Abstract
The mast cell-nerve unit has classically represented a fundamental neuroimmune axis in the development of itch due to the traditional prominence of histamine as a pruritogen. However, it is increasingly appreciated that most chronic itch disorders are likely non-histaminergic in nature, provoking the hypothesis that other novel effector itch mechanisms derived from mast cells are important. In this review, we present an overview of classical mast cell biology and put these concepts into the context of recent advances in our understanding of the regulation and function of the mast cell-nerve unit in itch biology.
Introduction
Originally described by Paul Ehrlich over a century ago, mast cells (MCs) have been viewed as important effector cells in allergic inflammatory processes that underlie diseases such as anaphylaxis, asthma, food allergy, and urticaria. Arising from pluripotent progenitor cells of the bone marrow, MC precursors circulate in the blood and enter tissues where they receive specific signals to undergo maturation and are long-lived (Galli et al., 2005, Pasparakis et al., 2014). Mature MCs are activated by binding of allergens to IgE, which is attached to the cell surface via the high-affinity receptor FcεRI. Binding of antigen results in crosslinking of IgE on FcεRI which then rapidly induces MC degranulation and the release of various preformed effector molecules (Amin, 2012). Although this is the most well described mode of MC activation, there are new pathways emerging that confer unique effector mechanisms and physiology.
MCs have been shown to reside in close proximity with neurons in multiple tissues including the bladder (Letourneau et al., 1996), gut (Stead et al., 1987), lung (Undem et al., 1995), and skin (Egan et al., 1998), provoking the hypothesis that a primary function of the MC-nerve unit is to regulate a variety of neuroimmune interactions. Indeed, many mediators released from MCs are classified as pruritogens. Although histaminergic itch elicited by MCs has been recognized as one of the most well-known physiologic processes, surprisingly, antihistamines have notoriously demonstrated poor efficacy for most chronic itch conditions. Thus, the clear role of MCs in many clinical itch disorders has yet to be defined. Additionally, the release of various factors like neuropeptides (NPs) from innervating neurons such as substance P (SP) and vasoactive intestinal peptide (VIP) have been reported to modulate MC function (Kulka et al., 2008), suggesting that previously unrecognized mechanisms of regulation and effector function may shed new light on the relevance of MCs to chronic itch.
In this review, we will focus on recent advances in MC biology that may explain new mechanisms by which MCs are regulated to elicit itch via histamine-independent pathways and have previously unrecognized roles in clinical itch disorders. These developments will likely open new avenues to novel therapeutic approaches for chronic itch.
Classical and emerging MC-derived pruritogens
Biogenic amines
Histamine is a classical pruritogen released from MCs (Figure 1) and acts on four distinct G protein-coupled receptors (GPCRs [H1R, H2R, H3R, and H4R]). Although neurons can broadly express H1R, H3R, and H4R, only H1R has been clearly demonstrated to be expressed by pruriceptive dorsal root ganglion (DRG) neurons in humans and mice, whereas H4R expression has been reported on rat DRG (Dimitriadou et al., 1994, Shim et al., 2007, Strakhova et al., 2009). The binding of histamine to H1R triggers the opening of the nonspecific cation channel transient receptor potential V1 (TRPV1) on sensory neurons, resulting in membrane depolarization, a subsequent action potential, and itch sensation. However, given that current antihistamines currently target H1R or H2R, the role of H4R in itch remains an open question. In vitro experiments demonstrate that TRPV1 is also involved in the histamine-H4R itch axis and that H4R contributes to itch in preclinical murine models (Jian et al., 2016, Mack and Kim, 2018). Indeed, H4R antagonists are currently in development for conditions like atopic dermatitis (AD) and future studies will be required to fully determine the role of histamine in chronic itch.
Figure 1. Various ligands and receptors known to stimulate the growth, migration, and/or activation of mast cells.
