Abstract
Objective
To determine the malignancy risk of isolated macrocalcifications (a calcified nodule with complete posterior acoustic shadowing) detected on ultrasonography (US) and to evaluate the postoperative American Thyroid Association (ATA) risk stratification of malignant tumors manifesting as isolated macrocalcifications.
Materials and Methods
A total of 3852 thyroid nodules (≥ 1 cm) of 3061 consecutive patients who had undergone biopsy between January 2011 and June 2018 were included in this study. We assessed the prevalence, malignancy rate, and size distribution of isolated macrocalcifications and evaluated the histopathologic features and postoperative ATA risk stratification of malignant tumors manifesting as isolated macrocalcifications.
Results
Isolated macrocalcifications were found in 38 (1.2%) of the 3061 patients. Final diagnosis was established in 30 (78.9%) nodules; seven malignant tumors were diagnosed as papillary thyroid carcinomas (PTCs). The malignancy rate of the isolated macrocalcifications was 23.3% in the 30 nodules with final diagnoses and 18.4% in all nodules. Among the six surgically-treated malignant tumors, five (83.3%) had an extrathyroidal extension (ETE) (minor ETE 1, gross ETE 4), and two (33.3%) had macroscopic lymph node metastasis. Four (66.7%) malignant tumors were categorized as high-risk tumors, one as an intermediate-risk tumor, and one as a low-risk tumor using the ATA risk stratification. Histopathologically, out of the six malignant tumors, ossifications were noted in four (66.7%) and predominant calcifications in two (33.3%).
Conclusion
The US pattern of isolated macrocalcifications (≥ 1 cm) showed an intermediate malignancy risk (at least 18.4%). All malignant tumors were PTCs, and most showed an aggressive behavior and a high or intermediate postoperative ATA risk.
Keywords: Thyroid nodule, Thyroid malignancy, Ultrasonography, Malignancy risk, Isolated macrocalcification
INTRODUCTION
Thyroid ultrasonography (US) is an essential diagnostic modality for assessing the malignancy risk of a thyroid nodule (1). Previous studies (2,3,4,5) have found that the following US features are predictive of thyroid malignancy: solid composition, hypoechogenicity, microcalcification, nonparallel orientation (taller than wide shape), and a spiculated/microlobulated (irregular) margin. Despite the fact that the presence of a macrocalcification might increase the risk for malignancy, it presented a variable malignancy rate and was not highly specific for malignancy (5,6,7,8). An isolated macrocalcification is defined as a calcified nodule with complete posterior acoustic shadowing in which any soft tissue component is not identified due to dense shadowing on US (9). Several studies delineated that an isolated macrocalcification was a US feature associated with benignity (8,10). Conversely, a previous study (9) reported that thyroid nodules with an isolated macrocalcification had a low-to-intermediate malignancy risk and should not be considered benign nodules.
An isolated macrocalcification is categorized as a nodule with an intermediate suspicion in the Korean Thyroid Imaging Reporting and Data System (TIRADS) (11,12) and a moderate suspicion in the American College of Radiology TIRADS (12,13,14). However, it is not specified in the American Thyroid Association (ATA) guideline and the European TIRADS (15,16). The malignancy risk of an isolated macrocalcification on US has not been established, and there has been little investigation on the behavior of malignant tumors that manifest as isolated macrocalcifications. Furthermore, the management of nodules with isolated macrocalcifications is controversial (12,14).
Therefore, this study was performed to determine the malignancy risk of isolated macrocalcifications and to evaluate the clinical postoperative risk stratification of malignant tumors that manifest as isolated macrocalcifications.
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board, and the requirement of patient informed consent was waived.
