Abstract
Background:
Alternate-day fasting (ADF) has gained popularity in recent years. The diet consists of a “fast day” where an individual consumes 0–25% of their energy needs, alternated with a “feast day” where a person is permitted to eat ad libitum.
Aim:
This study examined eating behavior traits of successful weight losers during alternate day fasting.
Methods:
Obese participants (n = 34) took part in 12 months of ADF and were grouped into a high (≥5%) or low-weight-loss (<5%) group post-treatment.
Results:
The high-weight-loss group demonstrated increased (p = 0.04) fullness, decreased (p = 0.03) hunger, increased dietary protein intake (15% to 20% of kcal, p = 0.04), and better adherence to fast-day calorie goals.
Conclusions:
Thus, individuals who achieve clinically significant weight loss with ADF demonstrate improved satiety, increased protein intake, and better adherence to fast-day calorie goals.
Keywords: Weight loss success, alternate day fasting, eating behavior traits, body weight, obese adults
Introduction
Alternate day fasting (ADF) has gained popularity as a weight loss therapy in recent years. The diet consists of a “fast day” where an individual consumes 0–25% of their energy needs as a lunch or dinner, alternated with a “feast day” where a person is permitted to eat ad libitum. Short-term studies of ADF demonstrate 3–8% weight loss after 2–3 months of treatment (Bhutani et al., 2013b; Eshghinia and Mohammadzadeh, 2013; Johnson et al., 2007; Varady et al., 2009). Our lab recently conducted a 12-month ADF trial in obese adults (Trepanowski et al., 2017) and we observed a mean weight loss of 6%. While this level of weight loss is not remarkable, it should be noted that weight change varied widely across participants, with some participants gaining +3.7% and others losing −17.5% of baseline body weight.
Individuals who are deemed “successful weight losers” generally exhibit a cluster of eating behavior traits. For instance, participants who report higher degrees of dietary restraint and self-efficacy tend to lose more weight than those who report lower levels of these traits (Batra et al., 2013; Johnson et al., 2012; Nakade et al., 2012). Likewise, participants who report low hunger ratings and high fullness ratings during diet interventions also tend to achieve greater weight loss (Batra et al., 2013; Nakade et al., 2012). The present study was undertaken to examine whether certain eating behavior traits (adherence to fast-day calorie goals, dietary restraint, self-efficacy, hunger, and fullness) differ between participants who achieved clinically significant weight loss (≥5% from baseline) (Jensen et al., 2014; Williamson et al., 2015) during 12 months of ADF, versus those who did not.
Our 12-month study (Trepanowski et al., 2017) also included a daily calorie restriction (CR) group. While the primary focus of this paper is to examine how changes in eating behaviors impact weight loss success in ADF participants, we have also included the CR data for the sake of comparison between interventions.
Methods
Participants and diets
Participants were recruited from the Chicago area by advertisements, as previously described (Trepanowski et al., 2017). Key inclusion criteria were as follows: male or female, age 18–65 y, BMI between 25 and 40 kg/m2, weight stable, previously sedentary or lightly active, non-diabetic, no history of cardiovascular disease, and non-smoker. Participants were randomized to an ADF or CR group for 12 months. The 12-month intervention was divided into a 6-month weight loss phase followed by a 6-month weight maintenance phase. During months 0 to 6, ADF participants were instructed to consume 25% of energy needs on the fast day and 125% of energy needs on the feast day, whereas CR participants were instructed to consume 75% of energy needs every day. During months 7 to 12, ADF participants were asked to consume 50% of energy needs on the fast day, and 150% of energy needs on the feast day, whereas CR participants were instructed to consume 100% of energy needs every day. Participants received dietary counselling from months 3 to 6. During these sessions, participants were taught how to monitor calories and portion sizes, while decreasing intake of high fat and high sugar foods. Experimental protocols were approved by the Office for the Protection of Research Participants at the University of Illinois, Chicago. All participants gave written informed consent to participate in the study.