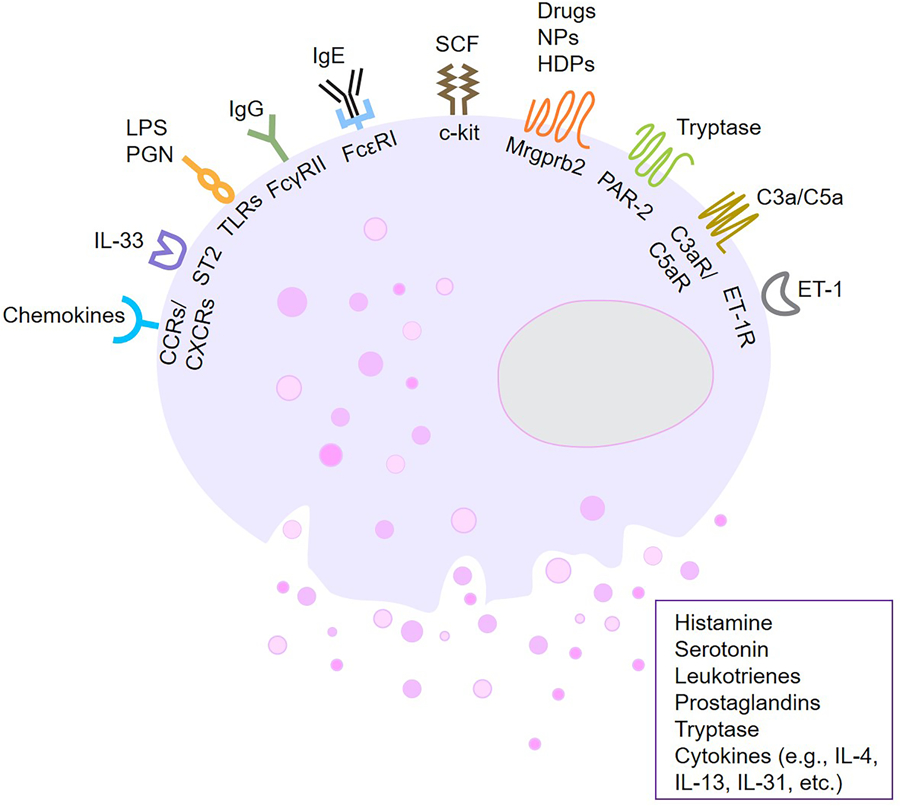
c-Kit (mast/stem cell growth factor receptor, CD117), which mediates responses to stem cell factor (SCF), is a key growth factor for the development of mast cells. IgE crosslinking of the high-affinity receptor FcεRI is the classical pathway leading to mast cell activation and degranulation. A recently identified receptor is Mrgprb2 (murine)/MRGPRX2 (human) which responds to cationic compounds, numerous drugs, and various neuropeptides (NPs) and host defense peptides (HDPs). Other receptors include protease-activated receptor (PAR)-2, chemokine receptors (CCRs/CXCRs) complement receptors, endothelin-1 receptor (ET-1R), FcγRII for IgG, Toll-like receptors (TLRs) for lipopolysaccharide (LPS) or peptidoglycan (PGN), ST2 for IL-33. Mast cell activation leads to the release of multiple mediators such as histamine, serotonin (5-hydroxytryptamine, 5-HT), leukotrienes (LTs), prostaglandins (PGs), tryptase and cytokines. Figure created with Biorender.
Although rodent MCs are described to be an important source of serotonin (5-hydroxytryptamine, 5-HT), release of serotonin from human MCs have been implicated in specific disease contexts such as mastocytosis (Herr et al., 2017, Kushnir-Sukhov et al., 2007). Notwithstanding of this, serotonin is defined as a pruritogen because cutaneous injection with serotonin successfully elicits itch in healthy humans and mice (Akiyama et al., 2010, Weisshaar et al., 1997). Indeed, serotonin signaling is not only associated with itchy skin disorders such as AD (Huang et al., 2004), allergic contact dermatitis (ACD) (Liu et al., 2013, Lundeberg et al., 1999), and psoriasis (Nordlind et al., 2006), but has also been linked to itch caused by systemic conditions like cholestasis, uremia, and morphine-induced pruritus (Aly et al., 2018, Kerr et al., 1992, Schworer et al., 1995). However, the precise and robust manner in which serotonin can be effectively manipulated to treat chronic itch disorders in patients remains to be shown.
Lipid mediators
Lipids are the major components of cell membranes. Phospholipase A2s (PLA2s) are a group of enzymes required for release of arachidonic acid (AA) and lysophosphatidic acid. More than 30 PLA2s are encoded in the mammalian system (Murakami and Taketomi, 2015). A recent RNA-sequencing study has found that the group IV PLA2 family is significantly enriched in itchy skin lesions compared to non-pruritic and non-lesional skin in either AD patients or psoriasis patients (Nattkemper et al., 2018). These data indicate that MC-derived lipid mediators are highly connected to itch mechanisms underlying inflammatory skin conditions.
Prostaglandins (PGs) are AA metabolites, and are broadly involved in inflammatory processes. Among the subtypes of PGs, PGD2 and PGE2 are the most significantly implicated in itch (Figure 1). Intradermal injection of PGE2 in human subjects has been shown to elicit itch via enhancing histamine- and serotonin-induced itching (Fjellner and Hagermark, 1979, Hagermark and Strandberg, 1977). However, intradermal injection of PGE2 does not induce itch behavior in mice (Andoh and Kuraishi, 1998), and even surprisingly, a topical application of PGE2 significantly suppressed spontaneous scratching in NC/Nga mice with AD-like disease (Arai et al., 2004). Similar to PGE2, application of PGD2 or the potent PGD2 agonist (BW245C) to the ocular surface can elicit itching and mild burning sensations in human subjects when treating glaucoma (Nakajima et al., 1991). In contrast, topical applications of PGD2 or TS-022, a DP1 receptor agonist, have shown suppressive effects on spontaneous scratching in NC/Nga mice (Arai et al., 2004, Arai et al., 2007). Clinically, mast cell activation syndrome (MCAS) is a newly recognized collection of disorders that typically involves multiorgan inflammation due to the release of mast cell mediators. Patients with MCAS can present with chronic and relapsing itch (Petra et al., 2014). Indeed, nonsteroidal anti-inflammatory drugs which inhibit PG synthesis, have been reported to be effective in MCAS-associated itch (Kesterson et al., 2018). However, future studies will be required to fully define the role of PGs in various chronic itch disorders.
