Abstract
Objective
Endovascular thrombectomy (EVT) fails in approximately 20% of anterior circulation large vessel occlusion (AC-LVO). Nonetheless, the factors that affect clinical outcomes of non-recanalized AC-LVO despite EVT are less studied. The purpose of this study was to identify the factors affecting clinical outcomes in non-recanalized AC-LVO patients despite EVT.
Materials and Methods
This was a retrospective analysis of clinical and imaging data from 136 consecutive patients who demonstrated recanalization failure (modified thrombolysis in cerebral ischemia [mTICI], 0–2a) despite EVT for AC-LVO. Data were collected in prospectively maintained registries at 16 stroke centers. Collateral status was categorized into good or poor based on the CT angiogram, and the mTICI was categorized as 0–1 or 2a on the final angiogram. Patients with good (modified Rankin Scale [mRS], 0–2) and poor outcomes (mRS, 3–6) were compared in multivariate analysis to evaluate the factors associated with a good outcome.
Results
Thirty-five patients (25.7%) had good outcomes. The good outcome group was younger (odds ratio [OR], 0.962; 95% confidence interval [CI], 0.932–0.992; p = 0.015), had a lower incidence of hypertension (OR, 0.380; 95% CI, 0.173–0.839; p = 0.017) and distal internal carotid artery involvement (OR, 0.149; 95% CI, 0.043–0.520; p = 0.003), lower initial National Institute of Health Stroke Scale (NIHSS) (OR, 0.789; 95% CI, 0.713–0.873; p < 0.001) and good collateral status (OR, 13.818; 95% CI, 3.971–48.090; p < 0.001). In multivariate analysis, the initial NIHSS (OR, 0.760; 95% CI, 0.638–0.905; p = 0.002), good collateral status (OR, 14.130; 95% CI, 2.264–88.212; p = 0.005) and mTICI 2a recanalization (OR, 5.636; 95% CI, 1.216–26.119; p = 0.027) remained as independent factors with good outcome in non-recanalized patients.
Conclusion
Baseline NIHSS score, good collateral status, and mTICI 2a recanalization remained independently associated with clinical outcome in non-recanalized patients. mTICI 2a recanalization would benefit patients with good collaterals in non-recanalized AC-LVO patients despite EVT.
Keywords: Cerebral infarction, Thrombectomy, Prognosis, Predictive factor
INTRODUCTION
Based on five randomized clinical trials, endovascular mechanical thrombectomy (EVT) is recommended as a standard treatment for anterior circulation large vessel occlusion (AC-LVO) (1,2). In addition, the diffusion-weighted imaging or CT perfusion assessment with clinical mismatch in the triage of wake up and late presenting stroke undergoing neurointervention with Trevo (DAWN) and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke-3 (DEFUSE-3) trials demonstrated the benefit of EVT in late-presenting AC-LVO through strict patient selection criteria (3,4).
The selection criteria in many clinical trials for EVT were developed to identify factors related to patient outcomes after EVT. Lower initial National Institutes of Health Stroke Scale (NIHSS), shorter procedure time, smaller early ischemic change on non-contrast computed tomography (CT), lower serum glucose, good collateral status, and recanalization success were predictive factors for good outcome after EVT (5,6,7,8,9). Among them, successful recanalization (defined as modified thrombolysis in cerebral infarction scale [mTICI] grade 2b or 3) was the most important factor for patient outcomes after EVT (9).
In an era of modern EVT, approximately 20% of patients do not achieve successful recanalization (3,4). However, to our knowledge, the prognosis and factors that affect clinical outcomes in patients without successful recanalization despite modern EVT are not well known. In this study, we intended to evaluate the prognostic factors in non-recanalized AC-LVO patients despite EVT.
MATERIALS AND METHODS
Patient Enrollment
This was a retrospective analysis of data from prospectively maintained registries collected between September 2010 and December 2015. The current study was initiated by the Korean Health Technology R&D Project and involved 16 stroke centers across Korea. The registries enrolled consecutive patients who underwent EVT with a stent retriever (Solitaire AB/FR, ev3, Irvine, CA, USA; Trevo Proview, Stryker, CA, USA), contact aspiration (Penumbra, Alameda, CA, USA), or a combined approach for acute ischemic stroke caused by AC-LVO. These patients had a modified Rankin Scale (mRS) score available at 3 months. AC-LVO was defined as occlusion of the internal carotid artery (ICA), M1 portion of the middle cerebral artery (MCA), or proximal M2 portion of the MCA.
