Abstract
Objective:
To estimate the risk of a multiple gestation pregnancy in ovarian stimulation intrauterine insemination (IUI) cycles when stratified by patient age and mature follicle number.
Methods:
We conducted a retrospective cohort study at a single private practice fertility center of IUIs performed from 2004 to 2017. Intervention(s) were ovarian stimulation and IUI if post wash total motile sperm count was > 8 million. Mature follicles were defined as ≥ 14 mm as measured on the day of ovulation trigger. Main outcomes and measures were rates of clinical pregnancy and multiple gestation.
Results:
We identified 24,649 women who underwent a total of 50,473 IUI cycles. Increasing the number of mature follicles from 1 to 5 at the time of IUI in women under 38 years of age increased the clinical pregnancy rate from 14.6% to 21.9% (aOR 1.6, 95%CI 1.4–1.9), almost entirely from a marked increase in multiple gestations per cycle from 0.6% to 6.5% (aOR 9.9, 95% CI 6.9–14.2). There was little increase in singleton pregnancies per IUI (14.1–16.4%) regardless of mature follicle number. The per pregnancy twin and higher order multiple gestation risk significantly increased (3.9% to 23.3%, P<0.01 and 0.2% to 10.6%, P<0.01, respectively) when comparing 1 versus 5 mature follicles present at the time of IUI (P<0.01). In women under 38 years with > 3 follicles present, over 1/4 of all pregnancies were multiples. Similar findings occurred in women 38 to 40 years. In women over 40, up to 4 follicles tripled the odds of pregnancy (aOR 3.1, 95%CI 2.1–4.5) while maintaining a less than 12% risk of multiple gestation per pregnancy, and a 1.0% absolute risk of multiples.
Conclusion:
Caution should be used in proceeding with IUI following ovarian stimulation when there are > 2 mature follicles in women under the age of 40 due to the substantially increased risk of multiple gestation without an improved chance of singleton clinical pregnancy.
Précis:
Ovulation induction with > 2 mature follicles in women under 40 substantially increases the risk of multiple gestation without improved singleton rates.
INTRODUCTION
Multiple gestation is associated with increased maternal and fetal morbidity and mortality. Fetal loss is as high as 5% for twins and 17% for triplets in the second and third trimesters1. Increased risks include pre-eclampsia, gestational diabetes, preterm labor and delivery2–8, fetal demise during third trimester, preterm birth, low (<2,500 grams) and very low (<1,500 grams) birth weight1,3,5,6,9. Preterm delivery is associated with cerebral palsy, retinopathy, bronchopulmonary dysplasia, polycythemia, hypoglycemia, and necrotizing enterocolitis10. The leading causes of maternal death in industrialized countries (pre-eclampsia, thromboembolic events, and postpartum hemorrhage) are nearly 3-fold higher in multiple gestations11. Twin and triplet gestations are associated with a 4-fold and 6-fold increased risk of perinatal mortality, respectively11,12.
Ovulation induction or ovarian stimulation with intrauterine insemination is a first line treatment for many types of infertility. However, the incidence of twins and high-order multiples resulting from ovarian stimulation has been reported to be over 20 and 100 times greater than natural conception births, respectively13. Multiple other studies report that ovulation induction by ovarian stimulation largely contribute to the observed rates of multiple gestation14–19. Current recommendations set forth by the American Society of Reproductive Medicine are to induce ovulation of 1 or 2 mature follicles (i.e. ovulation induction) in anovulatory patients such as women with polycystic ovarian syndrome (PCOS) or hypothalamic amenorrhea, and “multiple (often greater than 2) mature follicles” (i.e. ovarian stimulation) in patients with unexplained infertility or age-related subfertility in order to increase cycle fecundity10. Some prior studies have indicated that the number and size of mature follicles measured is unhelpful in predicting multiple gestation and there are not clear criteria set forth to avoid multiples20,21. Other studies demonstrate that increasing numbers of mature follicles are associated with both increased likelihood of pregnancy and increased risk of multiple gestation22–24. As suggested by a meta-analysis involving 11,599 ovarian stimulation with intrauterine insemination cycles, pregnancy rates increased by 5%, 8%, and 8% when recruiting two, three, and four mature follicles respectively. However, the increase in follicle number was associated with the risk of increasing multiples rate by 6%, 14%, and 10%, respectively24. The current literature is limited in that female age, the greatest predictor of fecundity, is often not accounted for in predicting the likelihood of pregnancy and multiple gestation.