Patients
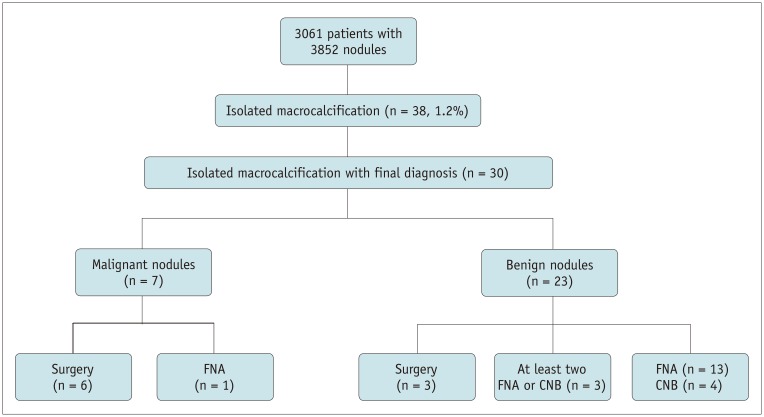
Between January 2011 and June 2018, 3309 consecutive patients underwent US-guided fine needle aspiration (FNA) or core needle biopsy (CNB) for thyroid nodules at a single institution. Among them, 3061 patients underwent US-guided FNA or CNB for nodules ≥ 1 cm, and 248 underwent US-guided FNA or CNB for nodules < 1 cm. Among the 3061 patients with 3852 nodules ≥ 1 cm, 38 patients with isolated macrocalcifications were finally included in our study (35 women and 3 men; mean age, 61.7 ± 9.3 years; age range, 40–73 years) (Fig. 1). Malignant nodules (n = 7) were finally diagnosed on the basis of the histopathologic results after surgery (n = 6) or a malignant FNA result (n = 1). Benign nodules (n = 23) were finally diagnosed according to the histopathologic results after surgery (n = 3), the presence of at least two benign FNA or CNB results (n = 3), and one benign FNA or CNB result (n = 17).
Fig. 1. Flow diagram of patient enrollment.
CNB = core needle biopsy, FNA = fine needle aspiration
US Examination and Image Analysis
All US examinations were performed using a 5- to 12-MHz linear-array transducer and a real-time US system (iU22 or EPIQ7, Philips Medial system, Bothell, WA, USA). One experienced radiologist with 21 years of experience in performing thyroid US and interventional procedures retrospectively reviewed all US images of the 3061 patients with nodules ≥ 1 cm. The reviewer, who was blinded to the cytopathologic biopsy diagnoses and final diagnoses, retrospectively assessed the presence of isolated macrocalcifications in all 3852 nodules. An isolated macrocalcification was defined as a calcified nodule with complete posterior acoustic shadowing and no identified soft tissue component within the calcified nodule. The reviewer retrospectively assessed the US features of isolated macrocalcifications including the size, location, presence of other thyroid nodules, and presence of focal disruption or lobulated contour at the anterior margin of a calcified nodule.
US-Guided FNA and CNB Procedures
US-guided FNA was performed by three radiologists using a 21- to 23-gauge needle and a 5-mL syringe. At least two passes were performed per nodule. Direct smear or liquid-based cytology was performed to prepare the FNA specimens for cytopathologic examination. The specimen was smeared on a slide and immediately fixed in 95% ethanol using the direct smear method. For liquid-based cytology, the specimen was prepared using the ThinPrep 2000 processor (Hologic Co., Inc., Marlborough, MA, USA).
CNB was performed using an 18-gauge, spring-activated, double-action needle (1.1-cm excursion; TSK Acecut, Create Medic, Yokohama, Japan) by one experienced radiologist, as described elsewhere (9,17). The specimen was immediately fixed in a 10% neutral buffered formalin solution and stained in the standard manner for histopathologic examination. After the patients underwent biopsy, the biopsy site was immediately compressed, and the patients were kept under observation as they provided self-manual compression of the biopsy site for 30 minutes.
The FNA cytology diagnosis was made based on the Bethesda system (18). The CNB pathology diagnosis was made based on the six categories of the CNB pathology reporting system (17,19).
