Abstract
Background.
Preoperative chemotherapy is important in the management of women with breast cancer, with the ability to downstage the breast primary tumor and axillary lymph nodes. Long-term studies are needed to identify late toxicities, recurrence patterns, and equivalency with postoperative chemotherapy for recurrence-free and overall survival.
Methods.
We conducted a single institution prospective randomized control trial comparing preoperative or post-operative FLAC/G-CSF chemotherapy for women with untreated clinical stage II (T1N1, T2N0 and T2N1) breast cancer. Long-term follow-up was conducted to define toxicities, recurrence patterns and recurrence-free and overall survival.
Results.
Fifty-three women with clinical stage II breast cancer were randomized, twenty-six patients to receive preoperative chemotherapy and twenty-seven to receive postoperative chemotherapy. Long-term follow-up, with a median of 25.3 years, was obtained. Local or systemic recurrence occurred in 8 women in the preoperative group and in ten women in the postoperative group, and recurrences were predominantly within 10 years of treatment. Late toxicities included local upper extremity paresthesia’s, upper extremity edema and congestive heart failure in one patient each. Analysis revealed no difference in recurrence-free survival (RFS; 20-year RFS probabilities, preoperative: 61.3%, postoperative: 54.7%, p=0.42), or in overall survival (OS) between the two treatment groups (20-year probabilities, preoperative: 64.6%, postoperative: 62.2%, p=0.44). Twenty five of 53 patients (47%) were alive and without disease at this follow-up.
Conclusions.
Twenty-five year follow up for this prospective randomized trial confirms the equivalency of preoperative vs. postoperative chemotherapy with FLAC/G-CSF for Stage II breast cancer for both RFS and OS.
Keywords: preoperative chemotherapy, neoadjuvant chemotherapy, Stage II breast cancer, breast cancer follow-up, long-term follow-up
Introduction
Breast cancer is the most common malignancy in women, with over 316,000 cases reported in 2017 in the United States alone. Over 60% of these breast tumors were invasive carcinoma, and many presented as TNM stage II or stage III breast cancer.1 Adjuvant chemotherapy has been an important component of the management of these women for many years; historically, chemotherapy was administered postoperatively as an adjuvant to local therapy in an effort to improve survival. Subsequently, as management approaches evolved, chemotherapy was administered preoperatively to women with locally advanced breast cancer in an effort to improve local control. From these studies it became clear that many breast cancers were responsive to preoperative chemotherapy, with reduction of tumor in the breast and regional lymph nodes, and increased potential for breast conservation. To further define this approach and to determine whether survival was altered by preoperative chemotherapy, prospective randomized trials were conducted to compare preoperative vs. postoperative chemotherapy in the management of women with regional breast cancer. At least 10 such trials with evaluable data were conducted and have been reported; these have recently been evaluated in a meta-analysis by the European Breast Cancer Trialists Collaborative Group (EBCTCG).2 This meta-analysis demonstrated that preoperative chemotherapy for local/regional breast cancer was associated with a high incidence of complete clinical response of the primary tumor, an increased frequency of breast-conserving therapy, more frequent local recurrence than for postoperative chemotherapy, and no significant difference in distant recurrence or overall survival between the two arms. The median follow-up for those 10 studies was 9 years, with only 1 trial reporting median follow-up beyond 10 years (10.3 years).3
In 1990 the National Cancer Institute (NCI) initiated at the Clinical Center, NIH, a prospective randomized control trial evaluating FLAC/G-CSF chemotherapy given preoperatively (preoperative) vs postoperatively (postoperative) for women with untreated clinical stage II breast cancer. In 2003 we reported the early results for this trial:4 with a median follow-up of 9 years, we found preoperative chemotherapy induced a 76-80% reduction in the size of the primary breast cancer and a corresponding 20% pathological complete response rate (pCR), along with reduction in the overall incidence and number of axillary metastasis, suggesting a benefit of preoperative chemotherapy administration as it relates to local control, downsizing of the primary tumor, and measurement of response4. There was no difference in recurrence-free (RFS) or overall survival (OS) between the preoperative vs postoperative chemotherapy groups.
The NCI trial was subsequently included in the metanalysis of 10 trials by the EBCTCG.2 The EBCTCG meta-analysis, while thorough and comprehensive but with median follow-up of 9 years, did not include any data for late recurrence events, long-term toxicity from therapy, long-term contralateral breast cancers, or long-term comparison of RFS or OS. This latter information is important for the evaluation of efficacy and toxicity of preoperative chemotherapy, for patient management and counseling, and for the design of new trials. To address these issues, we have conducted long-term follow-up for the NCI prospective randomized control trial. We now report our findings with a median follow-up of 25.3 years for this trial.
