Abstract
Background:
Unilateral offloading footwear prescribed to patients with diabetic foot ulcers elevates one limb relative to the other, which may lead to limp and abnormal gait. This study investigated whether the unilateral foot ulcer and offloading combination negatively impacts gait function beyond diabetic peripheral neuropathy.
Methods:
Eighty-six participants were recruited in 3 groups: 12 with diabetic peripheral neuropathy and unilateral foot ulcers wearing offloading footwear (offloading group, age=55.6±9.5years, BMI= 30.9±4.5kg/m2), 27 with diabetic peripheral neuropathy (neuropathy group, age=64.3±7.7years, BMI= 30.9±5.4kg/m2), and 47 non-diabetic controls (non-diabetic group, age=62.9±16.1years, BMI= 29.0±6.0kg/m2). Gait function was quantified during a habitual speed walking test using a validated wearable platform.
Findings:
The offloading group exhibited deteriorated gait function compared to the non-diabetic group (p<0.005, Cohen’s effect size d=0.90–2.61). They also had decreased gait speed (p<0.001, d=1.79) and stride length (p<0.001, d=1.76), as well as increased gait cycle time (p<0.001, d=1.67) and limp (p<0.050, d=0.72–1.49) compared to the neuropathy group. The offloading group showed increased gait unsteadiness compared to the neuropathy group, but the difference did not reach statistical significance in our samples.
Interpretation:
This study demonstrated that while diabetic peripheral neuropathy deteriorates gait function, including increasing gait unsteadiness and limp, the diabetic foot ulcer and offloading combination magnifies the deterioration beyond diabetic peripheral neuropathy. These findings promote caution of the current standards of care for treating diabetic foot ulcers with offloading footwear. However, it is possible that a contralateral shoe lift may remedy deteriorated gait function and improve quality of life for unilateral offloading users.
Keywords: diabetic foot ulcers, offloading, diabetic peripheral neuropathy, gait function, limp, gait unsteadiness
1. Introduction
Diabetic foot ulcers (DFUs) are a burden to people with diabetes and the health care system. Affecting up to one-third of people with diabetes, DFUs are the most common complication associated with diabetes mellitus [1–3]. These wounds often lead to infections [4] and lower extremity amputations, costing US health care systems greater than $10 billion annually [5]. DFUs cause deterioration in gait function, which is the major determinant of an independent and productive life and is essential for predicting poor quality of life, morbidity, and mortality [6]. Literature reports that people with DFUs ambulate with slower gait speed and shorter step lengths compared to healthy individuals [7]. DFUs are also reported to hinder gait initiation, resulting in a greater distance and number of steps to reach steady state ambulation [8, 9]. Without timely intervention, gait function deterioration among people with DFUs may lead to serious adverse outcomes, including worsening DFUs, amputation, early frailty, risk of falling, and loss of independency, all of which may further complicate their conditions [10–12].
Offloading devices are commonly prescribed to people with DFUs [13]. These devices redistribute plantar pressure and manage excessive plantar tissue stress in order to heal and prevent DFU formation [14–17]. While several studies have demonstrated the effectiveness of offloading devices in improving foot health, other studies have shed light on the detrimental effects that offloading devices impose on gait and mobility [15, 18–21]. Offloading devices reduce gait speed [22] and ankle motion if the device crosses the ankle joint, which limits propulsion during gait [16, 23]. More importantly, if used unilaterally, offloading footwear may elevate one limb relative to the other [22, 23]. This induced limb elevation is sometimes as high as 5 cm [24] and has been shown to decrease gait speed in both people with diabetes [25] and healthy individuals [22, 23]. Uneven limb elevation due to offloading footwear may also create asymmetry in step length between the right and left limbs, resulting in a limp while walking.