Data Analysis and Statistics
We assessed the prevalence, malignancy rate, and size distribution of the isolated macrocalcifications and evaluated the histopathologic features and postoperative ATA risk stratification (15) of malignant tumors that manifested as isolated macrocalcifications. The nodules were categorized into three groups based on size (group 1, 1–1.4 cm; group 2, 1.5–1.9 cm; and group 3, ≥ 2 cm). A pathologist retrospectively analyzed the histological features of ossification or calcification in the six malignant tumors that were surgically treated. In these tumors, the postoperative cancer stage was determined using the eighth American Joint Committee on Cancer staging system (20), and the clinical postoperative risk was estimated using the ATA risk stratification system (15).
The Mann-Whitney U test was used to compare the mean size between benign and malignant nodules. The Student's t test was used to compare the patients' age between benign and malignant nodules. The Fisher's exact test was used to compare sex and US features between benign and malignant nodules and to compare the rate of malignant tumors between group 1 and group 2 or 3. Statistical analyses were performed using the IBM Statistical Package for the Social Sciences for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA). A significant difference was defined as a p-value of < 0.05.
RESULTS
Demographic Data
Isolated macrocalcifications were detected in 38 (1.2%) of the 3061 patients who underwent FNA or CNB for thyroid nodules ≥ 1 cm. Among the 38 nodules with isolated macrocalcifications, final diagnoses were established in 30 nodules (78.9%). There were 23 benign nodules (76.7%) and seven malignant tumors (23.3%). All malignant tumors were papillary thyroid carcinomas (PTCs); there were five classic PTCs and one follicular-variant PTC.
FNA was performed in all patients, and CNB was performed in 13 patients. A nondiagnostic FNA result was found in 12 nodules (31.6%), and an atypia/follicular lesion of an undetermined significance (AUS/FLUS) was diagnosed in 4 (10.5%) of the 38 nodules. A nondiagnostic CNB result was found in one nodule (7.7%), and an indeterminate CNB result was found in five (38.5%) of the 13 nodules (Table 1). In 13 nodules which underwent both FNA and CNB, the nondiagnostic rates of FNA and CNB were 61.5% (n = 8) and 7.7% (n = 1), respectively (p = 0.016), and AUS/FLUS or indeterminate rates of FNA and CNB were 7.7% (n = 1) and 38.5% (n = 5), respectively (p = 0.385). The inconclusive rate including the nondiagnostic result and AUS/FLUS or intermediate result of FNA and CNB was 69.2% (n = 9) and 46.2% (n = 6), respectively (p = 0.375). There were no major complications among the patients who underwent FNA or CNB, and none of them required hospitalization. There was one patient with a minor complication of a small transient perithyroidal hematoma in the right thyroid lobe, which resolved after 20 minutes of manual compression.
Table 1. Diagnostic Results of Biopsy and Malignancy Risk of Isolated Macrocalcifications.
| Cytohistology Results | FNA (n = 38) | CNB (n = 13) | Final Diagnosis* (n = 30) | Malignancy Risk in All Nodules* (%) | Malignancy Risk in Nodules with Final Diagnoses* (%) | |
|---|---|---|---|---|---|---|
| Benign (n = 23) | Malignancy (n = 7) | |||||
| I. Nondiagnostic | 12 (31.6) | 1 (7.7) | 6 (26.1) | 1 (14.3) | 8.3 | 14.3 |
| II. Benign | 18 (47.4) | 7 (53.8) | 17 (73.9) | 1 (14.3) | 5.6 | 5.6 |
| III. AUS/FLUS, indeterminate | 4 (10.5) | 5 (38.5) | 0 | 1 (14.3) | 25.0 | 100 |
| IV. FN/SFN | 0 | 0 | 0 | 0 | - | - |
| V. Suspicious for malignancy | 1 (2.6) | 0 | 0 | 1 (14.3) | 100 | 100 |
| VI. Malignant | 3 (7.9) | 0 | 0 | 3 (42.9) | 100 | 100 |
| All | 38 | 13 | 23 | 7 | 18.4 | 23.3 |
Unless otherwise indicated, data are number of nodules and percentage in parenthesis. *Calculated according to FNA cytology results. AUS/FLUS = atypia of undetermined significance or follicular lesion of undetermined significance, CNB = core needle biopsy, FNA = fine needle aspiration, FN/SFN = follicular neoplasm or suspicious for follicular neoplasm
Malignancy Risk of Isolated Macrocalcifications in the Overall Nodules
The calculated malignancy rate of the isolated macrocalcifications was 23.3% (95% confidence interval [CI]: 8.2–38.5%) in the 30 nodules with a final diagnosis and 18.4% (95% CI: 6.1–30.7%) among all nodules with an isolated macrocalcification (Table 1).