Patients and Methods
Trial Design, Eligibility and Enrollment Criteria
The prospective randomized control trial (NCI 90-C-0044) was conducted between 1990 and 1998 according to approval by the NCI Institutional Review Board. All patients gave written, informed consent. The trial was designed to enroll 65 patients per arm for 130 patients but closed in 1998 due to slow enrollment. Follow-up continued through 2018. Fifty-three women with untreated clinical stage II breast cancer (T1N1, T2N0 or T2N1 based on the 1989 American Joint Committee on Cancer classification) were enrolled, with twenty-six patients randomized to receive preoperative chemotherapy and twenty-seven randomized to the postoperative chemotherapy group. Patients were required to have histologically confirmed invasive epithelial origin breast cancer. Estrogen receptor (ER) and progesterone receptor (PR) status of the primary tumor was determined and reported as either respective receptor positive, receptor negative, or unknown. Pregnant women and women with a previous history of malignant neoplasms, except for basal cell carcinoma or excised carcinoma in situ of the cervix, were excluded unless they had received curative surgery and had no evidence of recurrence for >10 years. Additional inclusion criteria included a leukocyte count >4000/mm³, platelet count >100,000/mm³, liver chemistries (AST, ALT, alkaline phosphatase and total bilirubin) <1.5 times the upper limit of normal, creatinine <1.7 mL/min and/or creatinine clearance >45mL/min and no evidence of metastasis. Table 1 summarizes the demographic information for those enrolled and treated. Each patient was evaluated by a surgeon, medical oncologist and radiation oncologist at which time they underwent a complete history and physical examination including laboratory studies to confirm inclusion criteria.
Table 1.
Demographic characteristics of subjects
| Characteristic | Preoperative Group | Postoperative Group |
|---|---|---|
| No. of subjects | 26 | 27 |
| Age (years) | ||
| Median |
49 |
43 |
| Range | 32-68 | 28-66 |
| Clinical Stage | ||
| T1N1 | 2 | 0 |
| T2N0 | 16 | 22 |
| T2N1 | 18 | 5 |
| Histology | ||
| Infiltrating ductal carcinoma | 21 | 25 |
| Infiltrating lobular carcinoma | 4 | 2 |
| Poorly differentiated | 1 | 0 |
| Clinical tumor number | ||
| Unifocal | 23 | 25 |
| Multifocal | 2 | 2 |
| Bilateral | 1 | 0 |
| Clinical tumor size (cm) | ||
| 1.5-2.0 | 2 | 0 |
| 2.1-3.0 | 15 | 12 |
| 3.1-4.0 | 6 | 11 |
| 4.1-5.0 | 3 | 4 |
| Estrogen receptor | ||
| Positive | 16 | 16 |
| Negative | 9 | 11 |
| Unknown | 1 | 0 |
| Progesterone receptor | ||
| Positive | 16 | 18 |
| Negative | 9 | 9 |
| Unknown | 1 | 0 |
Treatment, Follow Up and Statistical Analysis
The chemotherapy regimen consisted of a 21-day cycle of FLAC drug regimen; 5-fluorouracil , 400 mg/m² intravenous (IV) bolus days 1, 2 and 3, leucovorin, 500 mg/m² IV bolus on days 1, 2 and 3, doxorubicin, 15mg/m² IV bolus days 1, 2 and 3, and cyclophosphamide, 600 mg/m² IV bolus day 1. Patients in the preoperative arm receiving chemotherapy underwent local therapy after two cycles if progressive disease was observed. Patients with no assessable disease at the start of therapy or during therapy, or those who achieved a complete response (CR) after a few cycles of chemotherapy received a total of 5 cycles. For patients who were randomized to receive postoperative chemotherapy, the chemotherapy was initiated 2-3 weeks following surgery. Women with estrogen receptor (ER) positive or progesterone receptor positive tumors received tamoxifen (10 mg twice daily) for 5 years, starting upon completion of local therapy and chemotherapy.
