Abstract
In the last decade, several new therapies with different mechanisms of action have been approved for the management of moderate to severe Crohn’s disease (CD). However, there is limited guidance on optimal positioning of agents as first-or second-line therapies due to the absence of head-to-head trials. Furthermore, given the lack of comparative studies, treatment guidelines provide limited insight. In this review, we will discuss data on key treatment attributes, comparative efficacy and safety, factors predictive of response to each agent and propose an algorithm for positioning therapies for the management of patients with low-risk and high-risk CD.
The global incidence and prevalence of Crohn’s disease (CD) is rising, with steep increase in incidence in newly industrialized countries.1 It is associated with significant morbidity and potentially increased mortality, with high burden of hospitalizations and surgery, decreased work productivity and disability.2, 3 While clinically CD is characterized by relapsing and remitting course, subclinical inflammation often persists during periods of apparent clinical remission and leads to progressive bowel damage manifest as complications of stricture, fistula, abscess. In population-based cohorts of CD, majority of patients (56–81%) have luminal inflammatory disease at diagnosis, though 5–25% may present with stricturing or penetrating complications.4 However, over long periods of observation, only 20–30% of patients with CD will have a nonprogressive or indolent course; over 50% patients develop an intestinal complication by 20 years of diagnosis. The cumulative risk of surgery at 1, 5, and 10 years after diagnosis of CD is 16%, 33% and 47%, respectively; approximately 25% patients require repeat surgery within 5 years of first surgery.5, 6 Hence, the majority of patients will require active effort to identify therapies that achieve adequate control of bowel inflammation. Historically, patients were treated with corticosteroids, 5-aminosalicylates, and non-targeted immunosuppressive agents (such as thiopurines or methotrexate), with the goal of reducing disease-related symptoms. Over the last decade, with better understanding of natural history and risk stratification, as well as development of targeted immunosuppressive agents, treatment approach has evolved towards early introduction of highly effective therapy, to achieve clinical and endoscopic remission, with the intention of modifying the natural history of the disease.
In this review, we will discuss overall and comparative efficacy and safety of different agents in patients with low-and high-risk CD, based on clinical trials, indirect treatment comparisons, high quality observational studies and prospective registries. We will review potential predictors of response to specific agents that may facilitate positioning of therapies. Finally, based on our interpretation of evidence and clinical experience, we will propose a treatment algorithm for positioning these interventions in the management of mildly active CD at low risk of progression and moderate-to-severely active CD at high risk for progression. Trials of unapproved novel agents in development and biologic agents not frequently used in clinical practice (such as natalizumab) will not be included in this review.
CD Patients at High-risk vs. Low-risk for Disease Progression
Conventionally, clinical trials have focused on (cross-sectional) disease activity assessment, leading to regulatory approval and real-world use of immunosuppressive and/or biologic therapies based on symptomatic disease activity. However, over the last decade, there is increasing recognition that (longitudinal) disease severity assessment, which accounts for cumulative disease-related bowel damage and impact of disease on lifestyle is vital, to risk-stratify patients and ensure timely initiation of risk-congruent disease-modifying therapy.7
The International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) proposed an overall index of disease severity in patients with CD using a modified Delphi panel.8 In this, high-risk patients (high disease severity) were identified as those at increased risk of adverse disease-related complications including surgery, hospitalization and disability. Most important factors suggestive of high disease severity (in order of relative weights) based on structural damage, inflammatory burden and impact of quality of life are: large or deep mucosal lesions on endoscopy or imaging, presence of fistula and/or perianal abscess, intestinal resections, particularly of small intestinal segments >40cm, presence of stoma, extensive disease (ileal involvement >40cm, or pancolitis), at least 10 loose stools/week, presence of strictures, elevated C-reactive protein, lack of symptomatic improvement to prior treatment with biologics and/or immunosuppressive agents, significant impact of disease on activities of daily living, low albumin, presence of anorectal symptoms (anorectal pain, bowel urgency, incontinence, discharge, tenesmus), anemia, daily abdominal pain and corticosteroid use within the last 1 year (Table 1). In contrast, low-risk patients have limited anatomic involvement, with only superficial luminal ulceration, without prior surgery or exposure to corticosteroids and/or immunosuppressive therapy, and often have mild symptoms and low inflammatory burden. As is apparent, risk stratifying patients with CD warrants a thorough endoscopic and/or radiologic examination to ascertain burden, behavior and extent of disease.
Table 1.
Factors contributing to overall disease severity in patients with IBD, use to identify patients at high risk of disease-related complications including surgery, hospitalization and disability.
| High-risk Crohn’s disease | Low-risk Crohn’s disease | |
|---|---|---|
| Structural Damage | • Large or deep mucosal lesions • Fistula and/or perianal abscess • Prior intestinal resections, particularly of segments >40cm • Presence of strictures |
• Aphthous or small superficial ulcers • Absence of fistulae, abscess or strictures • No prior intestinal surgeries |
| Inflammatory burden | • Extensive disease (ileal involvement >40cm or pancolitis) • Elevated C-reactive protein • Low albumin |
• Limited anatomic involvement • Normal C-reactive protein • Normal albumin |
| Impact on quality of life | • Presence of stoma • >10 loose stools/week • Lack of symptomatic improvement with prior exposure to biologics and/or immunosuppressive agents • Significant impact of disease on activities of daily living • Presence of anorectal symptoms (anorectal pain, bowel urgency, incontinence, discharge, tenesmus) • Anemia • Daily abdominal pain • Corticosteroid use within last 1 year |
• Modest impact of disease on daily activities • No prior exposure to biologics and/or immunosuppressive agents • No prior disease-related hospitalization within the last 1 year • Absent to mildly active symptoms |
| Emerging predictors | • High number and titer of anti-microbial antibodies • Antimicrobial genetic peptide signature |
- |
Management of low-risk patients with CD