Among the 12 isolated macrocalcifications with FNA results of Bethesda system category 1, there was one malignant tumor, and the malignancy risk was 8.3% (1/12) in all nodules and 14.3% (1/7) in the nodules with a final diagnosis. Among the 18 nodules with benign FNA results, there was one malignant tumor, and the malignancy risk was 5.6% (1/18) in all nodules and in nodules with a final diagnosis. Among the four nodules with an AUS/FLUS FNA result, there was one malignant tumor, and the malignancy risk was 25.0% (1/4) in all nodules and 100% (1/1) in the nodule with a final diagnosis (Table 1). Among the five isolated macrocalcifications with indeterminate CNB results, there was one malignant tumor, and the malignancy risk was 20.0% (1/5) in all nodules and 100% (1/1) in the nodule with a final diagnosis. There were no malignant tumors among the isolated macrocalcifications with CNB results of category 1 and 2.
Malignancy Risk of Isolated Macrocalcifications according to Nodule Size
The size of the isolated macrocalcifications ranged from 10 to 46 mm (mean size, 13.7 ± 6.1 mm; median size, 12.0 mm). Of the 30 isolated macrocalcifications with a final diagnosis, the size of the benign nodules ranged from 10.0 to 17.0 mm (mean size, 12.4 ± 2.3 mm; median size, 12.0 mm) and that of the malignant nodules ranged from 11.0 to 20.0 mm (mean size, 14.6 ± 3.5 mm; median size, 15.0 mm). There was no significant difference in the mean size between the benign nodules and malignant tumors (12.4 mm vs. 14.6 mm, respectively; p = 0.087).
The size distribution of the isolated macrocalcifications was 71.1% in group 1, 21.1% in group 2, and 7.9% in group 3. The malignancy rate among all nodules was 11.1% in group 1, 37.5% in group 2, and 33.3% in group 3, and among the 30 nodules with a final diagnosis, the malignancy rate was 13.6% in group 1, 42.9% in group 2, and 100% in group 3 (Table 2). In the 30 isolated macrocalcifications with a final diagnosis, the frequency of malignant tumors was higher in nodules ≥ 1.5 cm (group 2 or 3) than in nodules < 1.5 cm (group 1), but the difference was not statistically significant (50.0% vs. 13.6%, respectively; p = 0.060). The tumor size was < 2 cm in six (85.7%) of the seven malignant tumors. The malignant tumors were equally distributed in group 1 and group 2 (Table 2).
Table 2. Malignancy Risk of Isolated Macrocalcifications according to Nodule Size.
| All (n = 38) | Final Diagnosis (n = 30) | Malignancy Risk in All Nodules (%) | Malignancy Risk in Nodules with Final Diagnoses (%) | ||
|---|---|---|---|---|---|
| Nodule Size | No. of Nodules | Benign (n = 23) | Malignancy (n = 7) | ||
| Group 1 | 27 (71.1) | 19 (82.6) | 3 (42.9) | 11.1 | 13.6 |
| Group 2 | 8 (21.1) | 4 (17.4) | 3 (42.9) | 37.5 | 42.9 |
| Group 3 | 3 (7.9) | 0 (0) | 1 (14.3) | 33.3 | 100 |
Unless otherwise indicated, data are number of nodules and percentage in parenthesis. Group 1: 1–1.4 cm, Group 2: 1.5–1.9 cm, Group 3: ≥ 2 cm.