Generated from AA via the 5-lipoxygenase (5-LO) pathway, LTs are divided into two classes, namely, the chemoattractant LTB4 and the cysteinyl LTs (CysLTs: LTC4, LTD4, and LTE4) (Luster and Tager, 2004). In mice, intradermal injections of LTB4 have been shown to induce scratching behavior (Andoh and Kuraishi, 1998, Fernandes et al., 2013). Additionally, TRPV1 or transient receptor potential ankyrin 1 (TRPA1) antagonists have been shown to inhibit itch behavior in this context, suggesting that these may be pruritogens (Fernandes et al., 2013). In the eye, subconjunctival injections of LTB4 have been shown to provoke site-directed scratching and application of the LTB4 receptor antagonist ONO-4057 inhibits ragweed pollen-associated ocular scratching in mice (Andoh et al., 2012). However, the role of CysLTs as pruritogens remains controversial. Studies have shown that applications of different CysLTs (LTC4, LTD4 or LTE4) to the eye do not induce itch behavior in guinea pigs (Woodward et al., 1995) and scratching does not increase in mice receiving intradermal injections of LTD4 (Andoh et al., 2001). Notwithstanding this, a recently published study has shown that intradermal injection of LTC4, causes robust itch behavior in mice (Solinski et al., 2019). Collectively, these studies indicate that the role of various LTs in mediating itch remains a complex area requiring further investigation.
MC-associated cytokines: IL-4, IL-13, and IL-31
Beyond classical pruritogens, specific cytokines are increasingly recognized for their ability to function as pruritogens. Indeed, a recent study demonstrated that the MC-associated type 2 cytokines IL-4 and IL-13 can directly stimulate peripheral sensory neurons in vitro. Further, conditional deletion of the gene for IL-4Rα, which mediates both IL-4 and IL-13 signaling, on sensory neurons resulted in attenuation of AD-like itch in mice (Oetjen et al., 2017). However, whether type 2 cytokines derived specifically from MCs mediate itch in vivo and in what contexts remains to be fully defined.
IL-31, first discovered in 2004 (Dillon et al., 2004), belongs to gp130/IL-6 cytokine family. It is the first cytokine to be defined as a pruritogen because of its ability to directly stimulate sensory neurons to evoke itch (Cevikbas et al., 2014). Although originally identified to be derived mainly from T helper type 2 cells, increasing evidence suggests that MCs might be a source of IL-31. Indeed, IL-31 mRNA was detected in a human MC line activated by the epithelial cell-derived antimicrobial peptides human β-defensin and LL-37 (Niyonsaba et al., 2010). More recently, IL-33 has been shown to activate human MCs to evoke the release of IL-31 (Petra et al., 2018). Additionally, in a number of chronic itch disorders closely associated with MC dysfunction including chronic spontaneous urticaria, mastocytosis, and myeloproliferative neoplasms, serum/plasma levels of IL-31 have been found to be elevated (Hartmann et al., 2013, Lin et al., 2017, Raap et al., 2010). Thus, studying the exact role of MCs in modulating IL-31 expression and the therapeutic potential of disruption of IL-31-IL31RA interactions for MC-related itch disorders remains and exciting area of investigation.
Regulation of MCs by Mrgprb2/MRGPRX2
Although classically activated by IgE-mediated FcεRI aggregation, MCs have been shown to respond to a variety of other stimuli including complement, chemokines, adenosine, nerve growth factor (NGF), SP, host defense peptides and basic peptides (Figure 1) (Metz et al., 2008, Serhan et al., 2019, Subramanian et al., 2016, Tatemoto et al., 2006). These diverse pathways of MC activation result in the release of a wide range inflammatory mediators (Tal and Liberman, 1997, Thangam et al., 2018). However, the precise contribution of these different pathways has remained poorly understood.
It has been well recognized since the 1950s that the compound 48/80 (48/80) is a rapid and potent activator of MCs (Paton, 1951). Indeed, intradermal injection of 48/80 has been used as a tool in both mice and humans to probe the mechanisms underlying MC-elicited itch (Fjellner et al., 1989, Goldberg et al., 1991). However, for decades the mechanism by which MCs responded to 48/80 was elusive. In 2015, McNeil et al. discovered that the Mas-related GPCR Mrgprb2 is a highly specific receptor for MCs and mediates 48/80-induced MC responses in vitro and pseudoanaphylactic responses in vivo in mice (McNeil et al., 2015). The human ortholog, MRGPRX2 is also present on human MCs. Beyond 48/80, many synthetic compounds, peptidomimetic drugs, and endogenous peptides and amines have demonstrated activity in stimulating Mrgprb2/MRGPRX2. More recently, Staphylococcus δ-toxin as well as antimicrobial peptides have been shown to directly stimulate this pathway to evoke MC activation (Azimi et al., 2017, Zhang and McNeil, 2019). We speculate that this highly conserved mechanism may underlie conditions such as contact urticaria, in which patients develop rapid urticarial reactions to classical haptens, but often independently of IgE, and much too rapid to be driven by a delayed hypersensitivity reaction. In addition, MRGPRX2 also has high fold changes on RNA level in itchy skin lesions biopsied from AD or psoriasis patients (Nattkemper et al., 2018), which indicates its broad involvement in itch-related dermatoses.