ADF and CR participants were categorized into one of two groups based on total weight loss at month 12: (a) low-weight-loss group (<5% loss of baseline body weight) and (b) high-weight-loss group (≥5% loss of baseline body weight). We chose to use 5% as a weight loss cut-off as this degree of weight reduction has been shown to produce improvements in risk factors for disease (i.e., glycemic measures, blood pressure, and plasma lipids) in “at risk” obese patients (Jensen et al., 2014; Williamson et al., 2015).
Measures
Body weight was measured monthly in a hospital gown using a balance beam scale (HealthOMeter, HealthOMeter Inc., Los Angeles, CA, USA) at the research center. Dietary restraint was assessed by the Three-Factor Eating Questionnaire (TFEQ) (Stunkard and Messick, 1985). Dietary restraint refers to the cognitive effort exerted by an individual to eat less than they would like (Schaumberg et al., 2016). Higher levels of dietary restraint may be beneficial in weight management, though this has been debated (Schaumberg et al., 2016). Self-efficacy was quantified by a validated questionnaire developed by Sallis et al. (1988). Self-efficacy refers to an individual’s perceived ability to motivate themselves to consistently accomplish one’s goals, such as consistently make healthy food choices and stick to energy targets (Sallis et al., 1988; Faghri et al., 2017). Hunger and fullness were measured by a validated (Flint et al., 2000) a visual analog scale (0–100 mm) during 3 fast days at baseline, months 3, 6, 9, and 12. Participants were instructed to record their feelings of hunger and fullness 5 minutes before going to bed. Energy and protein intake were assessed by a 7-day food record at baseline, months 3, 6, 9, and 12, and analyzed using Nutritionist Pro software (Axxya Systems, Stafford, TX, USA). Prescribed energy intake was calculated using the Mifflin equation (Mifflin et al., 1990). The proportion of adherent participants in the ADF and CR groups was also calculated. Intervention participants were considered ‘adherent’ when their actual energy intake, determined via 7-day food records at baseline, months 3, 6, 9, and 12, did not exceed 200 kcal of their prescribed daily energy goal. Physical activity was measured for 7 consecutive days at baseline and month 12 using an activity monitor (Sensewear Mini, Bodymedia, Pittsburgh, PA, USA).
Statistics
The primary outcome measure was body weight. We calculated that 30 participants per group would provide 80% power to detect a significant difference of 5% in body weight between the ADF and CR groups at month 6 (end of the weight loss phase), using a two-tailed independent-samples t-test with α = 0.05. Results are presented as mean ± SEM. We conducted an intention-to-treat analysis, which included data from all participants (n = 34 ADF; n = 35 CR) who underwent randomization. The last value carried forward was used for missing values. An independent samples t-test was used to test differences between groups at baseline and month 12. A paired t-test was used to test differences within group from baseline to month 12. Tests for normality were included in the model. No variables were found to be not normal. The Bonferroni correction for multiple comparisons was applied for t-test comparisons. Differences were considered significant at p < 0.05. All data were analyzed using SPSS software (version 24, SPSS Inc., Chicago, IL, US).
Results
Baseline characteristics and weight loss
Baseline characteristics did not differ between the weight loss groups for either ADF or CR (Table 1). Participants were primarily middle-aged females with obesity. In the ADF group, body weight change by month 12 ranged from +3.7% to −4.7% (mean −0.9% ± 0.6%) in the low-weight-loss group, and −5.5% to −17.5% (mean −9.9% ± 1.1%) in the high-weight-loss group (Figure 1). In the CR group, body weight change by month 12 ranged from 5.0% to −4.9% (mean −1.3% ± 0.7%) in the low-weight-loss group, and −5.4% to −19.8% (mean −9.2% ± 1.2%) in the high-weight-loss group.
Table 1.