Low-risk patients with CD may be asymptomatic or mildly symptomatic. In clinical trials, mildly active CD has been defined as patients with Crohn’s disease activity index (CDAI) score between 150–220, which largely corresponds to ambulatory patients, tolerating oral intake, stable weight and lack systemic symptoms and tenderness on abdominal exam. In these patients, therapies that have been best studied include 5-aminosalicylates and sulfasalazine, and budesonide. In a network meta-analysis of 25 trials comparing 5-ASA-based therapies and budesonide in patients with mild to moderately active CD, Moja and colleagues observed that only budesonide at doses of ≥9mg/d was superior to placebo in inducing symptomatic remission.9 Mesalamine, even at high doses was not effective in inducing symptomatic remission in patients with mild to moderately active CD. Similarly, among patients with quiescent CD, only budesonide 6mg/d was effective in decreasing the risk of symptomatic relapse; mesalamine was not effective in decreasing risk of symptomatic relapse. There is very limited data on the efficacy of budesonide or mesalamine for inducing or maintaining endoscopic response or remission in patients with CD. Older studies have suggested that sulfasalazine 3–6g/d may be effective in inducing symptomatic remission in patients with colon-dominant CD, though it’s efficacy in inducing endoscopic response is debatable. Based on these analyses, clinical guidelines from the American College of Gastroenterology and the European Crohn’s and Colitis Organization advise against the use of mesalamine in patients with active CD; guidelines suggest considering sulfasalazine for management of mild to moderately active colon-dominant CD.10 Despite these clear recommendations, mesalamine continues to be one of the most commonly used medications in patients with CD, being used in >50% patients with CD, highlighting an opportunity to alter low-value care in CD.11 Antibiotic therapy has also been studied in patients with mild to moderately active luminal CD in the absence of infection. Studies using metronidazole, ciprofloxacin and anti-mycobacterial therapy have failed to consistently demonstrate any benefit of these agents in inducing clinical and/or endoscopic remission in patients with CD.10 Exclusive enteral nutrition has been demonstrated to be as efficacious as corticosteroids for inducing symptomatic remission in pediatric patients, but not in adult patients with low-risk CD; however, in adults with CD.12 However, palatability and long-term compliance with exclusive enteral nutrition is poor limiting use for maintenance of remission. More recently, an oral CD-exclusion diet with partial enteral nutrition for 6 weeks was more effective than exclusive enteral nutrition in inducing sustained symptomatic remission in pediatric patients with mild to moderately active CD.13
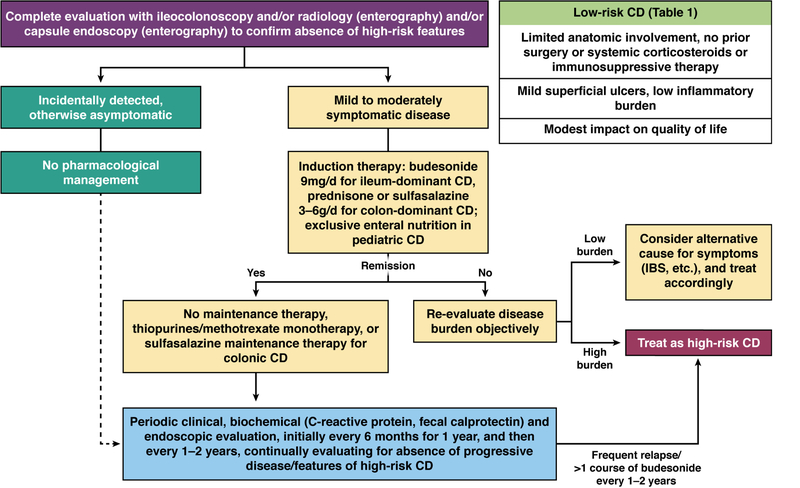
Our evidence-derived approach to the management of asymptomatic or mildly symptomatic patients with low-risk CD is summarized in Figure 1. In symptomatic patients with ileal and/or right colonic disease, we suggest induction therapy with budesonide 9mg/d for 8 weeks (or prednisone for patients with left colonic disease). In patients who achieve clinical remission, we do not use mesalamine or prednisone for maintenance, and maintenance therapy with budesonide 6 mg is limited; we recommend closely monitoring these patients clinically, biochemically (using C-reactive protein and fecal calprotectin) and endoscopically, off therapy, in 3–6 months. For patients with infrequent clinical relapse and non-progressive disease who continue to meet criteria for low-risk CD, we use budesonide episodically. For patients with frequent clinical relapse who require a course of budesonide every 1–2 years or more often, we consider these patients to be at high-risk for CD-related complications, and treat accordingly with biologic and/or antimetabolite therapy.
Figure 1.
Proposed algorithm for positioning therapies for patients with low-risk Crohn’s disease
Management of high-risk patients with CD
High-risk patients with CD are often symptomatic. In clinical trials, moderate-severely active CD has been defined as patients with CDAI score between 220–450, including patients with frequent diarrhea and/or abdominal pain, weight loss, extra-intestinal manifestations, as well as anorectal symptoms. Management of high-risk patients with requires choosing optimal immunosuppressive therapy for short-and long-term use to achieve remission and minimize risk of surgery, hospitalization and disease-related complications.
Comparative Efficacy of Pharmacological Therapies
There is paucity of head-to-head trials to inform comparative efficacy of different agents in patients with moderate to severely active CD. While such trials are ongoing, alternative approaches, such as indirect treatment comparison network meta-analysis and observational comparative effectiveness studies based on electronic health records, health care claims databases, etc., have been used to inform comparative effectiveness of different therapies.
In a recent network meta-analysis, Singh and colleagues separately analyzed the comparative efficacy of first-(biologic-naïve) and second-line biologic agents (patients with prior exposure to TNFα antagonists) for induction of remission, and for all agents for maintenance of remission.14 Based on eight RCTs in biologic-naïve patients with moderate to severely active luminal CD, using frequentist network meta-analysis and GRADE methodology for rating confidence in estimates, the investigators interpreted that infliximab and adalimumab were probably most effective, followed by ustekinumab and vedolizumab, for inducing clinical remission in biologic-naïve patients with moderate-severe CD (Table 2). Overall, there was moderate confidence in estimates supporting infliximab (OR, 4.33; 95% CI, 1.83–10.27), adalimumab (OR, 2.97; 95% CI, 1.16–6.70) and ustekinumab (OR, 2.02; 95% CI, 1.09–3.75) over certolizumab pegol. Similar results were observed for inducing clinical response. Based on subgroup data from six RCTs including 1606 patients with moderate to severely active CD with prior exposure to TNFα antagonists, the authors suggested that adalimumab and ustekinumab were ranked higher than vedolizumab for inducing clinical remission in patients with moderate to severely active luminal CD with prior exposure to TNFα antagonists. It is important to note, however, that while ustekinumab and vedolizumab have been studied in all patients with prior exposure to TNFα antagonists, adalimumab has primarily been studied in a subset of patients with prior response to, or intolerance to, infliximab; patients with primary non-response to infliximab were excluded.15 Moreover, this indirect comparison is unable to factor in the role of therapeutic drug monitoring and treatment optimization with different therapies as is usual clinical practice now. For example, patients with mechanistic failure of infliximab (persistent disease activity, despite adequate trough concentration) would benefit from switching out of class, whereas patients with pharmacokinetic failure, may benefit from optimization of index therapy (low infliximab trough concentration with no anti-drug antibodies) or switching within therapeutic class (undetectable infliximab trough concentration with high titer anti-drug antibodies).16, 17 Similarly, using network meta-analysis to compare different agents for maintenance of remission in a subset of patients with clinical response to induction therapy, adalimumab and infliximab were ranked highest. On indirect comparisons, moderate quality evidence supported adalimumab over certolizumab pegol (OR, 1.97; 95% CI, 1.04–3.73) and ustekinumab (OR, 2.19; 95% CI, 1.15–4.16) for maintenance of remission. When interpreting network meta-analysis, it is vital that included trials be conceptually similar in terms of key factors which determine treatment efficacy, including patients (similar disease characteristics and severity, prior failure of therapies), included interventions (standard dose and schedule), co-interventions (which can influence treatment efficacy) and outcome assessment (similar reporting indices, and definitions for outcome, assessed in standard manner); this comparability, however, is a matter of judgement.18, 19 There is limited data in published RCTs on the efficacy of these agents in achieving endoscopic remission, which precludes ability to inform the comparative efficacy for this endpoint. Despite findings from these indirect comparisons, in the absence of direct head-to-head comparative studies, providers and patients may entirely choose one agent over another out of personal choice and experience.