Local therapy included modified radical mastectomy or breast segmentectomy, complete axillary lymph node dissection, and whole-breast radiotherapy. Patients were given the option of mastectomy or breast conservation. Patients who were randomized to preoperative chemotherapy underwent local therapy 4-5 weeks following completion of 5 cycles of chemotherapy. Radiation therapy was administered after recovery from surgical therapy. Radiation therapy consisted of a minimum dose of 50.4 Gy in 1.8 Gy per fraction to the breast. Patients with a prechemotherapy clinically negative axilla or pathologically negative axilla after chemotherapy received only breast irradiation. Those with a prechemotherapy clinically positive axilla or pathologically positive axillary nodes received treatment to the supraclavicular nodes with their breast irradiation (the details of radiotherapy were described previously).4
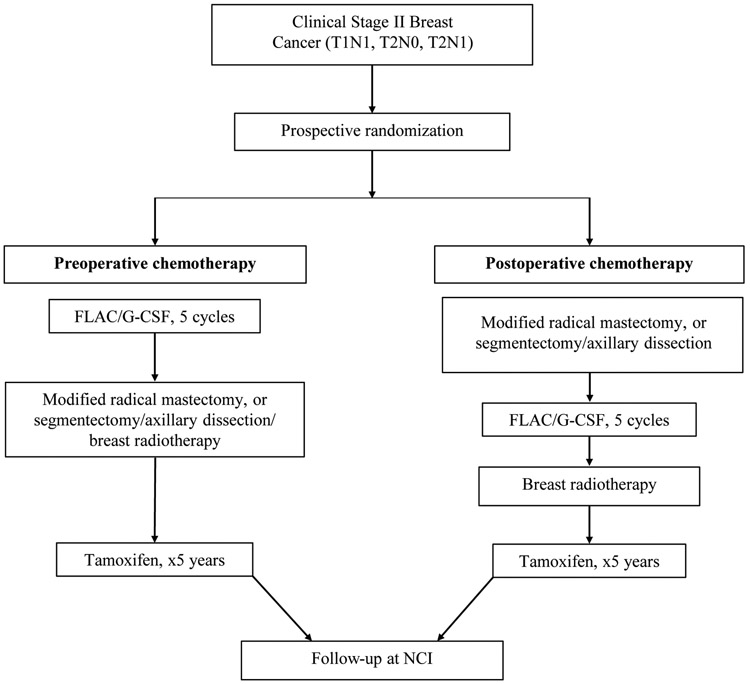
On completion of local therapy and chemotherapy, patients were followed with a history and physical examination every three months for the first three years, every six months for years 4 and 5, and yearly thereafter. Women receiving tamoxifen underwent yearly uterine ultrasound. For the present report, women were subsequently followed either by yearly physical exam performed at the NCI, direct contact, or evaluation of obituaries or available medical records. The questionnaire used to assess the status of patients included demographic information and assessment of overall health, issues with local or systemic therapy, routine surveillance, adverse events (AE) including congestive heart failure, paresthesias or lymphedema, and development of other malignancies. Local regional recurrence included in-breast, chest wall, surgical scar, supraclavicular, infraclavicular or internal mammary nodes, or recurrence in the ipsilateral axilla. Systemic recurrence include bone, liver, pulmonary or brain lesions. Figure 1 outlines the schema for the prospective, randomized trial and follow-up.
Fig 1.
Schema summarizing prospective randomized trial evaluating preoperative chemotherapy for stage II breast cancer using FLAC/G-CSF with subsequent follow-up. FLAC: fluorouracil, leucovorin calcium, doxorubicin, and cyclophosphamides; G-CSF, granulocyte-colony-stimulating factor.
The Kaplan-Meier method was used to determine the probability of RFS or OS as a function of time. For RFS, any recurrences or deaths without prior recurrence were considered events. RFS was calculated from the date of randomization until the date of recurrence or death without recurrence. Patients were censored if alive and without recurrence at the date they were last confirmed as being in that status. OS was calculated from the date of randomization until the date of death or last follow-up. The significance of the difference in RFS or OS curves was determined by a log-rank test. P-values are two-tailed.
Results
Fifty-three women met inclusion criteria and were enrolled in the clinical trial, with twenty-six randomized to receive preoperative chemotherapy followed by local therapy and twenty-seven randomized to receive local therapy first followed by postoperative systemic chemotherapy. The demographic characteristics of all patients in the two groups are summarized in Table 1. The median follow-up for the present report is 25.3 years (range, 11 months to 28 years). Follow-up was complete to death or current evaluation in 50 patients; six patients did not have a current evaluation, but were no evidence of disease (NED) at last evaluation at 7, 7, 12, 18, 21 and 21 years, respectively. Only one subject did not receive the allocated treatment: she received postoperative doxorubicin/cytoxan at an outside institution rather than postoperative FLAC/G-CSF at NCI.