Baseline characteristics.
| Low-weight-loss group (<5% weight loss) | High-weight-loss group (≥5% weight loss) | Difference between ADF weight loss groups (p-value1) | |
|---|---|---|---|
| ADF | n = 20 | n = 14 | |
| Female/male | 19/1 | 11/3 | 0.34 |
| Age (y) | 43 ± 2 | 46 ± 3 | 0.69 |
| Body weight (kg) | 94 ± 3 | 97 ± 4 | 0.26 |
| Height (cm) | 166 ± 2 | 167 ± 3 | 0.97 |
| BMI (kg/m2) | 34 ± 1 | 34 ± 1 | 0.85 |
| Ethnicity | |||
| African | 14 | 8 | 0.76 |
| American | |||
| Asian | 0 | 1 | |
| Caucasian | 5 | 4 | |
| Hispanic | 1 | 1 | |
| CR | n = 21 | n = 14 | Difference between CR weight loss groups (p-value1) |
| Female/male | 18/3 | 11/3 | 0.40 |
| Age (y) | 40 ± 2 | 46 ± 3 | 0.16 |
| Body weight (kg) | 102 ± 3 | 100 ± 5 | 0.74 |
| Height (cm) | 169 ± 2 | 169 ± 3 | 0.91 |
| BMI (kg/m2) | 36 ± 1 | 35 ± 1 | 0.51 |
| Ethnicity | |||
| African | 16 | 5 | 0.54 |
| American | |||
| Asian | 0 | 1 | |
| Caucasian | 4 | 8 | |
| Hispanic | 1 | 0 |
All values reported as mean ± standard error of the mean.
p-value between groups: independent samples t-test for continuous variables and McNemar’s test for categorical variables.
There was no difference between the ADF low-weight-loss group and the CR low-weight-loss group for any parameter. There was no difference between the ADF high-weight-loss group and the CR high-weight-loss group for any parameter.
BMI: body mass index; ADF: alternate-day fasting; CR: calorie restriction.
Figure 1.
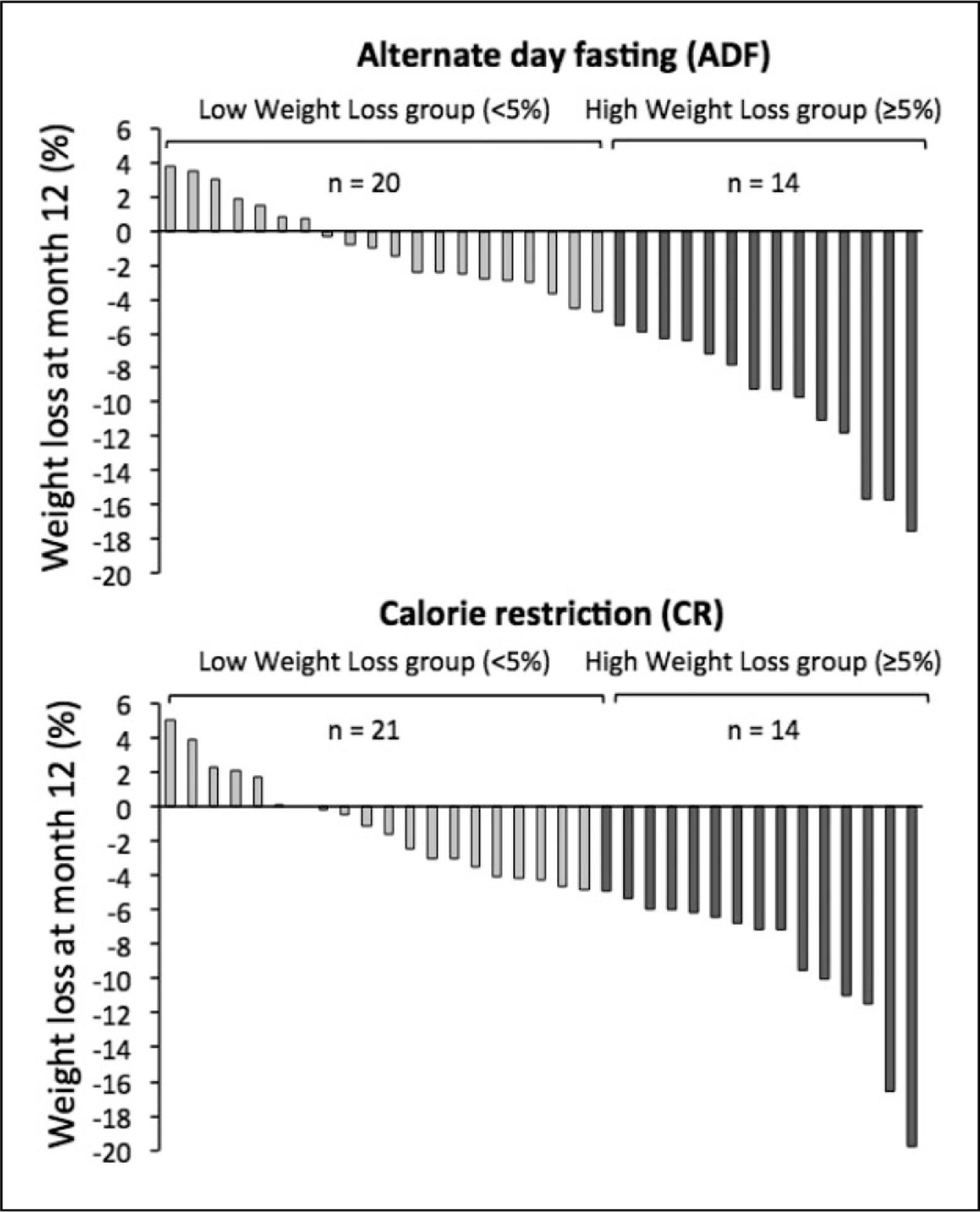
Weight loss by individual participants after 12 months of alternate-day fasting or daily calorie restriction.
Eating behavior traits
Restrained eating and self-efficacy did not differ between weight loss groups for ADF or CR (Table 2). Hunger decreased (p = 0.03) in the ADF high-weight-loss group from baseline to post-treatment but remained unchanged in the ADF low-weight-loss group. Hunger values did not differ between CR weight loss groups. Fullness increased (p = 0.04) over the course of the trial in the ADF high-weight-loss group but did not change in the ADF low-weight-loss group. Fullness ratings did not differ between CR weight loss groups.
Table 2.
