effect of Citrus limon peel aqueous methanol extract on experimentally induced urolithic rats. Pharm Biol. 2016;54(5):759–769. doi: 10.3109/13880209.2015.1079724 [DOI] [PubMed] [Google Scholar]
- 37.Hwang S-L, Shih P-H, Yen G-C. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012;60(4):877–885. doi: 10.1021/jf204452y [DOI] [PubMed] [Google Scholar]
- 38.Ayaz M, Sadiq A, Junaid M, et al. Flavonoids as prospective neuroprotectants and their therapeutic propensity in aging associated neurological disorders. Front Aging Neurosci. 2019;11:155. doi: 10.3389/fnagi.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyake Y, Yamamoto K, Osawa T. Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. f.) and its antioxidative activity. Food Sci Technol Int. 1997;3(1):84–89. [Google Scholar]
- 40.Hiramitsu M, Shimada Y, Kuroyanagi J, et al. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep. 2014;4(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minato K-I, Miyake Y, Fukumoto S, et al. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003;72(14):1609–1616. doi: 10.1016/S0024-3205(02)02443-8 [DOI] [PubMed] [Google Scholar]
- 42.Miyake Y, Suzuki E, Ohya S, et al. Lipid‐lowering effect of eriocitrin, the main flavonoid in lemon fruit, in rats on a high‐fat and high‐cholesterol diet. J Food Sci. 2006;71(9):S633–S637. doi: 10.1111/j.1750-3841.2006.00192.x [DOI] [Google Scholar]
- 43.Nogata Y, Ohta H, Ishii T, Sekiya K. Isolation of eriocitrin (eriodictyol 7‐O‐rutinoside) as an arachidonate lipoxygenase inhibitor from Lumie fruit (Citrus lumia) and its distribution in Citrus species. J Sci Food Agric. 2007;87(1):82–89. doi: 10.1002/jsfa.2674 [DOI] [Google Scholar]
- 44.Wang Z, Zhang H, Zhou J, et al. Eriocitrin from lemon suppresses the proliferation of human hepatocellular carcinoma cells through inducing apoptosis and arresting cell cycle. Cancer Chemother Pharmacol. 2016;78(6):1143–1150. doi: 10.1007/s00280-016-3171-y [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Huang H, Huang Z, et al. Eriocitrin in combination with resveratrol ameliorates LPS-induced inflammation in RAW264. 7 cells and relieves TPA-induced mouse ear edema. J Funct Foods. 2019;56:321–332. doi: 10.1016/j.jff.2019.03.008 [DOI] [Google Scholar]
- 46.Collier H, Dinneen L, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolfe G, MacDonald A. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J Pharmacol Exp Ther. 1944;80(3):300–307. [Google Scholar]
- 48.Loscalzo LM, Yow TT, Wasowski C, Chebib M, Marder M. Hesperidin induces antinociceptive effect in mice and its aglycone, hesperetin, binds to μ-opioid receptor and inhibits GIRK1/2 currents. Pharmacol Biochem Behav. 2011;99(3):333–341. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q, Mao L-N, Liu C-P, et al. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci Rep. 2016;6:19266. doi: 10.1038/srep19266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Zhang J, Liu Y, et al. Role of nitric oxide synthase in the development of bone cancer pain and effect of L‑NMMA. Mol Med Rep. 2016;13(2):1220–1226. doi: 10.3892/mmr.2015.4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaplan S, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 52.Rao BS, Reddy KE, Parveen K, Narendra BL, Shekhar SC, Mangala L. Effects of Cleome viscosa on hyperalgesia, oxidative stress and lipid profile in STZ induced diabetic neuropathy in Wistar rats. Pak J Pharm Sci. 2014;27(5):1137–1145. [PubMed] [Google Scholar]
- 53.Matsumoto H, Naraba H, Ueno A, et al. Induction of cyclooxygenase-2 causes an enhancement of writhing response in mice. Eur J Pharmacol. 1998;352(1):47–52. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda Y, Ueno A, Naraba H, Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69(24):2911–2919. [DOI] [PubMed] [Google Scholar]
- 55.Honore P, Mikusa J, Bianchi B, et al. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain. 2002;96(1):99–105. doi: 10.1016/S0304-3959(01)00434-1 [DOI] [PubMed] [Google Scholar]
- 56.Hunskaar S, Berge O-G, Hole HK. A modified hot-plate test sensitive to mild analgesics. Behav Brain Res. 1986;21(2):101–108. doi: 10.1016/0166-4328(86)90088-4 [DOI] [PubMed] [Google Scholar]
- 57.Carter RB. Differentiating analgesic and non-analgesic drug activities on rat hot plate: effect of behavioral endpoint. Pain. 1991;47(2):211–220. doi: 10.1016/0304-3959(91)90207-E [DOI] [PubMed] [Google Scholar]