Enrollment criteria for this study were as follows: 1) age ≥ 18 years; 2) initial NIHSS ≥ 4; 3) onset to puncture time ≤ 600 minutes; 4) mRS of 0 or 1 before the qualifying stroke; 5) collateral status assessable on computed tomography angiography (CTA); and 6) recanalization success or failure assessable on catheter angiogram during the procedure. Intracranial AC-LVO accompanying cervical ICA dissection or tandem occlusion was also included. However, AC-LVO caused by intracranial artery dissection and bilateral AC-LVO was excluded from this study. The Institutional Review Board of every participating hospital approved this study (IRB No: 4-2017-0511) and waived the requirement for informed consent for study inclusion based on the retrospective study design.
Data Collection and Assessment
Data collection and assessment methods were described in a previous report (10). Briefly, the Alberta Stroke Program Early CT Score (ASPECTS) was independently evaluated from 5-mm-thick non-contrast CT images by two neuroradiologists. The collateral status on single-phase CTA with 20-mm-thick maximum intensity projection images was assessed with a commercialized DICOM viewer (OsiriX, Pixmeo, Geneva, Switzerland). For multiphase CTA, only the first-phase CTA was used to evaluate the collateral status, which was categorized into either good or poor. According to the pial arterial filling score within the symptomatic ischemic territory using single phase CTA (11), good collateral status was defined as score 4 (slightly reduced prominence and extent of the pial vessel) and 5 (increased or normal prominence and extent of the pial vessels) within the ischemic territory in the symptomatic hemisphere. Patients with poorly identified vessel markings compared to the contralateral, unaffected hemisphere in CTA were excluded to reduce misclassification. In addition, if the occlusion site was located at the origin of the P-com of the distal ICA or the A1 orifice of the anterior cerebral artery in digital subtraction angiography, we classified it into a distal ICA involvement, as involvement of these sites could interfere with the collateral supply of brain. Beyond the distal ICA involvement was defined as the occlusion site located at the M1 or M2 segment of the MCA above the A1 orifice. In the case of tandem occlusion, the involvement of the distal ICA or beyond the distal ICA was determined based on where the distal occluded segment was located.
Two investigators blinded to the ASPECTS, the collateral status, and the clinical outcome independently assessed recanalization success. Recanalization failure was defined as mTICI grade 0–2a on the final control angiogram and further categorized as 0–1 and 2a. Kappa values for inter-rater agreement of the collateral grade (good or poor), mTICI (0–2a vs. 2b–3), and mTICI (0–1 vs. 2a) were 0.875, 0.813, and 0.905, respectively. Disagreement between raters was resolved by consensus. If the recanalization after EVT was not assessed due to poor image quality, the case was classified as undetermined and excluded from the analysis.