Eating behavior traits in alternate-day fasting and calorie restriction weight loss groups.
| Low-weight-loss group (<5% weight loss) | High-weight-loss group (≥5% weight loss) | ||||||
|---|---|---|---|---|---|---|---|
| ADF | Baseline | Month 12 | p-value1 within group | Baseline | Month 12 | p-value1 within group | p-value2 between groups |
| Eating behaviors | |||||||
| Restrained eating score | 34 ± 4 | 37 ± 5 | 0.64 | 36 ± 3 | 35 ± 4 | 0.81 | 0.81 |
| Self-efficacy score | 72 ± 5 | 75 ± 5 | 0.53 | 79 ± 3 | 80 ± 4 | 0.69 | 0.36 |
| Hunger (mm) | 32 ± 8 | 39 ± 7 | 0.43 | 45 ± 1 | 27 ± 8 | 0.03 | 0.24 |
| Fullness (mm) | 61 ± 7 | 53 ± 7 | 0.23 | 30 ± 9 | 53 ± 8 | 0.04 | 0.99 |
| Dietary intake | |||||||
| Energy intake, fast day (kcal) | – | 1229 ± 161 | – | – | 1058 ± 168 | – | 0.48 |
| Excess energy intake, fast day (kcal)3 | – | 414 ± 74 | – | – | 133 ± 98 | – | 0.03 |
| Energy intake, feast day (kcal) | 1617 ± 126 | 1601 ± 175 | 0.24 | 1787 ± 307 | 1647 ± 227 | 0.09 | 0.88 |
| Protein intake, fast day (%) | 17 ± 1 | 18 ± 1 | 0.07 | 15 ± 1 | 20 ± 1 | 0.04 | 0.37 |
| Protein intake, fast day (g) | 70 ± 5 | 73 ± 5 | 0.45 | 68 ± 4 | 84 ± 4 | 0.03 | 0.21 |
| Proportion of participants adherent (%) | – | 28 | – | – | 44 | – | 0.16 |
| Physical activity (steps/d) | 8112 ± 618 | 7384 ± 890 | 0.58 | 7439 ± 1151 | 7906 ± 1474 | 0.61 | 0.81 |
| CR | Baseline | Month 12 | p-value1 within group | Baseline | Month 12 | p-value1 within group | p-value2 between groups |
| Eating behaviors | |||||||
| Restrained eating score | 34 ± 4 | 36 ± 4 | 0.67 | 38 ± 5 | 34 ± 5 | 0.47 | 0.75 |
| Self-efficacy score | 78 ± 4 | 76 ± 3 | 0.60 | 71 ± 4 | 78 ± 4 | 0.55 | 0.84 |
| Hunger (mm) | 35 ± 6 | 29 ± 5 | 0.25 | 38 ± 8 | 38 ± 6 | 0.91 | 0.25 |
| Fullness (mm) | 66 ± 4 | 62 ± 4 | 0.38 | 50 ± 8 | 59 ± 6 | 0.10 | 0.67 |
| Dietary intake | |||||||
| Energy intake, every day (kcal) | 1890 ± 156 | 1726 ± 136 | 0.08 | 1902 ± 179 | 1882 ± 306 | 0.21 | 0.63 |
| Protein intake, every day (%) | 16 ± 1 | 17 ± 1 | 0.27 | 16 ± 1 | 16 ± 2 | 0.70 | 0.24 |
| Protein intake, every day (g) | 76 ± 6 | 73 ± 5 | 0.50 | 76 ± 5 | 74 ± 8 | 0.76 | 0.87 |
| Proportion of participants adherent (%) | – | 55 | – | – | 68 | – | 0.47 |