Comparison of Clinical and US Features of Isolated Macrocalcifications between Benign and Malignant Nodules
Table 3 demonstrates the clinical and US features of isolated macrocalcifications in benign and malignant nodules. Age and sex were not significantly associated with malignancy in isolated macrocalcifications with final diagnoses (p = 0.484 and p = 0.418, respectively). The location of isolated macrocalcification, presence of other nodules, and US features including focal disruption of calcification at the anterior margin and lobulated contour of the anterior margin were not significantly associated with malignancy (p > 0.05, respectively) (Table 3). In the five patients who underwent computed tomography (CT) of the neck, the nodules with isolated macrocalcifications correlated with coarse calcified nodules on CT images (Fig. 2).
Table 3. Comparison of Clinical and US Features of Isolated Macrocalcifications between Benign and Malignant Nodules.
| Clinical and US Features | Benign (n = 23) | Malignancy (n = 7) | P |
|---|---|---|---|
| Age* | 59.5 ± 10.2 | 62.4 ± 6.3 | 0.484 |
| Sex (female) | 18 (47.4) | 7 (53.8) | 0.418 |
| Size (mm)* | 12.4 ± 2.3 | 14.6 ± 3.5 | 0.087 |
| Location | 0.065 | ||
| Right lobe | 14 (60.9) | 2 (28.6) | |
| Left lobe | 9 (39.1) | 3 (42.9) | |
| Isthmus | 0 (0) | 2 (28.6) | |
| Multiple nodules | 21 (91.3) | 7 (100) | 0.999 |
| Anterior margin | |||
| Focal disruption | 13 (56.5) | 5 (71.4) | 0.669 |
| Lobulated contour | 12 (52.2) | 6 (85.7) | 0.193 |
Unless otherwise indicated, data are number of nodules and percentage in parenthesis. *Mean ± standard deviation. US = ultrasonography
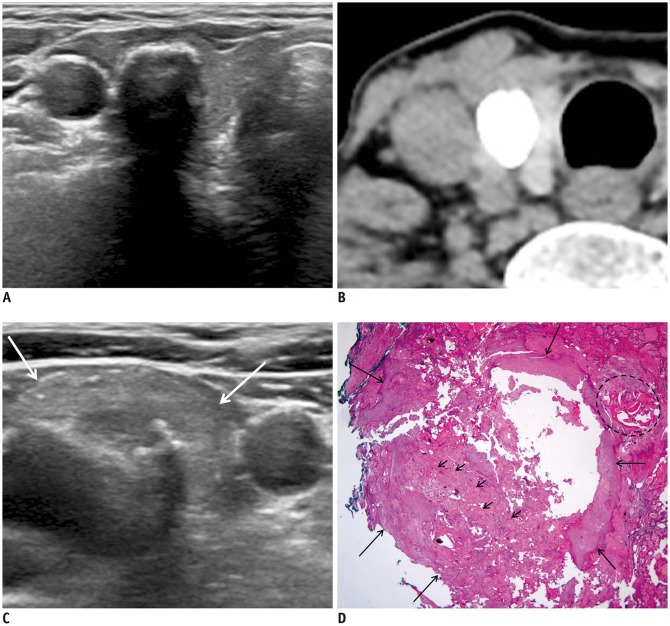
Fig. 2. 71-year-old woman with invasive encapsulated follicular variant papillary carcinoma.