Furthermore, Gaudenzio et al. showed that different stimuli influence the dynamics and features of MC degranulation in distinct ways. Stimulation of MRGPRX2 and other GCPRs (e.g., C3aR, C5aR, and endothelin-1R) resulted in the rapid release of smaller and uniformly sized granules from human MCs, while IgE-induced degranulation led to slower and sustained activation associated with the release of larger granules (Gaudenzio et al., 2016). Collectively, these findings provoked the hypothesis that Mrgprb2/MRGPRX2-mediated activation of MCs may elicit distinct processes from the classical IgE-histamine axis so closely attributed to the physiology of the MC-nerve functional unit.
The MC-itch nerve unit: beyond IgE and histamine
Due to the early history of histamine being defined as a pruritogen (Dale and Laidlaw, 1910), the MC-nerve unit has classically been viewed as the key neuroimmune interaction that mediates itch. Indeed, the discovery of numerous itch-specific pathways on neurons including gastrin-releasing peptide receptor (Sun and Chen, 2007), MrgprA3 (Liu et al., 2009), natriuretic polypep-tide b (Nppb) (Mishra and Hoon, 2013), IL-31 (Cevikbas et al., 2014), and thymic stromal lymphopoietin (Wilson et al., 2013) employed various methods to prove that these pathways were non-histaminergic and/or MC-independent. However, despite the prominence of MCs in the neuroimmune paradigm of itch, their precise contribution to various chronic itch disorders and the effector mechanisms employed by these cells to evoke itch have remained poorly understood.
To directly and simply address the role MCs in itch, Solinski et al. recently undertook an elegant approach whereby mice expressing Cre-recombinase expressed under MC-specific mast cell protease 5 were crossed to r26-LSL-hMRDq mice. This approach allowed for targeted insertion of the artificial Hm3Dq receptor into MCs, allowing for pharmacogenetic activation of MCs in a specific manner in response to clozapine-N-oxide (CNO). As hypothesized, CNO-induced activation of MCs was sufficient to induce itch, and in vitro activation of MCs led to the release of a variety of mediators including LTC4, serotonin, and sphingosine-1-phsophate. Strikingly, these mediators stimulated Nppb+ neurons which in turn depended on the canonical gastrin-releasing peptide-spinal cord circuit to ultimately evoke itch (Solinski et al., 2019).
In terms of regulation of MCs in itch, Meixiong et al. demonstrated that activation of Mrgprb2 by the endogenous pro-adrenomedullin peptide (PAMP) 9–20 resulted in itch that commenced independently of the IgE-histamine axis (Meixiong et al., 2019). Although PAMP is classically a vasoregulatory peptide released from the adrenal medulla, the authors found that it was highly expressed in keratinocytes from lesional skin of patients with ACD in conjunction with MC enrichment in the dermis. These findings provoked the hypothesis that epithelial cell-derived PAMP-dermal MC interactions may elicit ACD-associated itch. Indeed, ACD itch across three different murine models demonstrated dependence on Mrgprb2. Strikingly, PAMP-mediated Mrgprb2 stimulation resulted in preferential release of tryptase and lower release of histamine and serontonin from MCs, which is a distinct pattern of sensory neuronal activation from IgE-elicited itch (Figure 2), indicating that differential pathways to MC activation result in distinct neuronal responses. However, in contrast to PAMP, 48/80 was found to induce release of similar amounts of histamine as IgE-mediated stimulation (McNeil et al., 2015, Yao et al., 2014). Thus, it is possible that different ligands induce different effector functions on MCs even upon stimulation of the same Mrgprb2 receptor. Further, in humans, skin injection of PAMP was shown to be mitigated by co-injection with an antihistamine (Hasbak et al., 2006), demonstrating the complexity of how MC stimulation may result in itch responses.
Figure 2. The IgE-mediated versus Mrgprb2-mediated itch axis.