| Physical activity (steps/d) | 6884 ± 558 | 7268 ± 1038 | 0.27 | 6553 ± 754 | 8124 ± 1481 | 0.40 | 0.31 |
All values reported as mean ± standard error of the mean.
p-value within-weight-loss group from baseline to month 12: Paired samples t-test.
p-value between low- versus high-weight-loss groups at month 12: Independent samples t-test.
Excess intake on the fast day calculated as the mean difference between prescribed energy intake versus actual energy intake at month 12. There was no difference between the ADF low-weight-loss group and the CR low-weight-loss group for any parameter. There was no difference between ADF high-weight-loss group and CR high-weight-loss group for any parameter.
ADF: alternate-day fasting; CR: calorie restriction.
Dietary intake
Fast day and feast day energy intake did not differ between ADF weight loss groups (Table 2). By month 12, mean excess energy intake on the fast day was higher (p = 0.03) in the low-weight-loss group (414 ± 74 kcal) versus the high-weight-loss group (133 ± 98 kcal). Energy intake did not differ between CR weight loss groups. Protein intake increased (p < 0.05) in the ADF high-weight-loss group, with no change in the ADF low-weight-loss group. Protein intake did not differ between CR weight loss groups.
Adherence and physical activity
The proportion of participants adherent to their prescribed energy goals was 28% in the ADF low-weight-loss group and 44% in the ADF high-weight-loss group, with no significant differences between groups (Table 2). As for CR, the proportion of adherent participants was 55% in the CR low-weight-loss group and 68% in the CR high-weight-loss group, with no significant differences between groups. Physical activity level did not differ between weight loss groups for either ADF or CR.
Discussion
Results from this study indicate that obese individuals who lost a clinically significant amount of weight (≥5%) with 12 months of ADF augmented their dietary protein intake in a way that may have increased fullness and decreased hunger. These behavioral improvements may have helped these participants better adhere to their fast-day calorie goals, which may have contributed to their weight loss success with ADF.