Outcome Measurement
Clinical outcomes were evaluated by mRS at 3 months in all patients who fulfilled the enrollment criteria. Good outcome was defined as mRS 0–2 at 3 months after stroke. Patients were categorized into good or poor outcome groups. We analyzed factors associated with good outcomes in non-recanalized patients despite EVT.
Statistical Analysis
The data are presented as a mean ± standard deviation, median (interquartile range [IQR]), or as a number (percent), as appropriate. Differences between good and poor outcomes were compared with a chi-square test, Fisher's exact test, Student's t test, and a Mann-Whitney U test, as appropriate. To determine the independent prognostic factors for good outcomes in non-recanalized patients, binary logistic regression analysis was performed and summarized as an odd ratio (OR) and 95% confidential interval (CI). For multivariate analysis, variables with p < 0.2 in the univariate analysis were included in the binary logistic regression analysis. All tests were two-sided, and finally, p < 0.05 was considered statistically significant. In addition, the final model was fitted using the Hosmer-Lemeshow Goodness of Fit Test and p > 0.05 was considered as the model fit to the binary logistic regression analysis (12). We used the variance inflation factor to assess the multicollinearity among the variables included in the multivariate analysis. All statistical analyses were performed with IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA) and R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
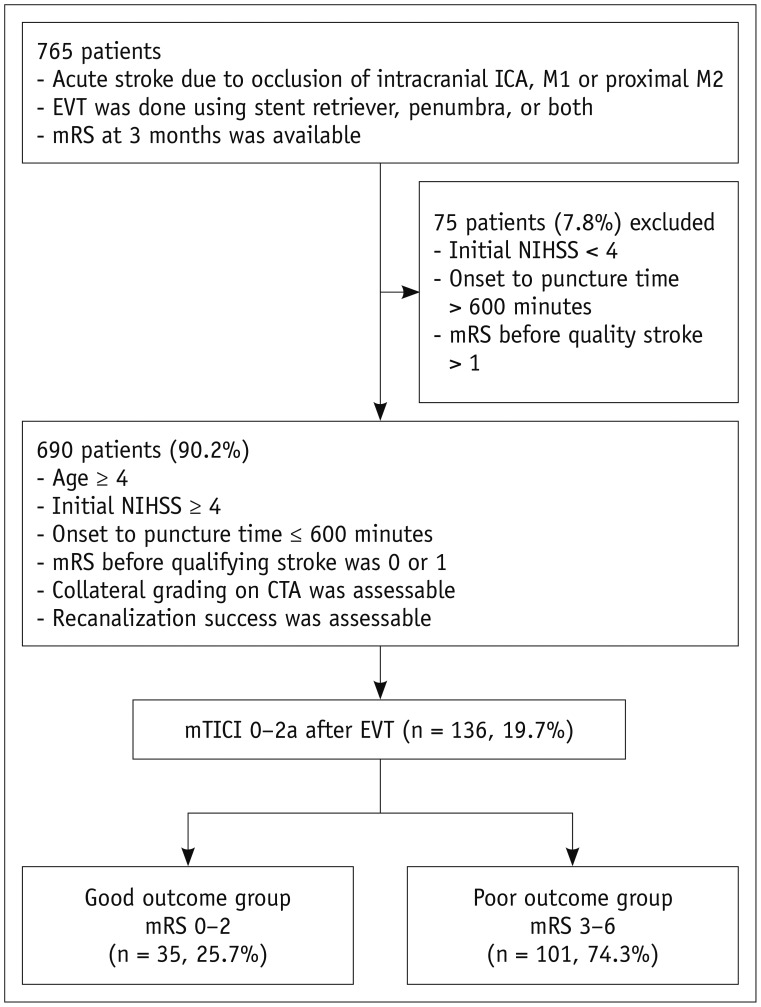
Altogether, 805 patients had undergone EVT for acute ischemic stroke with a stent retriever, penumbra, or both between September 2010 and December 2015. Among them, 765 patients (95.0%) had mRS available at 3 months, of whom 690 (90.2%) fulfilled the enrolment criteria. Of these 690 patients, 136 (19.7%) had recanalization failure despite EVT and were enrolled in this study (Fig. 1).
Fig. 1. Flow chart of patients' inclusion.
CTA = computed tomography angiography, EVT = endovascular thrombectomy, ICA = internal carotid artery, mRS = modified Rankin Scale, mTICI = modified thrombolysis in cerebral ischemia, NIHSS = National Institutes of Health Stroke Scale
Thirty-five (25.7%) of the non-recanalized patients had good outcome. The good outcome group was younger, had a lower incidence of hypertension, lower distal ICA involvement, and lower initial NIHSS and good collateral status (Table 1).
Table 1. Predictors for Good Outcomes in Patients with Recanlization Failure (n = 136).