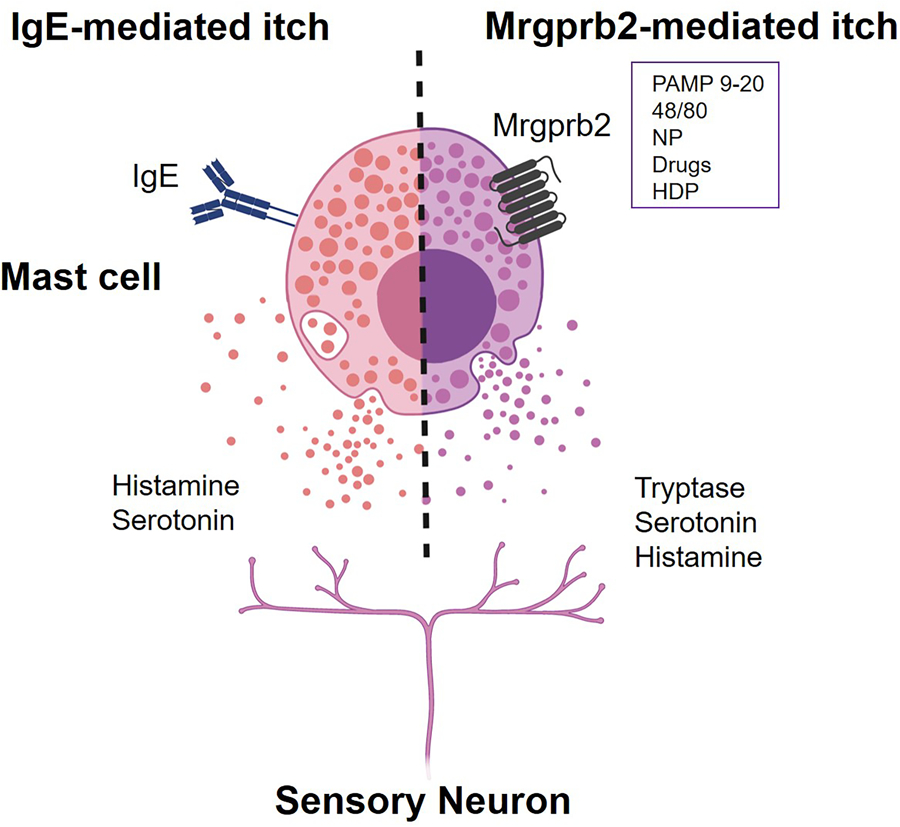
Classical activation of mast cells by IgE results in the release of the monoamines such as histamine and serotonin. Mrgprb2 (murine)/MRGPRX2 (human) can be activated by various cationic substances, such as pro-adrenomedullin peptide 9–20 (PAMP 9–20), compound 48/80, drugs, neuropeptides (NPs), and host defense peptides (HDPs). Mrgbprb2-mediated activation of mast cells elicits distinct mechanisms of itch from classical IgE stimulation, in which tryptase is a major mediator while others such as histamine and serotonin are also included. Figure created with Biorender.
In addition to pruritogens, MCs are also a source of other mediators that may contribute to neurite elongation or outgrowth of sensory neurons. Nerve growth factor (NGF) is one such molecule. Sensory nerve density has been shown to be increased in itchy skin lesions in AD and psoriasis and accompanied by increased numbers of degranulated MCs (Chang et al., 2007, Tominaga and Takamori, 2014). Thus, NGF released by MCs may be an underlying mechanism of this phenomenon.
Neurons promoting MC activation and neuroinflammation
The primary afferent neurons responding to MC-derived mediators consequently release NPs like calcitonin gene-related peptide (CGRP), SP, and VIP through calcium influx. Indeed, multiple prior studies have demonstrated that some NPs can directly stimulate MCs to evoke the release of various proinflammatory factors (Figure 1) (Lee et al., 2008, Manning et al., 2016, Roosterman et al., 2006, Serhan et al., 2019, Steinhoff et al., 2000). A recent study demonstrated that SP activates MCs via Mrgprb2 to evoke neurogenic inflammation and pain behavior (Green et al., 2019). It is increasingly appreciated that MCs can be activated by NPs to promote neurogenic inflammation in a variety of contexts. However, how itch-sensory neurons are directly involved in this process is an exciting field of future investigation.
A large proportion of primary afferent neurons express protease-activated receptor (PAR)-2, one of the known receptors for tryptase, whose activation promotes the release of CGRP and SP leading to neurogenic tissue inflammation and edema (Roosterman et al., 2006, Steinhoff et al., 2000). Other MC-derived itch mediators such as histamine, also interact with their specific receptors on neurons to cause the release of NPs as well (Gupta and Harvima, 2018, Subramanian et al., 2016). Both SP and VIP have been shown to activate murine and human MCs via Mrgprb2 and MRGPRX2, respectively (Subramanian et al., 2016). Thus, in addition to the new insights into the MC-nerve unit as a key mediator of itch sensation, understanding how sensory neurons directly regulate MC function and tissue inflammation is also a major outstanding area of inquiry.
It is well-known that stress aggravates itch symptoms, however, the mechanisms remain poorly understood. Intriguingly, animal experiments have shown that both brain and skin MCs are activated in the setting of stress (Esposito et al., 2002, Singh et al., 1999). Additionally, in AD patients, stress increases allergen-induced skin wheal reactions and serum levels of SP, VIP and NGF (Kimata, 2003). Thus, MCs could be key mediators of the stress-itch axis and remains a major gap in understanding itch behavior.
Summary and future directions
Although the MC-nerve unit has classically represented a fundamental link in the neuroimmune itch circuit, their precise contribution to various chronic itch disorders have remained poorly defined. Based on new understanding, several major questions remain: 1) What is the precise role of MCs in other chronic itch disorders like AD, chronic pruritus of unknown origin (Xu et al., 2016), and prurigo nodularis (Zeidler et al., 2018)? 2) Can antagonists for various GPCRs like Mrgprb2 be employed to treat such chronic itch disorders and which effector molecules are the most important therapeutic targets? Lastly, given that MC stabilizers have poor efficacy in chronic itch disorders such as AD (Benton et al., 1990) and the striking similarity between MCs and basophils, do homologous mechanisms exist within their rare, circulating counterparts? Ever since their original discovery, MCs continue to unveil their complex and important role in neurosensory biology and beyond.