Participants in the ADF high-weight-loss group increased their dietary protein intake over the course of the trial from 15% to 20% of energy. Although this is only a mild augmentation in protein intake, even small increases have been shown to improve fullness during energy restriction (Astrup et al., 2015; Paddon-Jones et al., 2008). In view of this, it is possible that a higher level of protein intake by the ADF high-weight-loss group contributed to their improved feelings of fullness (Astrup et al., 2015; Paddon-Jones et al., 2008). It can also be speculated that the increase in fullness and decrease in hunger helped these ADF participants better adhere to their fast-day calorie goals. Our data show that the ADF high-weight-loss group consumed less excess energy on the fast day (133 kcal) versus the ADF low-weight-loss group (413 kcal). This superior level of adherence may have translated into higher overall energy restriction and greater total weight loss.
Dietary restraint did not change in either of the ADF weight loss groups over the course of the trial. Only one other study of modified ADF (25% energy intake on the fast day) has examined dietary restraint. In this study by Bhutani et al. (2013a), dietary restraint increased after 3 months of modified ADF in males and females with obesity. It remains unclear why results from the present study conflict with previous findings (Bhutani et al., 2013a), particularly as both trials implemented similar population groups and provided standard nutrition education (Gidding et al., 2009). It is possible, however, that dietary restraint may increase during the first 3–4 months of ADF and then return to baseline as the trial progresses.
Self-efficacy was also assessed in the present trial. In the present study, self-efficacy did not differ between ADF weight loss groups. In a recent trial by Armitage et al. (2014), self-efficacy was shown to be a significant predictor of weight loss success with intermittent energy restriction in a larger sample (n = 222) of women with obesity. Since our study had a much smaller sample size, we may have lacked power to identify significant differences in self-efficacy with ADF.
We also compared how changes in eating behaviors and dietary intake differ between ADF and CR participants. Our parallel analysis revealed that neither of the CR weight loss groups experienced any beneficial changes in eating behaviors despite similar weight loss as ADF. Thus, it is possible that these improvements in eating behaviors may be unique to this intermittent fasting intervention.
During our initial exploration of the data, we used the median weight loss value (4%) as the cut-off between ADF weight loss groups, instead of 5% (Jensen et al., 2014; Williamson et al., 2015). In using the 4% cut-off, we did not see any statistically significant differences for any parameter. This prompted us to reanalyze our data using the 5% “clinically significant” weight loss cut-off (Jensen et al., 2014; Williamson et al., 2015). Interestingly, the 5% cut-off yielded statistically significant differences between weight loss groups, suggesting that this degree of weight loss may be a tipping point for changes in certain eating behaviors by ADF.
This study has some limitations. Hunger and fullness were assessed only at one time point on fast days. Implementing a meal challenge protocol to quantifying changes in subjective appetite may provide a more robust characterization of this construct (Flint et al., 2000). This study also failed to include objective measures of appetite and dietary restraint. Future studies should examine how gut peptides (ghrelin, leptin, GLP-1, and PYY) respond to different degrees of weight loss by intermittent fasting. Moreover, this is a secondary analysis of a larger study (Trepanowski et al., 2017), which primarily assessed the impact of ADF on body weight. Therefore, this study may not be adequately powered to detect differences in eating behavior traits between weight loss groups. A post hoc analysis revealed that our power to detect significant differences between the ADF weight loss groups was approximately 20% for dietary restraint, 20% for self-efficacy, and 40% for hunger, which is low.
It will be of interest in future studies to examine whether implementing multiple consecutive dietary interventions (e.g., intermittent fasting, followed by a low-carb diet, followed by low-fat diet … etc.) can help patients with obesity achieve a degree of weight loss that extends beyond 5%. Switching dietary interventions every ~3 months may prove to be a powerful way of continuing one’s weight loss curve. This type of intervention could help prevent the diet fatigue/boredom that is often encountered when individuals follow particular diet regimens for long periods of time. It may also be helpful to alternate periods of energy restriction with periods of energy balance, as this has been shown to produce greater weight loss than continuous energy restriction (Byrne et al., 2017).
In summary, individuals who are successful at losing a clinically significant amount of weight with ADF may demonstrate changes in eating behaviors, such as increased fullness, decreased hunger, and increased protein intake. Future confirmatory research may help elucidate whether successful weight loss with ADF is made easier by altered macronutrient intake, self-efficacy, or dietary restraint.