A. US image shows calcified nodule (14 mm) with posterior shadowing and smooth anterior margin in mid-right thyroid lobe. B. Unenhanced CT image shows densely calcified nodule in right thyroid lobe. C. US image shows 15-mm suspicious hyperechoic metastatic lymph node with macrocalcification (arrows) at level IV of right lateral neck. D. Well-circumscribed and encapsulated lesion (long arrows) shows follicular-patterned tumor cells (small arrows) mixed predominantly with dystrophic calcifications and focal ossification (dotted circle) (hematoxylin and eosin, 12.5 ×). TNM stage (AJCC cancer staging manual, 8th edition) was T1bN1bM0, and there was minor extrathyroidal extension of tumor. Cancer was postoperatively classified as American Thyroid Association intermediate risk. AJCC = American Joint Committee on Cancer, US = ultrasonography, TNM = tumor, node, metastasis
Tumor Size, Histology, and Clinical Features of the Six Surgically Diagnosed Malignant Tumors
Among the six malignant tumors with surgical pathologic diagnoses, an extrathyroidal extension (ETE) was found in five tumors (83.3%) (minor ETE 1, gross ETE 4), and macroscopic lymph node (LN) metastases were found in two tumors (33.3%). All tumors with an ETE or LN metastasis were < 2 cm, and three tumors were < 1.5 cm in size (Table 4). Among the four patients with a gross ETE, two patients had a tumor invasion of the anterior strap muscles (T3b), one patient had a tumor invasion of the trachea, and the other had a tumor invasion of both the recurrent laryngeal nerve and the trachea (T4a). Among the two patients with a macroscopic LN metastasis, one had an LN metastasis in the central neck region, and the other had LN metastases in the central and lateral neck regions (Fig. 2).
Table 4. Tumor Size, Histology, and Clinical Features of Surgically Diagnosed 6 Malignant Tumors.
| Size | Tumor Type (No.) | Histologic Feature | ETE (%) | LN Metastasis (%) | ETE or LN Metastasis (%) | AJCC 8th Edition TNM Stage (No.) | ATA Risk Stratification (No.) |
|---|---|---|---|---|---|---|---|
| Group 1 (n = 3) | Classic PTC (2) | Ossification (n = 2) | 3* (100) | 1‡ (33.3) | 3 (100) | T4aN0M0 (1) | High (2) |
| FVPTC (1) | Calcification (n = 1) | T3bN0M0 (1) | Intermediate (1) | ||||
| T1bN1bM0 (1) | |||||||
| Group 2 (n = 3) | Classic PTC (3) | Ossification (n = 2) | 2† (66.7) | 1‡ (33.3) | 2 (66.7) | T4aN0M0 (1) | High (2) |
| Calcification (n = 1) | T3bN1aM0 (1) | Low (1) | |||||
| T1bN0M0 (1) | |||||||
| Group 3 (n = 0) | - | - | - | - | - | - | - |
| All | PTC (6) | Ossification (n = 4) | 5 (83.3) | 2 (33.3) | 5 (83.3) | T4a/T3b (4), T1b (2) | High (4) |
| Calcification (n = 2) | N1b/N1a (2), N0 (4) | Intermediate (1) | |||||
| low (1) |
Group 1: 1–1.4 cm, Group 2: 1.5–1.9 cm, Group 3: ≥ 2 cm. *Minor ETE (n = 1), gross ETE (n = 2), †Gross ETE (n = 2), ‡Macroscopic LN metastasis. AJCC = American Joint Committee on Cancer, ATA = American Thyroid Association, ETE = extrathyroidal extension, FVPTC = follicular variant papillary carcinoma, LN = lymph node, PTC = papillary thyroid carcinoma, TNM = tumor, node, metastasis
The histopathological analysis showed ossifications in four malignant tumors (66.7%), and predominant calcifications mixed with a small portion of ossification were found in two (33.3%) of the six malignant tumors. Four (66.7%) malignant tumors were categorized as high-risk tumors, one as an intermediate-risk tumor, and one as a low-risk tumor using the clinical postoperative ATA risk stratification system (15) (Table 4). There were no distant metastases in the patients with malignant tumors.