Table 2.
Comparative efficacy of different therapies for inducing and maintaining clinical remission in patients with moderate to severely active Crohn’s disease, based on network meta-analysis by Singh et al
| First-line therapy in biologic-naïve patients |
In patients with clinical response to induction therapy |
|||
|---|---|---|---|---|
| Induction of clinical remission (odds ratio vs. placebo) |
Probability of remissiona; SUCRA ranking |
Maintenance of clinical remission (odds ratio vs. placebo) |
Probability of remissionb; SUCRA ranking |
|
| Infliximab | 5.90 (2.78–12.51) | 60%; 0.93 | 2.86 (1.71–4.80) | 48%; 0.68 |
| Adalimumab | 3.80 (1.76–8.18) | 49%; 0.75 | 4.42 (2.68–7.29) | 58%; 0.97 |
| Certolizumab pegol | 1.36 (0.89–2.08) | 25%; 0.20 | 2.25 (1.51–3.35) | 42%; 0.48 |
| Vedolizumab | 2.69 (1.36–5.32) | 40%; 0.55 | 2.32 (1.40–3.84) | 42%; 0.52 |
| Ustekinumab | 2.75 (1.76–4.32) | 41%; 0.56 | 2.02 (1.35–3.03) | 39%; 0.36 |
Pooled placebo rate of inducing clinical remission in biologic-naïve patients=25%;
Pooled placebo rate of maintaining clinical remission in patients with response to induction therapy=24%
[Abbreviations: SUCRA=surface under the cumulative ranking curve]
Observational studies have compared different TNFα antagonists, and more recently compared vedolizumab with TNFα antagonists. In a Danish population-based cohort of 827 biologic-naïve patients with CD, there were no significant differences in rate of CD-related hospitalization (HR, 0.81; 95% CI, 0.55–1.20) or major abdominal surgery (HR, 1.24; 95% CI, 0.66–2.33) between adalimumab-and infliximab-treated patients, though rate of all-cause hospitalization was lower in adalimumab-treated patients (HR, 0.74; 95% CI, 0.56–0.97).20 Other claims-and registry-based and multicenter cohort studies have confirmed lack of difference in clinical benefit between adalimumab and infliximab-treated patients.21–23 In contrast, in a U.S. administrative claims-based study of 3205 adults who were new users of TNFα antagonists, investigators observed that infliximab use was associated with lower rates of abdominal surgery and CD-related hospitalization as compared to certolizumab pegol.24 In a recent propensity score-matched study comparing vedolizumab vs. TNFα antagonists, performed using the VICTORY consortium, Bohm and colleagues observed no significant difference in risk of achieving clinical remission (HR, 1.27; 95% CI, 0.911.78) and corticosteroid-free remission (HR, 1.75; 95% CI, 0.90–3.43) after adjusting for concomitant steroid or immunomodulator use, disease location and number of prior TNFα antagonists used.25 In another multi-center study of older patients with IBD, Adar and colleagues observed no significant difference in rates of clinical remission and safety between vedolizumab and TNFα antagonist-treated patients.26
Combination Therapy vs. Monotherapy
Several RCTs have demonstrated superiority of combination of TNFα antagonists and immunosuppressive agents, specifically the combination of infliximab and thiopurines.27–29 In the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) trial of 508 biologic-and immunomodulator-naïve patients with moderate to severely active CD, combination therapy of infliximab and thiopurine was superior to infliximab monotherapy which in turn was superior to thiopurine monotherapy in achieving corticosteroid-free clinical remission (56.8% vs. 44.4% vs. 30.0%) and endoscopic remission (43.9% vs. 30.1% vs. 16.5%) at week 30.28 In another open-label randomized clinical trial of biologic-and thiopurine-naïve patients with moderate to severely active CD, the combination of adalimumab and thiopurine was more effective in achieving endoscopic remission (84.2% vs. 63.8%), but not clinical remission (71.8% vs 68.1%) as compared to adalimumab monotherapy at 26 weeks.30 However, the combination of infliximab and methotrexate was not superior to infliximab alone in the Combination of Maintenance Methotrexate-Infliximab Trial (COMMIT) trial in achieving corticosteroid-free clinical remission at week 50, in patients with CD treated with corticosteroid induction therapy in the preceding 6 weeks.31 This somewhat surprising and apparently discrepant finding from the SONIC trial was attributed to differences in trial design (lack of endoscopy criteria at trial inclusion, differences in ways corticosteroids were handled in trial), or perhaps a true lack of significant benefit of adding methotrexate to infliximab (although infliximab drug concentration was higher and the rate of immunogenicity was lower when used in combination with methotrexate vs. monotherapy). The observed benefit of combination therapy is at least partly attributed to achieving higher biologic trough concentration and lower immunogenicity as compared to biologic monotherapy. In a post-hoc analysis of SONIC trial, no differences in efficacy of combination therapy vs. infliximab were observed when evaluating patients by quartiles of infliximab trough concentration; however, currently this represents association rather than causation, and it is possible that superior remission rates drove higher trough concentration, rather than vice versa.32
Similar trials of combination therapy vs. biologic monotherapy have not been conducted for non-TNFα-targeting biologic agents, like vedolizumab and ustekinumab. Post-hoc analyses of clinical trials and observational studies suggest no consistent difference in efficacy of patients receiving these biologic agents alone vs. those who entered the trial on stable doses of immunosuppressive agents; however, it is important to note that patients in these trials who were on concomitant immunosuppressive agents had previously failed these therapies, were intrinsically more resistant to therapy, leading to confounding by disease severity.33, 34 While these newer agents have lower immunogenicity as compared to infliximab, the concept of exposure-response relationship probably holds true for these agents also, such that adding an anti-metabolite may result in achieving higher biologic trough concentration with these agents also.