- 58.Del Pozo E, Ruiz-Garcia C, Baeyens J. Analgesic effects of diltiazem and verapamil after central and peripheral administration in the hot-plate test. Gen Pharmacol Vasc Syst. 1990;21(5):681–685. doi: 10.1016/0306-3623(90)91017-L [DOI] [PubMed] [Google Scholar]
- 59.Sałat K, Więckowska A, Więckowski K, et al. Synthesis and pharmacological properties of new GABA uptake inhibitors. Pharmacol Rep. 2012;64(4):817–833. doi: 10.1016/S1734-1140(12)70877-0 [DOI] [PubMed] [Google Scholar]
- 60.Britto GF, Subash K, Rao NJ, Cheriyan BV, Kumar SV. A synergistic approach to evaluate the anti-nociceptive activity of a GABA agonist with opioids in albino mice. J Clin Diagn Res. 2012;6:682–687. [Google Scholar]
- 61.Sahbaie P, Sun Y, Liang D-Y, Shi X-Y, Clark JD. Curcumin treatment attenuates pain and enhances functional recovery after incision. Anesth Analg. 2014;118(6):1336–1344. [DOI] [PubMed] [Google Scholar]
- 62.Carballo-Villalobos AI, M-E G-T, Pellicer F, López-Muñoz FJ. Antihyperalgesic effect of hesperidin improves with diosmin in experimental neuropathic pain. Biomed Res Int. 2016;2016:8263463. doi: 10.1155/2016/8263463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borghi SM, Pinho-Ribeiro FA, Fattori V, et al. Quercetin inhibits peripheral and spinal cord nociceptive mechanisms to reduce intense acute swimming-induced muscle pain in mice. PLoS One. 2016;11(9):e0162267. doi: 10.1371/journal.pone.0162267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calixto-Campos C, Corrêa MP, Carvalho TT, et al. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal Cell Pathol. 2015;2015:Article ID 285708. doi: 10.1155/2015/285708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Oliveira Junior JO, de Freitas MF, de Andrade CB, Chacur M, Ashmawi HA. Local analgesic effect of tramadol is mediated by opioid receptors in late postoperative pain after plantar incision in rats. J Pain Res. 2016;9:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11–16. doi: 10.1177/2049463712438493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cunha TM, Souza GR, Domingues AC, et al. Stimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kγ/AKT/nNOS/NO signaling pathway. Mol Pain. 2012;8:1744-8069-1748-1710. doi: 10.1186/1744-8069-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anselmi L, Huynh J, Duraffourd C, et al. Activation of μ opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol Motil. 2015;27(4):509–523. doi: 10.1111/nmo.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein C, Kuchler S. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Design. 2012;18(37):6053–6069. doi: 10.2174/138161212803582513 [DOI] [PubMed] [Google Scholar]
- 70.Katavic PL, Lamb K, Navarro H, Prisinzano TE. Flavonoids as opioid receptor ligands: identification and preliminary structure− Activity relationships. J Nat Prod. 2007;70(8):1278–1282. doi: 10.1021/np070194x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higgs J, Wasowski C, Loscalzo LM, Marder M. In vitro binding affinities of a series of flavonoids for μ-opioid receptors. Antinociceptive effect of the synthetic flavonoid 3, 3-dibromoflavanone in mice. Neuropharmacology. 2013;72:9–19. doi: 10.1016/j.neuropharm.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 72.Zeilhofer HU, Möhler H, Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009;30(8):397–402. doi: 10.1016/j.tips.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 73.Enna S, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2005;54:1–27. [DOI] [PubMed] [Google Scholar]
- 74.Mirza NR, Munro G. The role of GABAA receptor subtypes as analgesic targets. Drug News Perspect. 2010;23(6):351–360. doi: 10.1358/dnp.2010.23.6.1489909 [DOI] [PubMed] [Google Scholar]
- 75.Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABA A receptor subtypes as novel targets. Trends Pharmacol Sci. 2009;30(9):453–459. doi: 10.1016/j.tips.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 76.McCarson KE, Enna S. GABA pharmacology: the search for analgesics. Neurochem Res. 2014;39(10):1948–1963. doi: 10.1007/s11064-014-1254-x [DOI] [PubMed] [Google Scholar]
- 77.DeFeudis F. Central GABA‐ergic systems and analgesia. Drug Dev Res. 1983;3(1):1–15. doi: 10.1002/ddr.430030102 [DOI] [Google Scholar]
- 78.Munro G, Hansen RR, Mirza NR. GABAA receptor modulation: potential to deliver novel pain medicines? Eur J Pharmacol. 2013;716(1–3):17–23. doi: 10.1016/j.ejphar.2013.01.070 [DOI] [PubMed] [Google Scholar]