Early and long-term toxicities were evaluated. We previously reported that, with a median follow-up of 9 years, there were no significant differences for the type and grade of toxicities between the two treatment groups, with the most prominent toxicity being Grade 4 neutropenia, without any long-term sequelae.4 The most prominent postoperative complication was seroma, which was comparable for the two groups. In the present report, with a median follow-up of 25.3 years, for the 25 women who were alive and without disease, three reported adverse events related to therapy including burning sensation in the medial side of the arm (paresthesias, n=1), congestive heart failure (n=1) likely due to systemic doxorubicin based chemotherapy,5 and upper extremity lymphedema (n=1), a well-known and reported sequela of axillary lymph node dissection6 (Table 2).
Table 2:
Treatment-related adverse events in women alive without disease (n=25)
| Adverse event | Number of patients | Treatment group |
|---|---|---|
| Paresthesias* | 1 | Preoperative |
| Congestive heart failure | 1 | Postoperative |
| Upper extremity lymphedema | 1 | Postoperative |
Paresthesias described as burning sensation in the intercostobrachial nerve distribution of upper arm.
Analysis of all patients revealed no difference in RFS between the preoperative and postoperative groups, with 10- and 20-year probabilities: 69.2% and 61.3 % for preoperative and 66.5% and 54.7% for postoperative, respectively (p=0.42; Figure 2A). Similarly, there was no difference in OS between the two treatment groups: 10- and 20-year probabilities, 84.6% and 64.6% for preoperative, and 77.8% and 62.2% for postoperative respectively (p=0.44; Figure 2B).
Fig 2.
Overall and Recurrence free survival during 25 Years of Follow-up after Surgery among Women who Received Preoperative Chemotherapy (dashed line) and those who received Postoperative Chemotherapy (straight line). a) Recurrence free survival P=0.42, preoperative: median RFS not reached 10-year RFS: 69.2% (95% CI: 47.8-83.3%), 20 year RFS: 61.3% (95% CI: 40.0-77.0%). postoperative: median RFS=20.5 years (95% CI: 6.5 years – not estimable) 10 year RFS: 66.5% (95% CI: 45.4-80.9%) 20 year RFS: 54.7% (95% CI: 34.2-71.3%). b) Overall Survival P=0.44, Preoperative Group: Median OS: not reached (10 year OS: 84.6% (95% CI: 64.0-93.9%), 20 year OS: 64.6% (95% CI: 42.9%-79.8%)), Postoperative Group: Median OS: 25.7 years (95% CI: 11.7 years – not estimable) 10 year OS: 77.8% (95% CI: 57.1-89.3%), 20 year OS: 62.2% (95% CI: 41.1-77.6%)
At the present follow-up, thirteen patients in the preoperative group and twelve in the postoperative group were alive and had no evidence of disease (NED). A total of eight patients in the preoperative group experienced recurrences, three of which were local/regional (in-breast [n=1], axillary lymph node [n=1], supraclavicular lymph node [n=1] and distant metastases [n=4] (Table 3). All local and systemic recurrences in the preoperative group occurred within 10 years from treatment. One woman who experienced recurrence, died from metastatic disease 20 years from treatment, but we were not able to identify the site of recurrence following medical and obituary review. Among the seven patients for whom recurrence sites were known, all had hormone receptor positive primary tumors and received tamoxifen; 6/7 had high grade (grade III) tumors. One woman in the preoperative group experienced primary contralateral breast cancer 17 years following initial treatment and is alive without disease to date.
Table 3:
Long-term follow up for patients receiving preoperative or postoperative chemotherapy.
| Characteristic | Preoperative Group | Postoperative Group |
|---|---|---|
| Number of patients | 26 | 27 |
| Clinical status | ||
| Alive without disease | 13 | 12 |
| Dead from disease | 6 | 7 |
| Dead, cause unknown | 2 | 2 |
| Dead, natural causes* | 0 | 2 |
| Dead, other causes** | 2 | 2 |
| NED at last available follow-up | 3 | 2 |
| Total recurrence | 8 | 10 |
| Recurrence, local/regional | 3 | 2 |
| Recurrence, systemic | 4 | 8 |
| Recurrence, site unknown | 1 | 0 |
| Site of recurrence | ||
| In-breast | 1 | 0 |
| Axillary lymph node | 1 | 0 |
| Supraclavicular lymph node | 1 | 0 |
| Chest wall | 0 | 2 |
| Bone alone | 1 | 5 |
| Bone/lung | 0 | 1 |
| Bone/liver | 0 | 1 |
| Lung | 3 | 0 |
| Brain | 0 | 1 |
| Site unknown | 1 | 0 |
| Contralateral breast primary | 1 | 4 |
Death from natural causes includes car accident.