Anti-Metabolite Monotherapy
Thiopurines and methotrexate have been evaluated for the management of moderate to severely active CD. Due to the slow onset of action of thiopurines, they are not recommended for induction of remission in patients with moderate to severely active CD.35 In Cochrane systematic review of 11 trials, Chande and colleagues reported that thiopurines may be modestly more effective than placebo in maintaining remission in patients with quiescent CD (RR, 1.19; 95% CI, 1.05 to 1.34).36 Parenteral methotrexate, but not low-dose oral methotrexate, may be effective in inducing and maintaining clinical remission in patients with moderate to severely active CD.37, 38 However, as noted below, anti-metabolites particularly thiopurines are associated with an increased risk of lymphoma and non-melanoma skin cancer, as well as an increased risk of serious and opportunistic infections. Hence, in our practice, thiopurines and methotrexate are rarely used as monotherapy in patients with CD. They may be considered in patients with low-risk CD who require repeated courses of budesonide or prednisone, especially in resource-limited settings.
Comparative Safety of Pharmacological Therapies
Besides efficacy, safety of treatment approaches is a vital factor for patients and physicians when choosing appropriate therapies. Unfortunately, data on comparative safety of different therapies used in the treatment of patients with moderate to severely active CD are limited. Clinical trials are underpowered to detect differences in risk of serious infections and malignancy between different agents. Observational studies have inherent biases related to inadequate assessment of disease phenotype and unmeasured confounding by indication and disease severity.
Risk of Serious and/or Opportunistic Infections
The most robust estimates on safety of biologic therapies from registries and real-world observational studies are available for TNFα antagonists. These agents may be associated with 1.5–2 times higher risk of serious infections as compared to immunosuppressive agents.39 In the TREAT registry of 6,273 patients with moderate to severely active CD (3,440 infliximab-treated and 2,833 other-treatments-only) with up to 13 years of follow-up, serious infections occurred at 2.2 events per 100 person-years (PY) in infliximab-treated patients compared to 0.9/100-PY in other-treatments-only patients.40 In the PYRAMID registry of 5,025 adalimumab-treated patients followed for up to 6 years, treatment emergent serious infections were reported at a rate of 4.7 events per 100-PY from 556 patients (11.1 %).41
In contrast, by virtue of gut-specificity of its receptor, vedolizumab is presumed to be a safer biologic. In an open-label extension study including data from four placebo-controlled and two open-label trials of vedolizumab in patients with IBD, Colombel and colleagues estimated that exposure-adjusted incidence rates of serious infections were similar for vedolizumab compared to placebo in patients with CD (5.6 vs. 3.0 per 100-PY).42 In a preliminary multi-center comparative safety study evaluating patients treated with vedolizumab versus TNFα antagonists, rates of serious infections was lower in vedolizumab-treated patients vs. TNFα antagonist-treated patients (6.9% vs. 10.1%; OR, 0.67 [0.41–1.07]); however this potential safety advantage dissipated with concomitant use of immunomodulators and corticosteroids (11.5% vs. 13.9%; OR 0.81 [0.31–2.07]).25, 43 Similar to vedolizumab, ustekinumab is believed to have lesser systemic immunosuppressive as compared to TNFα antagonists. Registry studies and large real-world observational studies of ustekinumab in CD are awaited. In an integrated safety analysis of data from 6 phase 2/3 trials of ustekinumab including 2574 patients with CD (1733-PY), incidence of serious infections was 5.0 per 100-PY (vs. 5.5 in placebo-treated patients).44 Extrapolating from other autoimmune diseases like psoriasis, the risk of serious infections with ustekinumab monotherapy may be lower as compared to TNFα antagonist monotherapy. In the PSOLAR (Psoriasis Longitudinal Assessment and Registry) registry with 12,093 patients (40,388-PY follow-up), absolute risk of serious infections with ustekinumab (0.93 per 100-PY) was lower as compared to infliximab (2.91 per 100-PY) and other biologic agents (1.91 per 100-PY).45 These findings on the relative safety of ustekinumab in patients with psoriasis should be interpreted with caution, though, since the dose of ustekinumab approved for use in CD is at least 50% higher than the dose used in psoriasis.
The combination of biologic agents with anti-metabolites is associated with a higher risk of serious infections as compared to monotherapy with either agent. In retrospective French population-based cohort study using the national health insurance database of 85,850 TNFα antagonist-and/or immunosuppressive-treated patients (178,155-PY), Kirchgesner and colleagues observed that the combination of TNFα antagonist and anti-metabolites (thiopurines or methotrexate) is associated with a higher risk of serious infections (requiring hospitalization) (2.2 per 100-PY) as compared to patients treated with TNFα antagonist monotherapy (1.9 per 100-PY) which itself is associated with higher risk of infection as compared to immunomodulator monotherapy (1.1 per 100-PY).46 Corresponding rates of opportunistic infections were 0.41 per 100-PY, 0.21 per 100-PY and 0.17 per 100-PY with combination therapy, TNFα antagonist monotherapy and immunomodulator monotherapy, respectively. After adjusting for important confounders, exposure to combination therapy was associated with higher risk of opportunistic infections as compared to monotherapy with either TNFα antagonist or immunomodulators. However, there was no difference in risk of opportunistic infections with TNFα antagonist monotherapy vs. immunomodulator monotherapy (HR, 1.08 [0.83–1.40]). The most common sites of serious infections were pulmonary (24.2%), gastrointestinal tract (22.5%) and skin (17.2%); the most common cause for opportunistic infections was viral. Approximately 3.9% of patients with serious infections died within 3 months. In a Danish propensity score matched population-based cohort study, Andersen and colleagues estimated that TNFα antagonist-based therapy is associated with 2.1 times higher risk of serious infections within 1 year, as compared to anti-metabolite-based therapy.47 In a meta-analysis of comparative studies including registries and observational comparative effectiveness studies, Singh and colleagues observed that risk of serious infections was modestly higher with combination therapy of TNFα antagonist and immunomodulators vs. TNFα antagonist monotherapy (6 cohorts, relative risk [RR], 1.19 [1.03–1.37]), and vs. immunomodulator monotherapy (2 cohorts, RR, 1.78 [1.24–2.57]).39 Based on 5 cohorts, median (range) of serious infections with TNFα antagonist monotherapy and immunomodulator monotherapy was 3.9 (0.4–11.1) and 2.2 (0.9–11.2) per 100-PY, respectively, with corresponding risk of serious infections being 64% higher with TNFα antagonist monotherapy (RR, 1.64 [1.19–2.27]). In a retrospective cohort study among Medicaid and Medicare beneficiaries from 2001 to 2013, Lewis and colleagues observed no difference in the risk of serious infections in patients treated with TNFα antagonists vs. those treated with chronic corticosteroids in patients with CD (6.6 vs. 7.7 per 100-PY; OR, 0.98 [0.87–1.10]);48 chronic corticosteroid use was, however, associated with an increased risk of mortality, major adverse cardiovascular events and fractures, as compared to TNFα antagonist use.