Diabetic peripheral neuropathy (DPN) is a common comorbidity seen among people with diabetes and DFUs [3, 26]. DPN is a dysfunction of the peripheral nerves, resulting in the loss of sensation of the extremities. This loss of sensation increases difficulty to maintain normal gait patterns [8, 9, 27–29] and increases the risk of developing DFUs [1, 4, 17, 30]. Currently, most studies only focus on how DPN deteriorates gait function, but few studies evaluate the impact of DFUs and offloading devices on gait function. Considering the frequent use of offloading devices for patients with DFUs and DPN, it is important for clinicians to be aware of any effects that DFUs and offloading devices impose on gait.
In this study, we used a validated wearable platform to objectively quantify gait function in patients with DPN and DFUs wearing offloading devices. We proposed to determine the magnitude of gait deterioration potentially caused by DFUs and offloading devices beyond diabetes and DPN. To examine the degree of degradation in gait function caused by DFUs and offloading devices, we compared gait performances among people with DPN and DFUs wearing offloading devices to those with DPN but without DFUs, as well as to age-matched non-diabetic controls. The hypotheses of this study were: (1) compared to age-matched, non-diabetic controls, people with DPN and DFUs using an offloading device have poorer gait function; (2) DFUs and offloading devices magnify the decline in gait function beyond DPN, including limp caused by offloading-induced limb elevation imbalance.
2. Methods
Eighty-six eligible participants were recruited in this study: 12 participants with DPN and unilateral DFUs wearing offloading footwear (Offloading), 27 participants with DPN but no foot ulcer (DPN), and 47 age-matched non-diabetic controls (ND). Status of an active foot ulcer was confirmed by the clinical investigator for the Offloading group. Participants in the Offloading group wore unilateral forefoot-offloading wedge shoes prescribed by their Podiatrist and their habitual closed-toe and closed-heel athletic shoe on the contralateral limb. Although contralateral footwear was not standardized, all contralateral footwear had a standard heel height and did not exhibit any characteristics that would alter gait. Participants were excluded from the study if they were non-ambulatory, had severe gait or balance problems (e.g., unable to walk a distance of 15 m independently with or without assistive devices or unable to stand still without moving feet), or were unwilling to participate. All participants signed a consent form prior to participation. This study was approved by the local institutional review board.
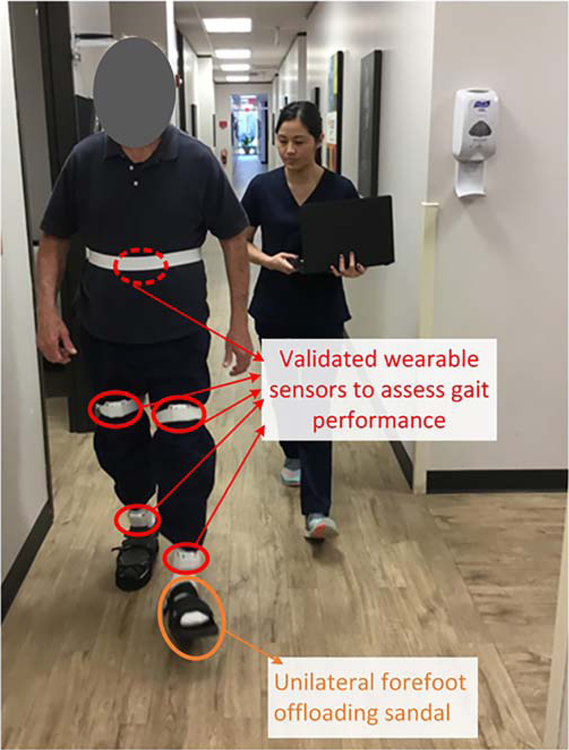
Participants’ demographics, including age, sex, height, weight, and body-mass-index (BMI), were collected. For all participants, five wearable sensors (LEGSys™, BioSensics, Watertown, MA, USA) were attached to the following locations: (1) dorsum of back; (2) anterior right thigh; (3) anterior left thigh; (4) anterior right shin; (5) anterior left shin, to quantify gait parameters of interest (Fig. 1) [31]. Participants were asked to walk at their habitual gait speed for 10 meters without any distraction using the protocol described in our previous studies [29, 32]. The gait test was performed with an investigator walking alongside the participant to aid in stability, if needed, during the trial. Gait parameters were calculated during steady state phase of walking using validated algorithms [33–35]. Steady state phase of gait was estimated using the algorithm suggested by Lindeman et al. [36].The evaluated gait parameters included gait speed and gait speed unsteadiness, stride length and stride length unsteadiness, gait cycle time, double support and double support limp, as well as step length limp. Gait speed unsteadiness was defined as coefficient of variation of stride-to-stride velocity during steady-state of walking [35, 37]. To determine gait symmetry, we estimated double support limp and step length limp. Double support limp was estimated by calculating the absolute difference between initial and terminal double support [34]. Step length limp was estimated by the percentage of difference between right and left step lengths [38].