DISCUSSION
Our study demonstrated that the isolated macrocalcifications (≥ 1 cm) detected in 1.2% of our cohort patients with thyroid nodules (≥ 1 cm) were associated with an intermediate malignancy risk, which ranged from 18.4% to 23.3%. Among the malignant tumors with surgical diagnoses, a gross ETE was found in 66.7% of the tumors, and a macroscopic LN metastasis was found in 33.3% of the tumors; 66.7% of the tumors were classified as high-risk tumors.
A previous study (9) reported that isolated macrocalcifications were detected in 1.1% of nodules, and that the malignancy risk ranged from 11.4% to 16.1% in all nodules, including subcentimeter nodules, and 16.7% in nodules (≥ 1 cm) with final diagnoses, which were similar to the results of our cohort data of thyroid nodules (≥ 1 cm).
The results of the previous study (9) and data from the present study suggest that the nodules with isolated macrocalcifications have an intermediate malignancy risk (approximately 10–20%), and the US pattern of the isolated macrocalcifications should be placed in an intermediate suspicion category of the risk stratification system of thyroid nodules.
Our study demonstrated that gross ETE or macroscopic LN metastasis was found in 5 (83.3%) of 6 malignant tumors < 2 cm and in all of 3 malignant tumors < 1.5 cm, and the majority (83.3%) of malignant tumors showed a high or intermediate ATA risk. Therefore, the malignant tumors that manifest as isolated macrocalcifications may have a potential for a poor prognosis and worse clinical course, such as tumor recurrence. Accordingly, although the malignancy risk of relatively small (< 1.5 cm) isolated macrocalcifications was modest (11.1–13.6%), a US-guided biopsy should be performed for nodules with isolated macrocalcifications ≥ 1 cm, considering the rare frequency and size distribution of malignant tumors with an aggressive behavior. Notably, the accurate evaluation of the posterior margin of the tumor is difficult because of dense posterior shadowing, which suggests that an enhanced neck CT is necessary to assess the ETE of tumors for preoperative evaluation when the biopsy result shows malignant nodules with isolated macrocalcifications.
In a previous study (9), CNB demonstrated a significantly lower rate of nondiagnostic and inconclusive results than FNA (7.7% vs. 53.8% and 15.4% vs. 57.7%, respectively) in 26 nodules that underwent both FNA and CNB. In our study, CNB also showed a lower nondiagnostic rate and a tendency of a lower inconclusive rate than FNA. However, CNB showed a relatively higher rate of inconclusive and indeterminate results than that of a previous study (9), which suggests that the diagnostic efficacy of CNB for nodules with isolated macrocalcifications may be lower than that of other thyroid nodules without isolated macrocalcifications. This result may be related to the small number of patients who underwent CNB in our study and the pathologists' experience in the interpretation of CNB specimens.
Thyroid nodules can undergo hemorrhagic, cystic, and fibrotic changes and become calcified or ossified. In our study, the histopathologic features of PTCs that manifested as isolated macrocalcifications more frequently showed ossifications rather than dystrophic calcification. Ectopic bone formation and osseous metaplasia in a thyroid nodule has been reported in benign nodules, such as nodular hyperplasia and follicular adenoma, and in malignant nodules (21,22,23). Among the malignant thyroid tumors, intratumoral heterotopic ossification was found exclusively in PTCs (22). Several studies (21,22) have examined the prevalence of intratumoral ossification in PTC, with reports of 13.0% (29/229) (21) and 23.1% (48/207) (22). Takeda et al. (22) reported that PTCs with intratumoral heterotopic ossification were associated with a higher incidence of LN metastases, multifocality, and ETE than PTCs without intratumoral heterotopic ossification, suggesting a different prognosis from that observed in other conventional PTCs. This finding may support our study's result that most nodules with isolated macrocalcifications represented the aggressive behavior of the PTC. The underlying pathogenesis of intratumoral ossification may involve the production of basic fibroblast growth factor by carcinoma cells, which stimulates myofibroblast proliferation that results in nodular fibrosis. Additionally, ossification is induced by the bone morphogenetic protein-2 from carcinoma cells (22).