Risk of malignancy
In a comprehensive systematic review including 23 RCTs of TNFα antagonists in IBD, with at least one reported cancer, there was no significant increase in risk of malignancy with TNFα antagonists (20/4,442) vs. placebo (16/2,778).49 In the TREAT registry of 6,273 patients with CD (3,420 treated with infliximab, 3,764 treated with conventional therapy, average follow-up 5.2 years), incidence of malignancy in infliximab-treated patients was 0.64 per 100-PY, which was not significantly different than the rate with conventional therapy (RR, 0.88 [0.66–1. 19]).50 Specifically, the risk of lymphoma was 0.05 per 100-PY in infliximab-treated patients (RR vs. conventional treatment, 0.98 [0.34–2.82]). On multivariable analysis, advanced age, longer disease duration and smoking were independently associated with increased risk of malignancy, whereas exposure to infliximab and/or anti-metabolite therapy was not associated with increased risk of malignancy. Similarly, in the PYRAMID registry of 5,025 patients with CD treated with adalimumab with mean follow-up of ~3 years, the incidence of any malignancy (including NMSC) was 0.8 per 100-PY.41 Risk of malignancy was higher in patients concomitantly on thiopurines vs. adalimumab monotherapy (3.1% vs. 1.9%, p=0.01). In a Danish nationwide registry-based cohort study of 56,146 patients with IBD followed over median 9.3 years, the incidence rate of malignancy in 4,553 patients exposed to TNFα antagonists was 0.43 per 100-PY.51 In a fully adjusted model, including age, calendar year, disease duration, baseline propensity scores, use of 5-aminosalicylates, local and systemic corticosteroids, and immunosuppressive agents, the overall risk of cancer was not different in patients exposed vs. unexposed to TNFα antagonists (RR, 1.07 [0.85–1.36]). There was no significant effect of age at time of exposure, and cumulative dose/duration of exposure, on overall risk of cancer. In a French population-based cohort using the National Health Insurance databases of 189,289 patients followed over a median 6.7 years, Lemaitre and colleagues identified 336 cases of lymphoma.52 Incidence rate (per 100-PY) of lymphoma in unexposed patients, patients exposed to thiopurine monotherapy, to TNFα antagonist monotherapy and to combination therapy was 0.026 (0.023–0.029), 0.054 (0.041–0.067), 0.041 (0.027–0.055), and 0.095 (0.045–0.145), respectively. In a multivariable Cox model, compared with unexposed patients, the risk of lymphoma was higher among those exposed to thiopurine monotherapy (HR, 2.60 [1.96–3.44]), TNFα antagonist monotherapy (HR, 2.41 [1.60–3.64]) and combination therapy (HR, 6.11 [3.46–10.8]). The risk was higher in patients exposed to combination therapy vs. those exposed to thiopurine monotherapy (HR, 2.35 [1.31–4.22]) or TNFα antagonist monotherapy (HR, 2.53 [1.35–4.77]).
There is very limited data on the risk of malignancy with vedolizumab or ustekinumab. In an open-label extension study including data from four placebo-controlled and two open-label trials of vedolizumab in patients with IBD, Colombel and colleagues reported malignancy in 18/2,830 (0.6%) patients treated with vedolizumab and 1/504 (0.2%) placebo-treated patients.42 Similarly, in an integrated safety analyses of phase II/III trials of ustekinumab for psoriasis, psoriatic arthritis and CD, the incidence of malignancy (excluding NMSC) was low and comparable among ustekinumab-treated patients (0.4 per 100-PY) and placebo-treated patients (0.2 per 100-PY).53 In psoriasis, in a nested case-control analysis of PSOLAR registry of 12,090 patients, 252 patients with malignancy were matched with 1,008 patients without malignancy. In this analysis, treatment with ustekinumab for 0–3 months, 3–12 months or >12 months was not associated with increased odds of malignancy versus no exposure; in contrast, in this registry, longer-term (≥12 months) exposure to TNFα antagonist was associated with increased odds of malignancy (OR, 1.54 [1.10–2.15]).54
Table 3 synthesizes the observed, non-comparative, incidence rates of serious infections and malignancy across different pharmacotherapies. Overall, TNFα antagonists may be more immunosuppressive than others, especially gut-selective vedolizumab, and may be associated with increased risk of serious infections and lymphoma. Moreover, the risk of lymphoma may be comparable between TNFα antagonists and thiopurines. However, comparative safety of pharmacotherapy for IBD should be viewed in conjunction with efficacy and in the context of treatment strategies/approach, rather than in the context of specific agents used. The potential safety advantage of non-TNFα-targeted biologics over TNFα antagonists may be lost when these agents are used in combination with thiopurines. Similarly, even though a specific agent may be safer in isolation, if it has lower efficacy as compared to another intervention, patients may fail to achieve remission, leading to ongoing severe inflammation which may lead to disease complications necessitate the need for corticosteroids, and eventually a higher risk of serious infections. Besides exposure to immunosuppressive therapies, most consistent risk factors for serious infections include use of corticosteroids, narcotics, moderate to severe disease activity, and older age.55
Table 3.