Fig. 1.
Study participant wearing forefoot offloading sandal and performing habitual speed walking test in the clinic. Gait performance was assessed using validated wearable sensors.
All continuous data were presented as mean (standard error). All categorical data were expressed as percentage of the sample size. Analysis of variance (ANOVA) was used for between-group comparison of continuous demographics. Analysis of Chi-square was used for comparison of categorical demographics. In order to control for the effects of demographics on gait performance, analysis of covariance (ANCOVA) was employed to compare between-group gait performance, with adjustment for BMI and gender. Fisher’s least significant difference-based post-hoc test was performed for pairwise comparison to explore significant main effects and interactions. Cohen’s d effect size was calculated to assess the magnitude of difference between each group. Values ranging from 0.20 to 0.49 indicated small, and values between 0.50 and 0.79 indicated medium effects. Values ranging from 0.80 to 1.29 indicated large, and values above 1.30 indicated very large effects. Values less than 0.20 were considered as having no noticeable effect [39]. For all comparisons, significance was accepted at p<0.050. All statistical analyses were performed using IBM SPSS Statistics 25 (IBM, Chicago, IL).
3. Results
Table 1 summarizes the analysis of demographic and clinical data. The three groups were matched with age and BMI. However, the DPN group and Offloading group had significantly less females than the ND group (p=0.015).
Table 1.
General characteristics of the study groups.
| Non-diabetic Control (ND, n = 47) | Diabetic Peripheral Neuropathy (DPN, n = 27) | Diabetic Foot Ulcer with Offloading (Offloading, n = 12) | p-value | |
|---|---|---|---|---|
| Age, years | 62.9 (2.3) | 64.3 (1.5) | 55.6 (2.7) | 0.156 |
| Female, % | 53% | 26% | 17% | 0.015 |
| Height, m | 1.68 (0.01) | 1.74 (0.02) | 1.77 (0.03) | 0.006 |
| Weight, kg | 82.4 (3.3) | 94.1 (3.8) | 96.9 (3.9) | 0.021 |
| BMI, kg/m2 | 29.0 (0.9) | 30.9 (1.0) | 30.9 (1.3) | 0.309 |
Significant differences between groups are indicated in bold
Data presented as mean (SE)
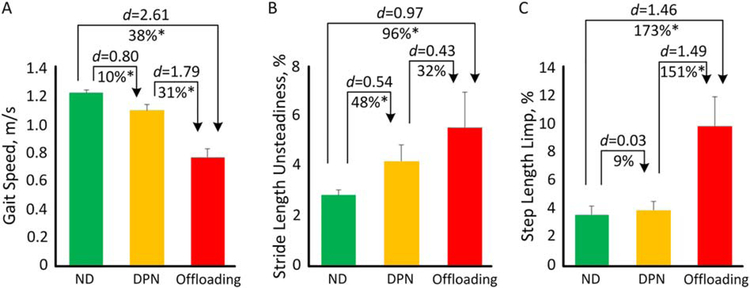
The Offloading group demonstrated deteriorated gait performance compared to both the ND and DPN groups (Table 2). Compared to the ND group, the Offloading group had reduced gait speed (p<0.001, d=2.61, Fig. 2A) and stride length (p<0.001, d=2.51), as well as increased gait cycle time (p<0.001, d=2.13) and double support (p=0.001, d=1.19). Compared to the DPN group, the Offloading group also had poorer gait performance in gait speed (p<0.001, d=1.79, Fig. 2A), stride length (p<0.001, d=1.76), and gait cycle time (p<0.001, d=1.67).