Acknowledgments
We thank all members in Kim lab for helpful comments and discussion.
Funding
This work is supported by the Doris Duke Charitable Foundation, LEO Pharma, and the National Institute of Arthritis Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health [NIH (K08AR065577 and R01AR070116)].
Abbreviations
- 48/80
compound 48/80
- 5-HT
5-hydroxytryptamine
- AA
arachidonic acid
- AD
atopic dermatitis
- ACD
allergic contact dermatitis
- CGRP
calcitonin gene-related peptide
- CNO
clozapine-N-oxide
- CysLTs
cysteinyl LTs
- DRG
dorsal root ganglion
- ET-1R
endothelin-1 receptor
- GCPR
G-protein-coupled receptor
- HDP
host defense peptide
- LPS
lipopolysaccharide
- LT
leukotriene
- MCAS
mast cell activation syndrome
- MCDPs
mast cell degranulating peptides
- MC
mast cell
- Mrgpr
MAS-related G protein-coupled receptor
- NGF
nerve growth factor
- NP
neuropeptide
- Nppb
natriuretic peptide b
- PAMP
pro-adrenomedullin peptide
- PAR
protease-activated receptor
- PGN
peptidoglycan
- PG
prostaglandin
- SCF
stem cell factor
- SP
substance P
- TLR
Toll-like receptor
- TRPA1
transient receptor potential A1
- TRPV1
transient receptor potential V1
- VIP
vasoactive intestinal peptide
Footnotes
Conflicts of Interest Disclosures
Dr. Kim has served as a consultant for AbbVie, Inc., Cara Therapeutics, Concert Pharmaceuticals, Incyte Corporation, Menlo Therapeutics, and Pfizer, Inc. He has also participated on the advisory board for Cara Therapeutics, Celgene Corporation, Kiniksa Pharmaceuticals, Menlo Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi, and Theravance Biopharma. He is also Founder, Chief Scientific Officer, and stockholder of Nuogen Pharma, Inc. He is stockholder of Locus Biosciences. All other authors declare that they have no relevant conflicts of interest.
References
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. Journal of neurophysiology 2010;104(5):2442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Ibrahim A, Farrag W, Abdelsalam K, Mohamed H, Tawfik A. Pruritus after intrathecal morphine for cesarean delivery: incidence, severity and its relation to serum serotonin level. International journal of obstetric anesthesia 2018;35:52–6. [DOI] [PubMed] [Google Scholar]
- Amin K. The role of mast cells in allergic inflammation. Respiratory medicine 2012;106(1):9–14. [DOI] [PubMed] [Google Scholar]
- Andoh T, Katsube N, Maruyama M, Kuraishi Y. Involvement of leukotriene B(4) in substance P-induced itch-associated response in mice. The Journal of investigative dermatology 2001;117(6):1621–6. [DOI] [PubMed] [Google Scholar]
- Andoh T, Kuraishi Y. Intradermal leukotriene B-4, but not prostaglandin E-2, induces itch-associated responses in mice. European journal of pharmacology 1998;353(1):93–6. [DOI] [PubMed] [Google Scholar]
- Andoh T, Sakai K, Urashima M, Kitazawa K, Honma A, Kuraishi Y. Involvement of leukotriene B4 in itching in a mouse model of ocular allergy. Experimental eye research 2012;98:97–103. [DOI] [PubMed] [Google Scholar]
- Arai I, Takano N, Hashimoto Y, Futaki N, Sugimoto M, Takahashi N, et al. Prostanoid DP1 receptor agonist inhibits the pruritic activity in NC/Nga mice with atopic dermatitis. European journal of pharmacology 2004;505(1–3):229–35. [DOI] [PubMed] [Google Scholar]
- Arai I, Takaoka A, Hashimoto Y, Honma Y, Koizumi C, Futaki N, et al. Effects of TS-022, a newly developed prostanoid DP1 receptor agonist, on experimental pruritus, cutaneous barrier disruptions and atopic dermatitis in mice. European journal of pharmacology 2007;556(1–3):207–14. [DOI] [PubMed] [Google Scholar]
- Azimi E, Reddy VB, Lerner EA. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch 2017;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton EC, McFarlane HA, Barnetson RS. Trial of nedocromil sodium in atopic eczema. The British journal of dermatology 1990;122(6):817–20. [DOI] [PubMed] [Google Scholar]
- Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. The Journal of allergy and clinical immunology 2014;133(2):448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Han SS, Jung HJ, Choi JH. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Brit J Dermatol 2007;156(6):1272–7. [DOI] [PubMed] [Google Scholar]
- Dale HH, Laidlaw PP. The physiological action of beta-iminazolylethylamine. J Physiol-London 1910;41(5):318–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology 2004;5(7):752–60. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V, Rouleau A, Dam Trung Tuong M, Newlands GJ, Miller HR, Luffau G, et al. Functional relationship between mast cells and C-sensitive nerve fibres evidenced by histamine H3-receptor modulation in rat lung and spleen. Clinical science 1994;87(2):151–63. [DOI] [PubMed] [Google Scholar]
- Egan CL, Viglione-Schneck MJ, Walsh LJ, Green B, Trojanowski JQ, Whitaker-Menezes D, et al. Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J Cutan Pathol 1998;25(1):20–9. [DOI] [PubMed] [Google Scholar]
- Esposito P, Chandler N, Kandere K, Basu S, Jacobson S, Connolly R, et al. Corticotropin-releasing hormone and brain mast cells regulate blood-brain-barrier permeability induced by acute stress. Journal of Pharmacology and Experimental Therapeutics 2002;303(3):1061–6. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, et al. Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B(4)-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27(4):1664–73. [DOI] [PubMed] [Google Scholar]