Incidence rate of serious infections and malignancy (per 100-PY) in patients with moderate to severe IBD treated with pharmacotherapy [Abbreviations: SI-serious infections; TNFα-Tumor necrosis factorα]
| Drug Class | Clinical Trials & Open Label Extensions (per 100-PY) |
Safety Registries (per 100-PY) |
Real-World Observational Studies (per 100-PY) |
|---|---|---|---|
| TNFα antagonists | SI: 3.4–6.1 Malignancy: 0.45 | SI: 2.2–4.7 Malignancy: 0.64–0.8 | SI: 1.9–10.9 Malignancy: 0.43 (lymphoma, 0.04) |
| Vedolizumab | SI: 4.3 Malignancy: 0.50 | - | SI: 5.2 Malignancy: 0.23 |
| Ustekinumab | SI: 5.5 Malignancy: 0.4 | (SI: 0.9–1.5 in psoriasis) | - |
Predictors of Response to Therapy
One of the key aspects that may inform personalized positioning of therapies is identification of factors that may be associated with response to different agents. Several post-hoc analyses of clinical trials, registries and cohort studies have evaluated these factors. Unfortunately, most commonly studied patient-, disease-and treatment-related factors associated with response to therapy appear to be common across agents, and agnostic of specific mechanisms of action, suggesting that they may be predictive of more difficult-to-treat IBD. In general, more severe disease activity, higher inflammatory burden, prior surgery and progression to complications of (fistula and/or stricture), prior exposure to TNFα antagonists and low serum trough concentration of biologic agent are associated with inferior response to all biologic agents. Agent-specific genetic, molecular and multi-omic markers hold promise and are currently being studied.
The cumulative and weighted effect of different factors associated with response to therapy have been modelled into clinical prediction tools to identify patients who may have a high vs. low likelihood of response to specific agents. Dulai and colleagues derived a multivariable model predicting response to vedolizumab at week 26 in patients with moderate-to-severely active CD, using data from phase III GEMINI 2 clinical trial and subsequently validated the model in a real-world observational cohort of patients with active CD treated with vedolizumab for 26 weeks (the VICTORY cohort).56 In the derivation analysis, absence of previous treatment with TNFα antagonists (+3 points), absence of prior bowel surgery (+2 points), absence of prior fistulizing disease (+2 points), baseline level of albumin (+0.4 points per g/L), and baseline concentration of C-reactive protein (reduction of 0.5 points for values between 3.0 and 10.0 mg/L and 3.0 points for values >10.0 mg/L) were associated with remission. In the validation set, the model identified patients in clinical remission, corticosteroid-free remission and mucosal healing with an area under the receiver operator curve (AUC) of 0.67, 0.66 and 0.72, respectively. In this clinical decision support tool, a cut-off value of ≤13 points identified patients at low likelihood of responding to vedolizumab with high sensitivity, whereas a cut-off >19 points identified patients at higher likelihood of responding to therapy with moderate specificity. In a subsequent analysis, this model predicted vedolizumab drug concentration with good accuracy in the GEMINI trial cohort, and predicted likelihood of surgery in patients with CD in an external real-world practice cohort with good accuracy.57 A similar prediction model identifying patients with high vs. low likelihood of response to ustekinumab is in development. In a matrix-based prediction model predicting primary non-response to infliximab in patients with CD, Billiet and colleagues observed that a matrix based on age at time of infliximab initiation, body mass index and prior surgery was able to accurately identify patients at high risk of primary non-response (AUC 0.80); addition of serological and genetic markers did not improve the performance of this prediction model.58
While these models have good accuracy in identifying patients at low vs. high likelihood of response to different therapies, it is unclear how well these models discriminate between relative likelihood of response to one agent vs. another, and hence may not help inform personalization of therapy. However, by identifying patients at low likelihood of response to a specific agent, they may help inform relative positioning of agents. More recently, machine learning-based models have recently been used as predictors of response to different therapies. Waljee and colleagues observed that random forest models (AUC 0.78) based on baseline and week 8 data performed better than conventional predictors of C-reactive protein and albumin in predicting biochemical response to ustekinimab based on phase III clinical trial data;59 however, this model had a modest accuracy in predicting clinical remission in ustekinumab-treated patients with CD. Similarly, based on data from clinical trials of vedolizumab, Waljee and colleagues observed that a random forest model prediction model based on baseline and week 6 data performed as well as a fecal calprotectin response at week 6.60
Besides known phenotypic and pharmacological factors predictive of response to biologic therapy, other factors including genetic factors, immune cell infiltrate patterns, drug target expression and microbiome signatures may influence likelihood of response to specific therapies, and provide opportunities for personalization of therapy.61 These factors have been best studied in predicting response to TNFα antagonists; similar studies predicting response to non-TNFα targeting biologics are awaited. However, it remains to be seen how discriminatory these factors might be in personalizing and choosing therapies in patients with IBD.