Table 2.
Between-group comparison for gait performance in ND, DPN, and Offloading groups
| ND (n = 47) | DPN (n = 27) | Offloading (n = 12) | DPN vs. ND |
Offloading vs. ND |
Offloading vs. DPN |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diff (%) | p-value * | d * | Diff (%) | p-value * | d * | Diff (%) | p-value * | d * | ||||
| Gait Speed, m/s | 1.22 (0.03) | 1.10 (0.03) | 0.76 (0.05) | −10% | 0.003 | 0.77 | −38% | <0.001 | 2.66 | −31% | <0.001 | 1.91 |
| Stride Length, m | 1.32 (0.02) | 1.24 (0.03) | 1.00 (0.04) | −6% | 0.003 | 0.77 | −24% | <0.001 | 2.52 | −19% | <0.001 | 1.75 |
| Gait Cycle Time, s | 1.09 (0.02) | 1.15 (0.02) | 1.37 (0.03) | 5% | 0.174 | 0.34 | 25% | <0.001 | 2.16 | 19% | <0.001 | 1.84 |
| Double Support, % | 22.52 (0.63) | 26.60 (0.82) | 27.75 (1.23) | 18% | <0.001 | 0.94 | 23% | <0.001 | 1.26 | 4% | 0.354 | 0.32 |
| Gait Speed Unsteadiness, % | 4.23 (.047) | 5.49 (0.62) | 6.96 (0.93) | 30% | 0.040 | 0.51 | 65% | 0.003 | 1.01 | 27% | 0.146 | 0.50 |
| Stride Length Unsteadiness, % | 2.83 (0.43) | 4.21 (0.56) | 5.55 (0.83) | 48% | 0.037 | 0.52 | 96% | 0.003 | 1.01 | 32% | 0.156 | 0.49 |
| Double Support Limp, % | 1.93 (0.30) | 2.12 (0.39) | 3.51 (0.59) | 10% | 0.423 | 0.20 | 82% | 0.008 | 0.90 | 65% | 0.043 | 0.70 |
| Step Length Limp, % | 3.61 (0.66) | 3.93 (0.87) | 9.85 (1.30) | 9% | 0.868 | 0.04 | 173% | <0.001 | 1.35 | 151% | <0.001 | 1.32 |
Results were adjusted by BMI and gender
Significant difference between groups were indicated in bold
Effect sizes were calculated as Cohen’s d
Data presented as mean (SE)
Fig. 2.
Comparison between ND, DPN, and Offloading groups for A) gait speed; B) stride length unsteadiness; and C) step length limp.
For gait variability, the Offloading group had increased gait speed unsteadiness (p=0.004, d=0.96) and stride length unsteadiness (p=0.004, d=0.97, Fig. 2B) compared to the ND group. At the same time, the Offloading group showed larger gait unsteadiness than the DPN group, however the between-group difference was not statistically significant (p>0.050).
For gait symmetry, the DPN group and the ND group had similar performances (p>0.050). However, compared to both the ND and DPN groups, the Offloading group showed significantly increased double support limp (p=0.007, d=0.90 and p=0.041, d=0.72, respectively) and step length limp (p<0.001, d=1.46 and p<0.001, d=1.49, respectively, Fig. 2C).