Acknowledgments
CMK conducted the clinical trial, analyzed the data, and wrote the manuscript. JFT, MCK, AB, and KG conducted the clinical trial. KAV designed the experiment, analyzed the data, and assisted with the preparation of the manuscript.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health (NHLBI) R01HL106228 and (NIDDK) (F32DK107157).
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Krista Varady is the author of the book “The Every Other Day Diet” published by the Hachette Book Group. The other authors have no competing interests to disclose.
References
- Armitage CJ, Wright CL, Parfitt G, et al. (2014) Self-efficacy for temptations is a better predictor of weight loss than motivation and global self-efficacy: Evidence from two prospective studies among overweight/obese women at high risk of breast cancer. Patient Education and Counseling 95: 254–258. [DOI] [PubMed] [Google Scholar]
- Astrup A, Raben A and Geiker N (2015) The role of higher protein diets in weight control and obesity-related comorbidities. International Journal of Obesity (London) 39: 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P, Das SK, Salinardi T, et al. (2013) Eating behaviors as predictors of weight loss in a 6 month weight loss intervention. Obesity (Silver Spring) 21: 2256–2263. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, et al. (2013a) Effect of exercising while fasting on eating behaviors and food intake. Journal of the International Society of Sports Nutrition 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani S, Klempel MC, Kroeger CM, et al. (2013b) Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity (Silver Spring) 21: 1370–1379. [DOI] [PubMed] [Google Scholar]
- Byrne NM, Sainsbury A, King NA, et al. (2017) Intermittent energy restriction improves weight loss efficiency in obese men: the MATADOR study. International Journal of Obesity (Lond) 2017 August 17. doi: 10.1038/ijo.2017.206. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshghinia S and Mohammadzadeh F (2013) The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. Journal of Diabetes and Metabolic Disorders 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghri PD, Simon J, Huedo-Medina T, et al. (2017) Perceived self-efficacy and financial incentives: factors affecting health behaviors and weight loss in a workplace weight loss intervention. Journal of Occupational and Environmental Medicine 59: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, et al. (2000) Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity and Related Metabolic Disorders 24: 38–48. [DOI] [PubMed] [Google Scholar]
- Gidding SS, Lichtenstein AH, Faith MS, et al. (2009) Implementing American Heart Association pediatric and adult nutrition guidelines: a scientific statement from the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation 119: 1161–1175. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, et al. (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Pratt M and Wardle J. (2012) Dietary restraint and self-regulation in eating behavior. International Journal of Obesity (Lond) 36: 665–674. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, et al. (2007) Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology and Medicine 42: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, et al. (1990) A new predictive equation for resting energy expenditure in healthy individuals. American Journal of Clinical Nutrition 51: 241–247. [DOI] [PubMed] [Google Scholar]
- Nakade M, Aiba N, Morita A, et al. (2012) What behaviors are important for successful weight maintenance? Journal of Obesity 2012: 202037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Westman E, Mattes RD, et al. (2008) Protein, weight management, and satiety. American Journal of Clinical Nutrition 87: 1558S–1561S. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Pinski RB, Grossman RM, et al. (1988) The development of self-efficacy scales for health related diet and exercise behaviors Health Education Research 3: 283–292. [Google Scholar]
- Schaumberg K, Anderson DA, Anderson LM, et al. (2016) Dietary restraint: what’s the harm? A review of the relationship between dietary restraint, weight trajectory and the development of eating pathology. Clinical Obesity 6: 89–100. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ and Messick S. (1985) The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research 29: 71–83. [DOI] [PubMed] [Google Scholar]
- Trepanowski JF, Kroeger CM, Barnosky A, et al. (2017) Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. Journal of the American Medical Association Internal Medicine 177: 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Bhutani S, Church EC, et al. (2009) Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. American Journal of Clinical Nutrition 90: 1138–1143. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Bray GA and Ryan DH (2015) Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring) 23: 2319–2320. [DOI] [PubMed] [Google Scholar]