Positioning Therapies in the Management of High-risk CD
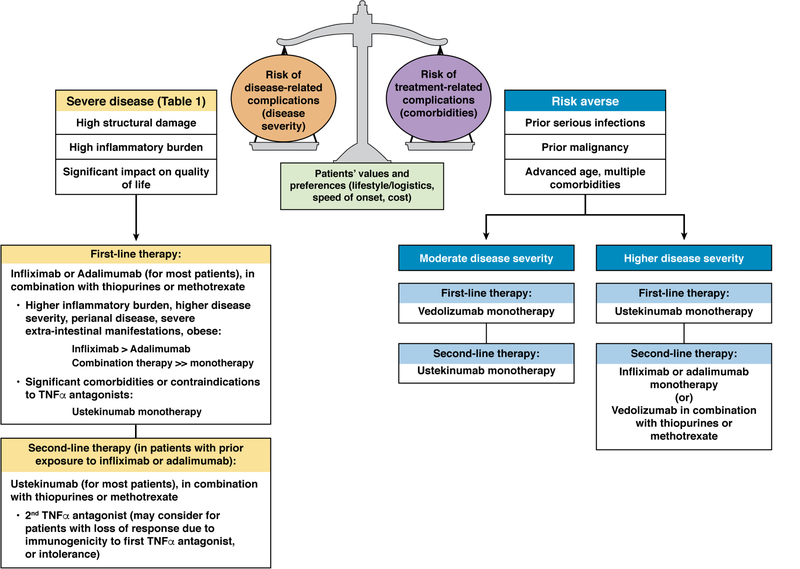
In the absence of well-defined biomarkers that discriminate likelihood of responding to one medication over another, positioning therapies in patients with high-risk CD combines a science that weighs the risk of disease-related complications (relative efficacy) vs. treatment-related complications (relative safety), with the art of understanding patients’ overall health state, values and preferences, and costs of therapy. Factors that may play into values and preferences may be lifestyle and logistics (impacts mode of delivery), speed of onset of action, and comorbid conditions (using specific medications that may target two related health conditions, or may impact drug-drug interactions). We propose our evidence-informed approach to positioning therapies in high-risk patients with CD (Figure 2). In many patients with moderate-to-severely active CD, with high disease severity (as defined above), we prefer using TNFα antagonists, specifically infliximab or adalimumab, as first-line therapy; infliximab may be preferred over adalimumab in patients with high body mass index, higher inflammatory burden or perianal disease, whereas adalimumab may be preferred out of convenience of self-administration for some patients. Most often, we use these medications in combination with immunosuppressive agents (thiopurines or oral or subcutaneous methotrexate) at conventional doses, particular in patients with high inflammatory burden, and/or those with multiple disease severity factors. In a subset of patients with moderate-to-severely active CD, with high disease severity, who have relative contraindications to using TNFα antagonists (active malignancy particularly hematologic malignancies, demyelinating diseases, severe heart failure, multiple repeated non-CD-related serious infections or multiple serious comorbidities), we prefer to use ustekinumab monotherapy as first-line biologic agent; however, if the balance of such a patient falls more towards a higher risk of treatment-related complications with relatively moderate risk of disease-related complications, we prefer vedolizumab monotherapy. In high disease severity patients failing initial infliximab or adalimumab as their first-line agent (ongoing evidence of moderate to severe inflammation), we attempt to optimize their index therapy through therapeutic drug monitoring. In patients previously exposed to TNFα antagonists, particularly those who experience loss of response despite high serum trough concentrations (mechanistic failures), we prefer to switch to ustekinumab as second-line agent, most often in combination with immunosuppressive agents; a second TNFα antagonist (infliximab or adalimumab, whichever of them has not been used) may be an effective option in patients with prior response to TNFα antagonists who develop loss of response due to immunogenicity (pharmacokinetic failure). We favor using ustekinumab as preferred agent in patients with comorbid moderate to severe psoriasis, or vedolizumab in immunosuppressed patients who may be on other high-risk therapies, or patients who are very risk averse and otherwise have moderate disease activity and severity.
Figure 2.
Proposed algorithm for positioning therapies for patients with high-risk Crohn’s disease
These treatment preferences are rather generic and individualized decision-making in the context of the entire scenario is warranted. Regardless of initial treatment decision, we continually and closely monitor clinical and objective treatment responses (biochemical markers like fecal calprotectin and C-reactive protein) within the first 6–12 weeks, with an endoscopic assessment performed readily within 4–6 months of treatment initiation and/or optimization with the objective of achieving endoscopic remission. With evolving paradigms on treat-to-target of symptomatic and endoscopic remission, discussions on treatment escalation in asymptomatic patients with ongoing moderate to severe endoscopic activity become more nuanced weighing risk and benefits of treatment escalation in the face of uncertainties of risk of disease progression and treatment-related complications or even treatment failure.
Acknowledgments
Disclosures: Dr. Nguyen is supported by NIH/NIDDK (T32 DK007202). Dr. Singh is supported by NIH/NIDDK (K23DK117058), ACG Junior Faculty Development Award and the Crohn’s and Colitis Foundation Career Development Award (#404614)
NHN - None to declare
SS - Research grants from AbbVie, Consulting fees from AbbVie, Takeda, Pfizer, AMAG Pharmaceuticals
WJS - research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (owned by Precision IBD); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Precision IBD (owns Prometheus Laboratories), Progenity, Prometheus Laboratories (owned by Precision IBD), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD (owns Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech-consultant, stock options; Progenity-consultant, stock; Oppilan Pharma-employee, stock options; Escalier Biosciences-employee, stock options; Precision IBD (also owns Prometheus Laboratories)-employee, stock options; Ventyx Biosciences-employee, stock options; Vimalan Biosciences-employee, stock options.
Footnotes
Conflicts of Interest:
REFERENCES
- 1.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Olen O, Askling J, Sachs MC, et al. Increased Mortality of Patients With Childhood-Onset Inflammatory Bowel Diseases, Compared With the General Population. Gastroenterology 2019;156:614–622. [DOI] [PubMed] [Google Scholar]
- 3.Olen O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Loftus EV Jr., Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol 2010;105:289–97. [DOI] [PubMed] [Google Scholar]
- 5.Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 6.Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109:1739–48. [DOI] [PubMed] [Google Scholar]
- 7.Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol 2016;14:348–354 e17. [DOI] [PubMed] [Google Scholar]
- 8.Siegel CA, Whitman CB, Spiegel BMR, et al. Development of an index to define overall disease severity in IBD. Gut 2018;67:244–254. [DOI] [PubMed] [Google Scholar]
- 9.Moja L, Danese S, Fiorino G, et al. Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Alimentary Pharmacology & Therapeutics 2015;41:1055–65. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 11.Ma C, Dutton SJ, Cipriano LE, et al. Systematic review with meta-analysis: prevalence, risk factors and costs of aminosalicylate use in Crohn’s disease. Aliment Pharmacol Ther 2018;48:114–126. [DOI] [PubMed] [Google Scholar]
- 12.Narula N, Dhillon A, Zhang D, et al. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2018;4:CD000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine A, Wine E, Assa A, et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019;157:440–450 e8. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Fumery M, Sandborn WJ, et al. Systematic review and network meta-analysis: first-and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther 2018;48:394–409. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn W, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Annals of internal medicine. Volume 146, 2007:829–838. [DOI] [PubMed] [Google Scholar]
- 16.Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology 2017;153:835–857.e6. [DOI] [PubMed] [Google Scholar]
- 17.Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 18.Singh S. How to Conduct and Interpret Systematic Reviews and Meta-Analyses. Clin Transl Gastroenterol 2017;8:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130–7. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Andersen NN, Andersson M, et al. Comparison of infliximab with adalimumab in 827 biologic-naive patients with Crohn’s disease: a population-based Danish cohort study. Alimentary Pharmacology & Therapeutics 2018;47:596–604. [DOI] [PubMed] [Google Scholar]