| mRS (0–2) (n = 35) | mRS (3–6) (n = 101) | P | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||||
| Age, years | 63.5 ± 12.2 | 69.4 ± 11.6 | 0.012 | 0.962 (0.932–0.992) | 0.015 | 0.968 (0.909–1.030) | 0.304 |
| Male | 20 (57.1) | 45 (44.6) | 0.276 | 1.659 (0.764–3.605) | 0.201 | ||
| Hypertension | 17 (48.6) | 72 (71.3) | 0.026 | 0.380 (0.173–0.839 | 0.017 | 0.778 (0.162–3.741) | 0.778 |
| Diabetes | 8 (22.9) | 30 (29.7) | 0.576 | 0.701 (0.285–1.720) | 0.438 | ||
| Hyperlipidemia | 3 (8.6) | 11 (10.9) | 0.947 | 0.767 (0.201–2.926) | 0.698 | ||
| Smoking | 11 (31.4) | 27 (26.7) | 0.753 | 1.256 (0.543–2.906) | 0.594 | ||
| CAOD | 7 (20.0) | 16 (15.8) | 0.761 | 1.328 (0.496–3.558) | 0.573 | ||
| Atrial fibrillation | 15 (42.9) | 51 (50.5) | 0.560 | 0.735 (0.339–1.596) | 0.437 | ||
| Previous ischemic stroke | 3 (8.6) | 18 (17.8) | 0.301 | 0.432 (0.119–1.568) | 0.202 | ||
| Initial NIHSS | 12 (5–19) | 17 (9–25) | < 0.001 | 0.789 (0.713–0.873) | < 0.001 | 0.760 (0.638–0.905) | 0.002 |
| ASPECT | 8 (6–10) | 8 (6–10) | 0.093 | 1.399 (0.942–2.079) | 0.096 | 1.023 (0.556–1.883) | 0.942 |
| mTICI 2a | 17 (48.6) | 36 (35.6) | 0.250 | 1.705 (0.783–3.712) | 0.179 | 5.636 (1.216–26.119) | 0.027 |
| Good collateral | 32 (91.6) | 44 (43.6) | < 0.001 | 13.818 (3.971–48.090) | < 0.001 | 14.130 (2.264–88.212) | 0.005 |
| IV-tPA | 16 (45.7) | 47 (46.5) | 1.000 | 0.968 (0.447–2.093) | 0.999 | ||
| Distal ICA involvement | 3 (8.6) | 39 (38.6) | 0.002 | 0.149 (0.043–0.520) | 0.003 | 0.205 (0.033–14.271) | 0.464 |
| Beyond distal ICA inolvement | 32 (91.4) | 73 (72.3) | 0.036 | 4.091 (1.159–14.439) | 0.029 | 0.229 (0.003–16.070) | 0.497 |
| M1 segment of MCA | 21 (65.6) | 64 (87.7) | |||||
| M2 segment of MCA | 11 (34.4) | 9 (12.3) | |||||
| Initial glucose, mg/dL | 129.6 ± 54.2 | 151.6 ± 102.5 | 0.124 | 0.994 (0.984–1.004) | 0.227 | ||
Data are shown as number (%), mean ± standard deviation, or median (interquartile range). ASPECT = Alberta Stroke Program Early CT score, CAOD = coronary artery occlusive disease, CI = confidence interval, ICA = internal carotid artery, IV = intravenous, MCA = middle cerebral artery, mRS = modified Rankin Scale, mTICI = modified thrombolysis in cerebral ischemia, NIHSS = National Institute of Health Scale, OR = odd ratio, tPA = tissue plasminogen activator
In univariate analysis, the initial NIHSS, good collateral status, and beyond distal ICA involvement were associated with good outcome. In addition, the distal ICA involvement was negatively associated with good outcome (Table 1). After adjusting for potentially associated factors in univariate analysis (p < 0.20), including age, hypertension, initial NIHSS, mTICI 2a recanalization, distal ICA involvement, beyond distal ICA involvement and good collateral status, the initial NIHSS (OR, 0.760; 95% CI, 0.638–0.905, p = 0.002), good collateral status (OR, 14.130; 95% CI, 2.264–88.212, p = 0.005) and mTICI 2a recanalization (OR, 5.636; 95% CI, 1.216–26.119, p = 0.027) remained independent factors with good outcome in non-recanalized patients (Table 1). The model was fairly calibrated (p = 0.599 by the Hosmer-Lemeshow test) with reasonable discrimination (C statistics = 0.915).
DISCUSSION
The patients with mTICI 2a recanalization and good collateral had a relatively higher probability of good outcome in the recanalization failure group. This study is, to our knowledge, the first to identify prognostic factors associated with clinical outcomes in patients with recanalization failure, despite modern EVT.
Recanalization success is one of the most important factors affecting clinical outcome in patients who receive EVT (9). Conventionally, recanalization is defined as mTICI 2b (contrast agent filling more than 50% of the affected artery) or 3 (complete filling). The mTICI 2a recanalization is defined as the contrast agent filling less than 50% of the affected vascular territory. The mTICI 0 and 1 are defined as no or minimal recanalization (perfusion past the initial obstruction but limited distal branch filling with little or no distal perfusion), respectively. For example, if a patient had an occlusion of the right MCA M1 segment, recanalization of either the superior or the inferior M2 division alone might be graded as mTICI 2a. Intuitively, mTICI 2a reperfusion can benefit patients more than mTICI 0 or 1.