- Fjellner B, Hagermark O. Pruritus in Polycythemia-Vera - Treatment with Aspirin and Possibility of Platelet Involvement. Acta dermato-venereologica 1979;59(6):505–12. [DOI] [PubMed] [Google Scholar]
- Fjellner B, Lindelof B, Wahlgren CF, Lengstam I. Influence of grenz rays and psychological factors on experimental pruritus induced by histamine and compound 48/80. Archives of dermatological research 1989;281(2):111–5. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature immunology 2005;6(2):135–42. [DOI] [PubMed] [Google Scholar]
- Goldberg A, Korzets Z, Bernheim J, Mekori YA. Cutaneous responses to histamine, compound 48/80, and codeine in patients with chronic renal failure. Annals of allergy 1991;67(5):525–8. [PubMed] [Google Scholar]
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019;101(3):412–20 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunological reviews 2018;282(1):168–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagermark O, Strandberg K. Pruritogenic Activity of Prostaglandin-E2. Acta dermato-venereologica 1977;57(1):37–43. [PubMed] [Google Scholar]
- Hartmann K, Wagner N, Rabenhorst A, Pflanz L, Leja S, Forster A, et al. Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. The Journal of allergy and clinical immunology 2013;132(1):232–5. [DOI] [PubMed] [Google Scholar]
- Hasbak P, Eskesen K, Lind H, Holst J, Edvinsson L. The vasorelaxant effect of adrenomedullin, proadrenomedullin N-terminal 20 peptide and amylin in human skin. Basic & clinical pharmacology & toxicology 2006;99(2):162–7. [DOI] [PubMed] [Google Scholar]
- Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li G, Xiang J, Yin D, Chi R. Immunohistochemical study of serotonin in lesions of chronic eczema. International journal of dermatology 2004;43(10):723–6. [DOI] [PubMed] [Google Scholar]
- Jian T, Yang N, Yang Y, Zhu C, Yuan X, Yu G, et al. TRPV1 and PLC Participate in Histamine H4 Receptor-Induced Itch. Neural plasticity 2016;2016:1682972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PG, Argiles A, Mion C. Whole blood serotonin levels are markedly elevated in patients on dialytic therapy. American journal of nephrology 1992;12(1–2):14–8. [DOI] [PubMed] [Google Scholar]
- Kesterson K, Nahmias Z, Brestoff JR, Bodet ND, Kau A, Kim BS. Generalized pruritus relieved by NSAIDs in the setting of mast cell activation syndrome. Journal of Allergy and Clinical Immunology-in Practice 2018;6(6):2130–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata H. Enhancement of allergic skin wheal responses in patients with atopic eczema/dermatitis syndrome by playing video games or by a frequently ringing mobile phone. Eur J Clin Invest 2003;33(6):513–7. [DOI] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123(3):398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Brown JM, Wu YL, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. Journal of Allergy and Clinical Immunology 2007;119(2):498–9. [DOI] [PubMed] [Google Scholar]
- Lee MG, Dong XZ, Liu Q, Patel KN, Choi OH, Vonakis B, et al. Agonists of the mas-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. Journal of immunology 2008;180(4):2251–5. [DOI] [PubMed] [Google Scholar]
- Letourneau R, Pang X, Sant GR, Theoharides TC. Intragranular activation of bladder mast cells and their association with nerve processes in interstitial cystitis. Brit J Urol 1996;77(1):41–54. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhou Q, Liu C, Ying M, Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Scientific reports 2017;7(1):17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27(9):3549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009;139(7):1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg L, Liang Y, Sundstrom E, Nordlind K, Verhofstad A, Liden S, et al. Serotonin in human allergic contact dermatitis. An immunohistochemical and high-performance liquid chromatographic study. Archives of dermatological research 1999;291(5):269–74. [DOI] [PubMed] [Google Scholar]
- Luster AD, Tager AM. T-CELL trafficking in asthma: Lipid mediators grease the way. Nature Reviews Immunology 2004;4(9):711–24. [DOI] [PubMed] [Google Scholar]
- Mack MR, Kim BS. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends in immunology 2018;39(12):980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BM, Gruba SM, Meyer AF, Haynes CL. Neuropeptide-Induced Mast Cell Degranulation and Characterization of Signaling Modulation in Response to IgE Conditioning. ACS chemical biology 2016;11(11):3077–83. [DOI] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015;519(7542):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019;50(5):1163–71 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology 2008;213(3–4):251–60. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 2013;340(6135):968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Taketomi Y. Secreted phospholipase A2 and mast cells. Allergology international : official journal of the Japanese Society of Allergology 2015;64(1):4–10. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Goh Y, Azuma I, Hayaishi O. Effects of prostaglandin D2 and its analogue, BW245C, on intraocular pressure in humans. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 1991;229(5):411–3. [DOI] [PubMed] [Google Scholar]
- Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, et al. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. The Journal of investigative dermatology 2018;138(6):1311–7. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. Journal of immunology 2010;184(7):3526–34. [DOI] [PubMed] [Google Scholar]
- Nordlind K, Thorslund K, Lonne-Rahm S, Mohabbati S, Berki T, Morales M, et al. Expression of serotonergic receptors in psoriatic skin. Archives of dermatological research 2006;298(3):99–106. [DOI] [PubMed] [Google Scholar]
- Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017;171(1):217–28 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nature reviews Immunology 2014;14(5):289–301. [DOI] [PubMed] [Google Scholar]
- Paton WDM. Compound 48 80 - a Potent Histamine Liberator. Brit J Pharm Chemoth 1951;6(3):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Stewart JM, Conti P, Theoharides TC. Spectrum of mast cell activation disorders. Expert review of clinical immunology 2014;10(6):729–39. [DOI] [PubMed] [Google Scholar]
- Petra AI, Tsilioni I, Taracanova A, Katsarou-Katsari A, Theoharides TC. Interleukin 33 and interleukin 4 regulate interleukin 31 gene expression and secretion from human laboratory of allergic diseases 2 mast cells stimulated by substance P and/or immunoglobulin E. Allergy and asthma proceedings 2018;39(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raap U, Wieczorek D, Gehring M, Pauls I, Stander S, Kapp A, et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Experimental dermatology 2010;19(5):464–6. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol Rev 2006;86(4):1309–79. [DOI] [PubMed] [Google Scholar]
- Schworer H, Hartmann H, Ramadori G. Relief of cholestatic pruritus by a novel class of drugs: 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists: effectiveness of ondansetron. Pain 1995;61(1):33–7. [DOI] [PubMed] [Google Scholar]
- Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nature immunology 2019. [DOI] [PMC free article] [PubMed]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007;27(9):2331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh LK, Pang XZ, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav Immun 1999;13(3):225–39. [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Kriegbaum MC, Tseng PY, Earnest TW, Gu X, Barik A, et al. Nppb Neurons Are Sensors of Mast Cell-Induced Itch. Cell reports 2019;26(13):3561–73 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Tomioka M, Quinonez G, Simon GT, Felten SY, Bienenstock J. Intestinal Mucosal Mast-Cells in Normal and Nematode-Infected Rat Intestines Are in Intimate Contact with Peptidergic Nerves. Proceedings of the National Academy of Sciences of the United States of America 1987;84(9):2975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nature medicine 2000;6(2):151–8. [DOI] [PubMed] [Google Scholar]
- Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, et al. Localization of histamine H4 receptors in the central nervous system of human and rat. Brain research 2009;1250:41–8. [DOI] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. Journal of Allergy and Clinical Immunology 2016;138(3):700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007;448(7154):700–3. [DOI] [PubMed] [Google Scholar]
- Tal M, Liberman R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neuroscience letters 1997;221(2–3):129–32. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochemical and biophysical research communications 2006;349(4):1322–8. [DOI] [PubMed] [Google Scholar]
- Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Frontiers in immunology 2018;9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Takamori K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J Dermatol 2014;41(3):205–12. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Riccio MM, Weinreich D, Ellis JL, Myers AC. Neurophysiology of Mast Cell-Nerve Interactions in the Airways. International archives of allergy and immunology 1995;107(1–3):199–201. [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res 1997;46(10):412–6. [DOI] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155(2):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Nieves AL, Spada CS, Williams LS, Tuckett RP. Characterization of a Behavioral-Model for Peripherally Evoked Itch Suggests Platelet-Activating-Factor as a Potent Pruritogen. Journal of Pharmacology and Experimental Therapeutics 1995;272(2):758–65. [PubMed] [Google Scholar]
- Xu AZ, Tripathi SV, Kau AL, Schaffer A, Kim BS. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. Journal of the American Academy of Dermatology 2016;74(5):1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JH, Cui M, Li MT, Liu YN, He QH, Xiao JJ, et al. Angiopoietin1 inhibits mast cell activation and protects against anaphylaxis. PloS one 2014;9(2):e89148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler C, Tsianakas A, Pereira M, Stander H, Yosipovitch G, Stander S. Chronic Prurigo of Nodular Type: A Review. Acta dermato-venereologica 2018;98(2):173–9. [DOI] [PubMed] [Google Scholar]
- Zhang L, McNeil BD. Beta-defensins are proinflammatory pruritogens that activate Mrgprs. The Journal of allergy and clinical immunology 2019;143(5):1960–2 e5. [DOI] [PubMed] [Google Scholar]