- 21.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for crohn’s disease. Clinical Gastroenterology and Hepatology 2014;12:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macaluso FS, Fries W, Privitera AC, et al. A Propensity Score-matched Comparison of Infliximab and Adalimumab in Tumour Necrosis Factor-alpha Inhibitor-naive and Non-naive Patients With Crohn’s Disease: Real-Life Data From the Sicilian Network for Inflammatory Bowel Disease. J Crohns Colitis 2019;13:209–217. [DOI] [PubMed] [Google Scholar]
- 23.Narula N, Kainz S, Petritsch W, et al. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-alpha naive Crohn’s disease. Alimentary Pharmacology & Therapeutics 2016;44:170–80. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Heien HC, Sangaralingham LR, et al. Comparative Effectiveness and Safety of Anti-Tumor Necrosis Factor Agents in Biologic-Naive Patients With Crohn’s Disease. Clinical Gastroenterology and Hepatology 2016;14:1120–1129.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohm M SS, Fischer M, Kadire S, Tran G, Rahal M, Aniwan S, Meserve J, et al. Comparative effectiveness of vedolizumab and tumour necrosis factor-antagonist therapy in Crohn’s disease: a multicentre consortium propensity score-matched analysis, In European Crohn’s and Colitis Congress, Vienna, 2018. [Google Scholar]
- 26.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor alpha antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther 2019;49:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology 2006;130:1054–61. [DOI] [PubMed] [Google Scholar]
- 28.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. New England Journal of Medicine 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 29.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: A prospective, randomized trial. Journal of Crohn’s and Colitis 2016;10:1259–1266. [DOI] [PubMed] [Google Scholar]
- 31.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–688.e1. [DOI] [PubMed] [Google Scholar]
- 32.Colombel JF, Adedokun OJ, Gasink C, et al. Combination Therapy With Infliximab and Azathioprine Improves Infliximab Pharmacokinetic Features and Efficacy: A Post Hoc Analysis. Clin Gastroenterol Hepatol 2019;17:1525–1532 e1. [DOI] [PubMed] [Google Scholar]
- 33.Hedin C, Halfvarson J. Should we use vedolizumab as mono or combo therapy in ulcerative colitis? Best Pract Res Clin Gastroenterol 2018;32–33:27–34. [DOI] [PubMed] [Google Scholar]
- 34.Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s disease: results of the ICC Registry, a nationwide prospective observational cohort study. J Crohns Colitis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chande N, Townsend CM, Parker CE, et al. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2016;10:CD000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2015:CD000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald JW, Wang Y, Tsoulis DJ, et al. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database of Systematic Reviews 2014:CD003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel V, Wang Y, MacDonald JK, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2014:CD006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh S, Facciorusso A, Dulai PS, et al. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREATTM registry. American Journal of Gastroenterology 2012;107:1409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Haens G, Reinisch W, Panaccione R, et al. Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients With Crohn’s Disease With up to 6 Years of Follow-Up in the Pyramid Registry. Am J Gastroenterol 2018;113:872–882. [DOI] [PubMed] [Google Scholar]
- 42.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faleck D SP, Meserve J, Rahal M, Kadire S, Tran G, Weiss A, Winters A, et al. Comparative Effectiveness of Vedolizumab and Tumor Necrosis Factor-Antagonist Therapy in Ulcerative Colitis: A Multicenter Consortium Propensity Score-Matched Analysis. Gastroenterology 2018;154:S82. [Google Scholar]
- 44.Ghosh SSB, O’Brien CD, Tikhonov I, Zhou Y, Volger S, Marano C, Ott E, Gasink C, Sandborn WJ, Danese S. Safety of Ustekinumab in Inflammatory Bowel Diseases: Integrated Safety Analysis of Results from Phase 2 and 3 Studies in Crohn’s Disease and Ulcerative Colitis, In United European Gastroenterology Week; 2019, 2019. [Google Scholar]
- 45.Papp K, Gottlieb AB, Naldi L, et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol 2015;14:706–14. [PubMed] [Google Scholar]
- 46.Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018;155:337–346 e10. [DOI] [PubMed] [Google Scholar]
- 47.Nyboe Andersen N, Pasternak B, Friis-Moller N, et al. Association between tumour necrosis factor-alpha inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ 2015;350:h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis JD, Scott FI, Brensinger CM, et al. Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-alpha-Directed Therapy for Inflammatory Bowel Disease. American Journal of Gastroenterology 2018; 113:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonovas S, Fiorino G, Allocca M, et al. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clinical Gastroenterology and Hepatology 2016;14:1385–1397.e10. [DOI] [PubMed] [Google Scholar]
- 50.Lichtenstein GR, Feagan BG, Cohen RD, et al. Drug therapies and the risk of malignancy in crohn’s disease: Results from the TREAT™ registry. American Journal of Gastroenterology 2014;109:212–223. [DOI] [PubMed] [Google Scholar]
- 51.Andersen NN, Pasternak B, Basit S, et al. Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA-Journal of the American Medical Association 2014;311:2406–2413. [DOI] [PubMed] [Google Scholar]
- 52.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab Safety in Psoriasis, Psoriatic Arthritis, and Crohn’s Disease: An Integrated Analysis of Phase II/III Clinical Development Programs. Drug Saf 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017;77:845–854.e5. [DOI] [PubMed] [Google Scholar]
- 55.Holmer A, Singh S. Overall and comparative safety of biologic and immunosuppressive therapy in inflammatory bowel diseases. Expert Rev Clin Immunol 2019;15:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dulai PS, Boland BS, Singh S, et al. Development and Validation of a Scoring System to Predict Outcomes of Vedolizumab Treatment in Patients With Crohn’s Disease. Gastroenterology 2018;155:687–695 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dulai PS AA, Peyrin-Biroulet L, Singh S, Serrero M, Jairath V, Filippi J, et al. A Simple Scoring Tool Predicts Exposure-Response Relationship, Onset of Action, Response to Interval Shortening, and Surgical Risk with Vedolizumab Therapy for Crohn’s Disease. Gastroenterology 2019;156:S843. [Google Scholar]
- 58.Billiet T, Papamichael K, de Bruyn M, et al. A Matrix-based Model Predicts Primary Response to Infliximab in Crohn’s Disease. Journal of Crohn’s & colitis 2015;9:1120–6. [DOI] [PubMed] [Google Scholar]
- 59.Waljee AK, Wallace BI, Cohen-Mekelburg S, et al. Development and Validation of Machine Learning Models in Prediction of Remission in Patients With Moderate to Severe Crohn Disease. JAMA Netw Open 2019;2:e193721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waljee AK, Liu B, Sauder K, et al. Predicting corticosteroid-free endoscopic remission with vedolizumab in ulcerative colitis. Aliment Pharmacol Ther 2018;47:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atreya R, Neurath MF. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2018;3:790–802. [DOI] [PubMed] [Google Scholar]