Other cancer related deaths in the preoperative group included ovarian cancer (n=2). Other cancer related deaths in the postoperative group included colon (n=1) and pancreatic cancer (n=1).
A total of ten patients in the postoperative group experienced recurrences, two of which were local/regional and eight involved distant metastases (Table 3). Two women developed chest wall recurrences at 2 and 10 years, respectively, from randomization. Eight women experienced a systemic recurrence, seven of which occurred within the first 5 years following treatment and one involving bone and lung occurred seven years from initial treatment. All local/regional and distant recurrences in the postoperative group occurred within 10 years. Four women in the postoperative group developed contralateral primary breast cancer in the 7-23 year range from initial treatment (Table 3). Of these women, one developed a second contralateral breast recurrence seven years later. This woman died free of breast cancer 16 years following initial treatment of her primary tumor. The remaining three women were alive without disease. Among the 10 patients in the postoperative group who recurred, eight had hormone receptor positive tumors and received tamoxifen, and 7/10 had high grade (grade III) tumors.
In the preoperative group, six women had breast cancer progression that eventually lead to their death; three deaths occurred within 10 years at 1, 7 and 8 years, and three deaths occurred after the original follow-up period at 16, 17 and 20 years, respectively. Two women died from unknown causes 20 and 22 years from initial treatment, and two died from ovarian cancer at 2 and 17 years, respectively, from randomization. Three patients in the preoperative group who were censored as NED had follow-up information available through 2004, 2012 and 2014, respectively, but we were not able to contact or locate documents suggestive of death in 2018. As a result, their last follow up information was used for data analysis. Among 11 patients with an initial cCR, 9 (81.8%) remained NED, 1 died of breast cancer and 1 died of ovarian cancer. Among 6 women with either a partial response or no change, 2 had no change in status, 3 died of breast cancer and 1 died of ovarian cancer.
Of the twenty-seven women who received postoperative chemotherapy, twelve were alive and without evidence of disease. Seven patients died from breast cancer, six of whom died within 7 years and were described in the original report,4 and one who died 11 years from date of randomization. The cause of death was not determined in two patients who died at 14 and 21 years from date of randomization, both of whom were NED at their last follow up of 7 and 9 years, respectively. Two women died from other cancers, one of pancreatic cancer at 12 years and one of colon cancer at 21 years from date of randomization. We were not able to contact or find evidence of recurrence or death for 2 women in the postoperative group. As a result, their last clinic follow-up date information was used for analysis. These women had no clinical evidence of disease at their last follow up 7 and 18 years from initial randomization.
Discussion
In this study we conducted a single institution prospective randomized trial evaluating an intensive chemotherapy regimen, FLAC/G-CSF, given preoperatively or postoperatively for clinical stage II breast cancer. We now report the outcome results with a median potential follow-up of 25.3 years. This is the longest follow-up reported for any randomized trial evaluating preoperative chemotherapy for breast cancer. Fifty-three patients were enrolled, 26 in the preoperative arm and 27 in the postoperative arm. Only one patient did not receive the allocated treatment. The present follow-up includes local/regional and distant recurrences, death from breast cancer or other malignancies or causes, in-breast and contralateral recurrences, and long-term toxicity. Detailed demographic information is available for each subject at trial entry to further clarify the significance of long-term events. This follow-up expands considerably on our previous analysis with a median follow-up of 9 years,4 and also expands on the follow-up for the ten randomized trials analyzed in the EBCTCG meta-analysis, where the median follow-up was 9 years and only one trial reported a median follow-up >10 years (10.3 years3).