The collateral status has been suggested as a strong predictor for both recanalization success and functional outcome (13,14,15,16,17,18,19). Retrograde collateral filling may help thrombolytic agents to access the distal aspects of a clot (20). Moreover, the degree of ischemic vascular injury after vessel occlusion can be minimized by the collateral supply. Thus, the response to intravenous tissue plasminogen activator and EVT could be improved in patients with good collaterals (12). In addition, the time to reperfusion to improve the likelihood of good outcome can be adjusted for the collateral status (10), which was an important background factor in the late window trials, such as DAWN and DEFUSE-3 (3,4).
In our study, we also confirmed that good collateral status was significantly associated with good outcomes even in the case of a failed EVT. In addition, mTICI 2a recanalization provided benefit for the outcome of patients with failed EVT. According to previous reports, the presence of proximal artery occlusion without absence of early recanalization led to neurological deterioration through hemodynamic compromise (21). This result indicates that good collateral status does not completely protect brain tissue; rather delays ischemic injury in various ways. Alternatively, timely recanalization in addition to good collateral status is essential for achieving a good outcome. Similarly, mTICI 2a recanalization may have a more positive effect on clinical outcome in patients with good collaterals; however, non-recanalization in all poor collateral patients resulted in poor outcome regardless of the mTICI grade (0–1 vs. 2a).
Previous studies identified younger age, lower NIHSS, good collateral, lower serum glucose, lower ASPECTS, and successful recanalization as independent predictors for good outcome (5,6,7,8,9). In our study, age and baseline ASPECTS were not associated with good outcomes in the non-recanalization group. Good outcome is less common in patients > 80 years (22). In this study, most patients were younger than 80 years, and age did not differ significantly between the groups. In addition, baseline ASPECTS was similar in the two groups in this study. Thus, ASPECTS did not affect patient outcome. Lower NIHSS was an independent predictive factor for good outcome after successful recanalization (23). As the collateral status was categorized as good or poor in this study, it might not reflect a detailed status. Initial neurological severity may reflect a more precise collateral status even among the good collateral group and, thus, remain independently associated with the clinical outcome.
Finally, the use of tissue plasminogen activator (tPA) was not associated with good outcome in this study. Currently, the question whether EVT without prior tPA is better or worse than EVT with tPA has been of concern. (24,25) The benefits of tPA before EVT is that tPA can lead to spontaneous successful recanalization before EVT, especially in the case of near-occlusion or short thrombi and significantly lowers the number of passes required to achieve successful recanalization (26). Alternatively, tPA can help achieve successful recanalization, which may lead to a good outcome. In our study, we only included the non-recanalized AC-LVO patients despite EVT. Thus, the effect of tPA may be limited.
This study had some strengths and limitations. Many attributable reasons regarding failure to achieve successful recanalization, defined as mTICI 2b–3, have been suggested (27). We did not collect the information regarding reasons of failure of EVT and the point when EVT attempts were discontinued, as the EVT strategy of each participating hospital was different. Nevertheless, the primary focus of this study was to determine the predictors for a good outcome despite a failed EVT. The collateral status was assessed with single-phase CTA, as it is the most widely used modality at the participating centers (93.5% of enrolled patients). Due to delayed circulation time after beginning the CTA scan, contrast agent filling of cerebral arteries through collaterals on single-phase CT is insufficient. Thus, using single-phase CTA can result in good collaterals misclassified as poor collaterals. However, we only included patients with clearly visible vessel markings in the contralateral normal hemisphere. In addition, we only included the first phase image for patients who underwent multiphase CTA to increase inter-rater agreement. Finally, mTICI 2a recanalization and good collateral illustrated a wide range of confidence interval due to our relatively small sample size. Thus, future studies with a large sample size are required. Therefore, interrater agreement was excellent for the discrimination of poor and good collateral status and for of mTICI 0–1 and mTICI 2a. Despite the retrospective nature of our study, our study population represents at least 10% of all patients who received EVT in Korea during the study period, based on nationwide data from the Health Insurance Review and Assessment Service. Therefore, the results of this study reflect real-world practice in Korea.
In conclusion, lower initial NIHSS, good collateral, and mTICI 2a recanalization were independent predictors of good outcome in patients with recanalization failure despite EVT. This result suggests that mTICI 2a recanalization would benefit patients with good collateral, even if recanalization were not successful despite EVT.
Footnotes
This work supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1056).