4. Discussion
In this study, we examined how unilateral DFUs and offloading footwear may alter gait function beyond sensory feedback alteration caused by diabetes and peripheral neuropathy. We were able to confirm our hypothesis that people with DPN and DFUs wearing offloading devices have poorer gait function compared to healthy controls. We were also able to confirm our hypothesis that DFUs and offloading devices further deteriorate gait beyond DPN, specifically for performance in gait speed, stride length, gait cycle time, and gait symmetry. However, the effects of the DFU and offloading footwear combination on other gait parameters of interest, including double support, gait speed unsteadiness, and stride length unsteadiness, were small to medium (d=0.32–0.50), which didn’t achieve statistical significance in our sample. A few previous studies have reported deteriorated gait in people with DFUs [7, 8], and those wearing offloading devices [15, 22, 23], which is consistent with findings in this current study. However, none of the previous studies compared gait function of the DFU and offloading population with cohorts of well-established gait impairment models, such as people with DPN, as this current study did.
Compared to the ND group, the Offloading group had significantly worsened gait performance, including gait unsteadiness and limp. However, when comparing between the Offloading group and the DPN group, gait unsteadiness was not found to significantly differ in our sample. A previous study demonstrated similar findings in regards to the coefficient of variability for stride velocity [8]. This shows that the DFU and offloading footwear combination may alter gait speed and gait symmetry, but may not have much influence beyond DPN to cause inter-stride difference. We speculate that gait unsteadiness is mainly related to cognitive performance and autonomic nervous system [40], but is less affected by limb discrepancy. However, the altered gait symmetry could lead to potential adverse outcomes, such as increased risk of falls, reduced gait efficiency, joint pain, and formation of DFUs on the contralateral side [10–12].
Previous studies have posed potential remedies for improving gait function in people who wear offloading footwear. Crews et al. demonstrated that using a contralateral shoe lift with unilateral offloading footwear reduces variability in walking velocity [25]. Another study reported that normal gait patterns were achieved in unilateral offloading users when a walking boot heel-sole profile matched the height of the contralateral walking shoe [23]. It is possible that using a shoe lift could potentially reduce gait unsteadiness and asymmetry for those with unilateral DFUs who wear offloading footwear. Future studies are needed to verify this hypothesis.
The small sample size was the major limitation in this study. Other limitations include a relatively shorter walking distance for the testing protocol compared to protocols used in other studies that studied gait variability in general older adults [35, 36]. However, for patients with diabetic neuropathy and (risk of) diabetic foot ulcers, the relatively shorter walking distance of 10-meter has been suggested [41–43]. Additionally, the type of footwear worn by participants was not standardized. In this study, all people with diabetes wore prescribed diabetic shoes and participants in the control group were asked to wear regular footwear without excessive heels (higher than 2.5cm). No participants wore sandals or other types of footwear that may alter gait. We used this test protocol, as we believe testing participants with their daily footwear can more accurately represent gait presentation in daily life compared to a standardized shoe. Furthermore, duration of experience wearing an offloading device was not considered when analyzing results. However, this allows our results to reflect how offloading footwear may affect gait performance regardless of wear experience.
5. Conclusions
This study demonstrated that while DPN deteriorates gait function, including increasing gait unsteadiness and gait asymmetry (limp), the DFU and offloading combination magnifies the deterioration beyond DPN. These findings promote caution of the current standards of care for treating DFUs with offloading footwear. However, it is possible that a contralateral shoe lift may remedy deteriorated gait function and improve quality of life for unilateral offloading users. Future studies are needed to verify the findings of this study in a larger sample size.
Highlights.
Unilateral foot ulcers and offloading footwear deteriorate gait beyond neuropathy
Unilateral foot ulcers and offloading footwear deteriorate gait speed and symmetry
A contralateral shoe lift may improve gait in unilateral offloading users
7. Acknowledgments
We thank Hanger, Inc. for permitting participant recruitment and data collection at their facilities.