We found no significant differences in RFS or OS between the preoperative and postoperative groups. All local/regional recurrences in both groups occurred within 10 years, which is consistent with that reported for the ten trials included in the meta-analysis of the EBCTCG (and which included the present trial).2 Overall, among the 18 women that recurred in both groups in the present trial, 15 (83.3%) were hormone receptor positive and had been treated with tamoxifen; as noted above there were no late (>10 years) recurrences among these women, such as what has been reported in studies with long-term follow-up for HR positive breast cancer treated with tamoxifen.7 Our finding of equivalent RFS between preoperative and postoperative groups differed from the EBCTCG meta-analysis which noted a higher local recurrence rate at 15 years for the neoadjuvant chemotherapy group (15 .5% vs 5.9% for postoperative chemotherapy, p=0.0001).2 These differences were found to be greatest in the two trials in which many women did not receive breast surgery after neoadjuvant chemotherapy (IB Bordeaux3 and Institut Curie S68), but the rate ratios did not differ significantly between the three classes of chemotherapy (with anthracycline but no taxane, with anthracycline and taxane, without anthracycline or taxane) used in these trials, or between the use or not of tamoxifen. The reason for the equivalent outcomes between the two arms for recurrences in our study (which is also in agreement with the findings for the NSABP-B18 trial9) may relate to the fact that all patients in the preoperative arm of our study (and all but 3 patients in B-18) had breast surgery after chemotherapy. Both studies used anthracycline without taxane chemotherapy, and postmenopausal women received tamoxifen. Our findings were confirmed out to 25 years.
The drug combination used in our trial, FLAC/G-CSF, and the drug combinations used in the nine other trials in the EBCTCG meta-analysis, are not commonly used today. However, it should be recognized that several of the individual drugs, including cyclophosphamide, doxorubicin, epirubicin, and 5-fluorouracil, are still being utilized and are very active against breast cancer. Importantly, these trials and the drug combinations which were used not only confirmed the responsiveness of the primary tumors to chemotherapy, but also the ability of multiple regimens to improve breast conservation options and downstage axillary lymph node metastases without a detriment in long-term RFS or OS. These contributions alone have had a substantial impact on the management of women with breast cancer. Current approaches would include the use of agents for Her-2/neu positive tumors, carboplatin, and the taxanes. Many women will continue to present with more advanced stage II or stage III breast cancer and will be candidates for neoadjuvant therapy; the NCCN guidelines recommend neoadjuvant treatment for stage II and stage III tumors, precisely the same group which were evaluated in the trials included in the meta-analysis. Although the effects on survival will continue to be evaluated, one would anticipate high locoregional response rates with these current regimens. Another important advance since many of these trials were initiated has been the application of sentinel lymph node biopsy (SLNB). We showed, in the initial analysis of our trial, that neoadjuvant chemotherapy reduced the incidence of axillary metastases from 59.3% in the postoperative arm to 34.6 % in the preoperative arm.4 This downstaging of axillary metastases has important implications for management of these patients. SLNB is now recommended after neoadjuvant chemotherapy in patients with a clinically negative axilla; because of this downstaging, which was learned from these trials, a negative SLNB will spare many patients the need for an axillary lymph node dissection.
In summary, this single institution prospective randomized trial provides a comprehensive comparison of the treatment of women with stage II breast cancer with preoperative vs. postoperative chemotherapy, indicating both early results and long-term follow-up. This trial adds to our understanding of outcome for neoadjuvant chemotherapy by showing, compared to adjuvant chemotherapy, the equivalency of RFS and OS out to 25 years. We showed that preoperative chemotherapy is associated with a high response rate in the breast primary and axillary lymph nodes without an increase in postoperative or long-term complications. Most local-regional and distant recurrences from either preoperative or postoperative chemotherapy occurred within the first 10 years, which provides an important and rather early measure for assessing the efficacy of newer regimens. Neoadjuvant chemotherapy, originally designed for locally-advanced breast cancer, now has widespread application to include many women with early stage breast cancer. With its value for assessing response of newer drug regimens, and the ability of oncoplastic surgery to obtain good cosmetic results with tumors achieving even a partial response, the application of preoperative chemotherapy may expand even further.
Acknowledgments
Research Funding Acknowledgement: This research was supported by the Intramural Research Program, National Cancer Institute, Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH), Bethesda, MD.
Footnotes
Disclosure: The authors have no disclosures.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures. 2017-2018:1–41. [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018; 19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauriac L, MacGrogan G, Avril A, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann. Oncol 1999; 10(1):47–52. [DOI] [PubMed] [Google Scholar]
- 4.Danforth DN Jr., Cowan K, Altemus R, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Ann. Surg. Oncol 2003; 10(6):635–644. [DOI] [PubMed] [Google Scholar]
- 5.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003; 97(11):2869–79. [DOI] [PubMed] [Google Scholar]
- 6.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 2007; 25(24):3657–63. [DOI] [PubMed] [Google Scholar]
- 7.Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017; 377(19):1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholl SM, Asselain B, Palangie T, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991; 27(12):1668–71. [DOI] [PubMed] [Google Scholar]
- 9.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. J. Natl. Cancer Inst. Monogr 2001(30):96–102. [DOI] [PubMed] [Google Scholar]