There are some limitations in our study. First, because our study was conducted retrospectively, there is an inevitable limitation in the interpretation of the isolated macrocalcifications. Second, the US images were interpreted by one experienced thyroid radiologist, and the interobserver agreement on the isolated macrocalcification could not be evaluated. Third, the reference standard for a benign diagnosis was mainly based on the FNA results as well as the histologic findings of the surgical specimen, which might have led to a false-negative result. Fourth, we could not assess if the CT characteristics of isolated macrocalcification helped to predict malignant tumors because only a few patients underwent neck CT. Fifth, our study did not assess the malignancy risk of subcentimeter isolated macrocalcifications; thus, further investigation may be necessary.
In conclusion, isolated macrocalcification (≥ 1 cm) showed an intermediate malignancy risk (at least 18.4%). All malignant tumors were PTCs, and majority of tumors showed an aggressive behavior and a high or intermediate postoperative ATA risk. These findings will provide helpful information for risk stratification and management of nodules with isolated macrocalcification.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Ha EJ, Lim HK, Yoon JH, Baek JH, Do KH, Choi M, et al. Primary imaging test and appropriate biopsy methods for thyroid nodules: guidelines by Korean Society of Radiology and National Evidence-based Healthcare Collaborating Agency. Korean J Radiol. 2018;19:623–631. doi: 10.3348/kjr.2018.19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol. 2014;170:R203–R211. doi: 10.1530/EJE-13-0995. [DOI] [PubMed] [Google Scholar]
- 4.Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25:538–550. doi: 10.1089/thy.2014.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid Imaging Reporting and Data System risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid. 2016;26:562–572. doi: 10.1089/thy.2015.0460. [DOI] [PubMed] [Google Scholar]
- 6.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 7.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 8.Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J, et al. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg. 2011;35:122–127. doi: 10.1007/s00268-010-0827-3. [DOI] [PubMed] [Google Scholar]
- 9.Na DG, Kim DS, Kim SJ, Ryoo JW, Jung SL. Thyroid nodules with isolated macrocalcification: malignancy risk and diagnostic efficacy of fine-needle aspiration and core needle biopsy. Ultrasonography. 2016;35:212–219. doi: 10.14366/usg.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography. 2016;35:25–38. doi: 10.14366/usg.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon SJ, Na DG, Gwon HY, Paik W, Kim WJ, Song JS, et al. Similarities and differences between Thyroid Imaging Reporting and Data Systems. AJR Am J Roentgenol. 2019;213:W76–W84. doi: 10.2214/AJR.18.20510. [DOI] [PubMed] [Google Scholar]
- 13.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14:587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Comparison of performance characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association guidelines. AJR Am J Roentgenol. 2018;210:1148–1154. doi: 10.2214/AJR.17.18822. [DOI] [PubMed] [Google Scholar]
- 15.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na DG, Baek JH, Jung SL, Kim JH, Sung JY, Kim KS, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol. 2017;18:217–237. doi: 10.3348/kjr.2017.18.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 19.Jung CK, Min HS, Park HJ, Song DE, Kim JH, Park SY, et al. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med. 2015;49:288–299. doi: 10.4132/jptm.2015.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing; 2017. pp. 873–890. [Google Scholar]
- 21.Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol. 2009;22:887–894. doi: 10.1038/modpathol.2009.38. [DOI] [PubMed] [Google Scholar]
- 22.Takeda M, Mikami T, Numata Y, Okamoto M, Okayasu I. Papillary thyroid carcinoma with heterotopic ossification is a special subtype with extensive progression. Am J Clin Pathol. 2013;139:587–598. doi: 10.1309/AJCPQZQN50HKIAHA. [DOI] [PubMed] [Google Scholar]
- 23.Aurora N, Hashmi I, Misra S, Aydin N. A rare presentation: a case report of osseous metaplasia and mature bone formation in a follicular adenoma of the thyroid. Int J Surg Case Rep. 2017;37:83–86. doi: 10.1016/j.ijscr.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]