6. Funding
Partial support was provided by the National Institutes of Health/National Institute on Aging (Award No. R42-AG032748) and the Qatar National Research Foundation (Award No. NPRP 7-1595-3-405). The content is solely the responsibility of the authors and does not necessarily represent the official views of sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest:
None
9. References
- 1.Boulton AJ, The diabetic foot: a global view. Diabetes/metabolism research and reviews, 2000. 16(S1): p. S2–S5. [DOI] [PubMed] [Google Scholar]
- 2.Yazdanpanah L, Nasiri M, and Adarvishi S, Literature review on the management of diabetic foot ulcer. World journal of diabetes, 2015. 6(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong DG, Boulton AJ, and Bus SA, Diabetic foot ulcers and their recurrence. New England Journal of Medicine, 2017. 376(24): p. 2367–2375. [DOI] [PubMed] [Google Scholar]
- 4.Hicks CW, et al. , Burden of infected diabetic foot ulcers on hospital admissions and costs. Annals of vascular surgery, 2016. 33: p. 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghav A, et al. , Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Therapeutic advances in endocrinology and metabolism, 2018. 9(1): p. 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou H, et al. , Hemodialysis Impact on Motor Function beyond Aging and Diabetes—Objectively Assessing Gait and Balance by Wearable Technology. Sensors, 2018. 18(11): p. 3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernando ME, et al. , Gait parameters of people with diabetes-related neuropathic plantar foot ulcers. Clinical Biomechanics, 2016. 37: p. 98–107. [DOI] [PubMed] [Google Scholar]
- 8.Grewal GS, et al. , Diabetic peripheral neuropathy and gait: does footwear modify this association? 2013, SAGE Publications Sage CA: Los Angeles, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrobel JS and Najafi B, Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol, 2010. 4(4): p. 833–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews RT, et al. , A growing troubling triad: diabetes, aging, and falls. Journal of aging research, 2013 2013. [Google Scholar]
- 11.Kelly C, et al. , Fear of falling is prevalent in older adults with diabetes mellitus but is unrelated to level of neuropathy. Journal of the American Podiatric Medical Association, 2013. 103(6): p. 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves ND, et al. , Aging and type 2 diabetes: consequences for motor control, musculoskeletal function, and whole-body movement. Journal of aging research, 2013 2013. [Google Scholar]
- 13.Morona JK, et al. , Comparison of the clinical effectiveness of different off‐loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta‐analysis. Diabetes/metabolism research and reviews, 2013. 29(3): p. 183–193. [DOI] [PubMed] [Google Scholar]
- 14.Lozano-Platonoff A, et al. , The gold standard in diabetic foot treatment: total contact cast. Gaceta medica de Mexico, 2014. 150(1): p. 58–64. [PubMed] [Google Scholar]
- 15.Albright BC and Woodhull-Smith WM, Rocker bottom soles alter the postural response to backward translation during stance. Gait & posture, 2009. 30(1): p. 45–49. [DOI] [PubMed] [Google Scholar]
- 16.Mrdjenovich DE, Off-loading practices for the wounded foot: concepts and choices. The Journal of the American College of Certified Wound Specialists, 2010. 2(4): p. 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzarini PA, et al. , Measuring Plantar Tissue Stress in People With Diabetic Peripheral Neuropathy: A Critical Concept in Diabetic Foot Management. J Diabetes Sci Technol, 2019: p. 1932296819849092. [DOI] [PMC free article] [PubMed]
- 18.Chakraborty PP, et al. , A comparative study between total contact cast and pressure-relieving ankle foot orthosis in diabetic neuropathic foot ulcers. Journal of diabetes science and technology, 2014. 9(2): p. 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafi B, et al. , Can’t Stand the Pressure: The Association Between Unprotected Standing, Walking, and Wound Healing in People With Diabetes. J Diabetes Sci Technol, 2017. 11(4): p. 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crews RT, Sayeed F, and Najafi B, Impact of strut height on offloading capacity of removable cast walkers. Clin Biomech (Bristol, Avon), 2012. 27(7): p. 725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Deursen R, Footwear for the neuropathic patient: offloading and stability. Diabetes/metabolism research and reviews, 2008. 24(S1): p. S96–S100. [DOI] [PubMed] [Google Scholar]