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e99. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 2.Hong KS, Ko SB, Yu KH, Jung C, Park SQ, Kim BM, et al. Update of the Korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2016;18:102–113. doi: 10.5853/jos.2015.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 5.Costalat V, Lobotesis K, Machi P, Mourand I, Maldonado I, Heroum C, et al. Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 Patients prospective study. Eur J Radiol. 2012;81:4075–4082. doi: 10.1016/j.ejrad.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Soize S, Barbe C, Kadziolka K, Estrade L, Serre I, Pierot L. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology. 2013;55:977–987. doi: 10.1007/s00234-013-1191-4. [DOI] [PubMed] [Google Scholar]
- 7.Daou B, Chalouhi N, Starke RM, Dalyai R, Hentschel K, Jabbour P, et al. Predictors of outcome, complications, and recanalization of the solitaire device: a study of 89 cases. Neurosurgery. 2015;77:355–361. doi: 10.1227/NEU.0000000000000830. [DOI] [PubMed] [Google Scholar]
- 8.Ozdemir O, Giray S, Arlier Z, Bas¸ DF, Inanc Y, Colak E. Predictors of a good outcome after endovascular stroke treatment with stent retrievers. ScientificWorldJournal. 2015;2015:403726. doi: 10.1155/2015/403726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon W, Kim SK, Park MS, Baek BH, Lee YY. Predictive factors for good outcome and mortality after stent-retriever thrombectomy in patients with acute anterior circulation stroke. J Stroke. 2017;19:97–103. doi: 10.5853/jos.2016.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BM, Baek JH, Heo JH, Nam HS, Kim YD, Yoo J, et al. Collateral status affects the onset-to-reperfusion time window for good outcome. J Neurol Neurosurg Psychiatry. 2018;89:903–909. doi: 10.1136/jnnp-2017-317627. [DOI] [PubMed] [Google Scholar]
- 11.Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275:510–520. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 12.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 13.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon BK, Smith EE, Modi J, Patel SK, Bhatia R, Watson TW, et al. Regional leptomeningeal score on CT angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. AJNR Am J Neuroradiol. 2011;32:1640–1645. doi: 10.3174/ajnr.A2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009;40:3001–3005. doi: 10.1161/STROKEAHA.109.552513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 17.Nambiar V, Sohn SI, Almekhlafi MA, Chang HW, Mishra S, Qazi E, et al. CTA collateral status and response to recanalization in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2014;35:884–890. doi: 10.3174/ajnr.A3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang YH, Kang DH, Kim YW, Kim YS, Park SP, Liebeskind DS. Impact of time-to-reperfusion on outcome in patients with poor collaterals. AJNR Am J Neuroradiol. 2015;36:495–500. doi: 10.3174/ajnr.A4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol. 2017;81:849–856. doi: 10.1002/ana.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 21.Castonguay AC, Zaidat OO, Novakovic R, Nguyen TN, Taqi MA, Gupta R, et al. Influence of age on clinical and revascularization outcomes in the North American solitaire stent-retriever acute stroke registry. Stroke. 2014;45:3631–3636. doi: 10.1161/STROKEAHA.114.006487. [DOI] [PubMed] [Google Scholar]
- 22.Tisserand M, Seners P, Turc G, Legrand L, Labeyrie MA, Charron S, et al. Mechanisms of unexplained neurological deterioration after intravenous thrombolysis. Stroke. 2014;45:3527–3534. doi: 10.1161/STROKEAHA.114.006745. [DOI] [PubMed] [Google Scholar]
- 23.Shi ZS, Liebeskind DS, Xiang B, Ge SG, Feng L, Albers GW, et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke. 2014;45:1977–1984. doi: 10.1161/STROKEAHA.114.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Maria F, Mazighi M, Kyheng M, Labreuche J, Rodesch G, Consoli A, et al. Intravenous thrombolysis prior to mechanical thrombectomy in acute ischemic stroke: silver bullet or useless bystander? J Stroke. 2018;20:385–393. doi: 10.5853/jos.2018.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broeg-Morvay A, Mordasini P, Bernasconi C, Bühlmann M, Pult F, Arnold M, et al. Direct mechanical intervention versus combined intravenous and mechanical intervention in large artery anterior circulation stroke: a matched-pairs analysis. Stroke. 2016;47:1037–1044. doi: 10.1161/STROKEAHA.115.011134. [DOI] [PubMed] [Google Scholar]
- 26.Desilles JP, Loyau S, Syvannarath V, Gonzalez-Valcarcel J, Cantier M, Louedec L, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46:3241–3248. doi: 10.1161/STROKEAHA.115.010721. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Hong KS. Endovascular therapy: the second round begins. J Stroke. 2017;19:119–120. doi: 10.5853/jos.2017.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]