- 22.Gulgin H, et al. , 3D gait analysis with and without an orthopedic walking boot. Gait & posture, 2018. 59: p. 76–82. [DOI] [PubMed] [Google Scholar]
- 23.Pollo FE, Gowling TL, and Jackson RW, Walking boot design: a gait analysis study. Orthopedics, 1999. 22(5): p. 503–507. [PubMed] [Google Scholar]
- 24.Kipp D, Village D, and Edwards KJ, Effectiveness of Evenup™ Shoe-Lift Use Among Individuals Prescribed a Walking Boot. Journal of allied health, 2017. 46(2): p. 104–110. [PubMed] [Google Scholar]
- 25.Crews RT and Candela J, Decreasing an Offloading Device’s Size and Offsetting Its Imposed Limb Length Discrepancy Lead to Improved Comfort and Gait. Diabetes care, 2018: p. dc172584. [DOI] [PMC free article] [PubMed]
- 26.Alam U, et al. , Diabetic neuropathy and gait: a review. Diabetes therapy, 2017. 8(6): p. 1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allet L, et al. , The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia, 2010. 53(3): p. 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang GE, et al. , Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults. Clin Biomech (Bristol, Avon), 2019. 72: p. 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najafi B, et al. , The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J Am Podiatr Med Assoc, 2013. 103(3): p. 165–73. [DOI] [PubMed] [Google Scholar]
- 30.Pham H, et al. , Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes care, 2000. 23(5): p. 606–611. [DOI] [PubMed] [Google Scholar]
- 31.Zahiri M, et al. , Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults - Toward an early diagnosis of CIPN. J Geriatr Oncol, 2019. 10(6): p. 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou H, et al. , Instrumented Trail-Making Task: Application of Wearable Sensor to Determine Physical Frailty Phenotypes. Gerontology, 2018: p. 1–12. [DOI] [PMC free article] [PubMed]
- 33.Najafi B, Khan T, and Wrobel J, Laboratory in a box: wearable sensors and its advantages for gait analysis. Conf Proc IEEE Eng Med Biol Soc, 2011. 2011: p. 6507–10. [DOI] [PubMed] [Google Scholar]
- 34.Aminian K, et al. , Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech, 2002. 35(5): p. 689–99. [DOI] [PubMed] [Google Scholar]
- 35.Najafi B, et al. , Does walking strategy in older people change as a function of walking distance? Gait & posture, 2009. 29(2): p. 261–266. [DOI] [PubMed] [Google Scholar]
- 36.Lindemann U, et al. , Distance to achieve steady state walking speed in frail elderly persons. Gait & posture, 2008. 27(1): p. 91–96. [DOI] [PubMed] [Google Scholar]
- 37.Dubost V, et al. , Stride-to-stride variability while enumerating animal names among healthy young adults: result of stride velocity or effect of attention-demanding task? Gait Posture, 2008. 27(1): p. 138–43. [DOI] [PubMed] [Google Scholar]
- 38.Aminian K, et al. , Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Posture, 2004. 20(1): p. 102–7. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J, Statistical power analysis for the behavioural sciences. 1988, Hillsdale, NJ: erlbaum. [Google Scholar]
- 40.Bahureksa L, et al. , The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology, 2017. 63(1): p. 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katoulis EC, et al. , Gait abnormalities in diabetic neuropathy. Diabetes Care, 1997. 20(12): p. 1904–7. [DOI] [PubMed] [Google Scholar]
- 42.Akashi PM, et al. , The effect of diabetic neuropathy and previous foot ulceration in EMG and ground reaction forces during gait. Clin Biomech (Bristol, Avon), 2008. 23(5): p. 584–92. [DOI] [PubMed] [Google Scholar]
- 43.Bacarin TA, Sacco IC, and Hennig EM, Plantar pressure distribution patterns during gait in diabetic neuropathy patients with a history of foot ulcers. Clinics (Sao Paulo), 2009. 64(2): p. 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]