Overall, there is a lack of data on the age of the patient and follicle number stratified risks of pregnancy and multiple gestation in IUI cycles with ovarian stimulation. Lack of such data may result in ambiguity in clinical judgement when deciding whether a cycle should be canceled based on the number of mature follicles present in order to prevent the risks associated with a multiple gestation. Establishing age-based data regarding mature follicle number on the day of trigger can enable more patients to safely achieve a singleton pregnancy prior to considering further intervention and expense, such as in vitro fertilization (IVF). This study evaluated the risk of a multiple gestation pregnancy based on the number of mature follicles on the day of ovulation trigger and patient age, in IUI cycles with ovarian stimulation.
METHODS:
All ovarian stimulation-or ovulation induction-IUI cycles were performed at Shady Grove Fertility Center, a private practice fertility center in Rockville, MD. This study was performed with Institutional Review Board approval (Advara CIRBI, Pro00027148). All clinical data was stored in the same electronic medical record system during the study period. The same clinical variables were collected over the entire period of the study. The electronic medical record was queried to capture all IUI cycles and desired variables were extracted. All ovarian stimulation- or ovulation induction-IUI cycles were included regardless of stimulation protocol (clomiphene citrate or letrozole or gonadotropins or combination). These are referred to going forward as ovulation induction-IUI cycles. Ovarian stimulation and ovulation induction protocols did not change during the study period. Ovulation induction medications were prescribed per the discretion of the health care provider. Letrozole (2.5–7.5 milligrams (mg)), clomiphene citrate (50–150 mg), recombinant follitropin or menotropin (75 international units(IU)) were initiated on cycle day 3 after a baseline pelvic ultrasound was performed, and taken daily for 5 days unless inadequate follicle response. If gonadotropins were supplemented to either clomiphene or letrozole cycles, 75 IU of follitropin or menotropin were added on cycle days 8 to 10, after completion of a 5 day regimen of an oral agent. A follicle scan was performed between cycle day 9 and 12, and then every 1 to 3 days as needed until the lead follicle reached 18–20 millimeters (mm) in greatest diameter. All patients received recombinant human chorionic gonadotropin injections (250 microgram subcutaneous or 10,000 units intramuscular per health care provider preference) when the lead follicle was 18–20mm If a serum luteinizing hormone was obtained and a surge was noted to have occurred (> 20 IU), the trigger was omitted. The BMI cut off was above 44 kilogram per meter2. All infertility diagnoses outside of significant male factor were included. To exclude significant male factor infertility, cycles with a post wash count of < 8 million total motile sperm were excluded, as the pregnancy rates were stable over 8 million motile sperm25. A subgroup analysis was performed in patients with unexplained infertility, as multifollicular recruitment may be used more frequently in this patient group as a strategy to increase pregnancy rates. Additional analyses were also done on patients with polycystic ovarian syndrome (PCOS) or oligo-ovulation Clinical pregnancy was defined as the presence of an intrauterine gestational sac with fetal cardiac activity, and multiple gestation rates were defined as the presence of two or more intrauterine gestational sacs with fetal cardiac activity per IUI cycle. Multiple gestations were further analyzed by twin gestation and high order multiple gestation. Data was missing on infertility diagnosis for 2,264 cycles (4.4%). These cycles were included in the overall study analysis but not the subgroup analysis of unexplained infertility and anovulation.
Duration of infertility was defined as the number of reported months without contraception while being sexually active. The relationship of duration of infertility with clinical pregnancy and multiple gestation was assessed by subgroups (<12 months, 12–23 months, 24–25 months, and ≥36 months) and as a continuous variable. Year of treatment was assessed to examine if practice pattern changes (for example more letrozole use in later years) was associated with differences in clinical pregnancy or multiple gestation.
Mature follicles were defined as those measuring ≥ 14 mm on the day of trigger.22,26 The total number of follicles ≥ 14 mm was recorded as a field in each patient’s medical record. Follicles <14mm on the day of trigger were not recorded in the majority of patient records. Participants were initially stratified using standard SART (Society for Assisted Reproductive Technology) age ranges (< 35 years, 35–37 years, 38–40 years, 41–42 years, > 42 years)27. However, the decision was made to collapse age categories (< 38 years, 38–40 years, and > 40 years) due to similar results in the <35 and 35–37 year old age groups (Figure 1).
Figure 1:
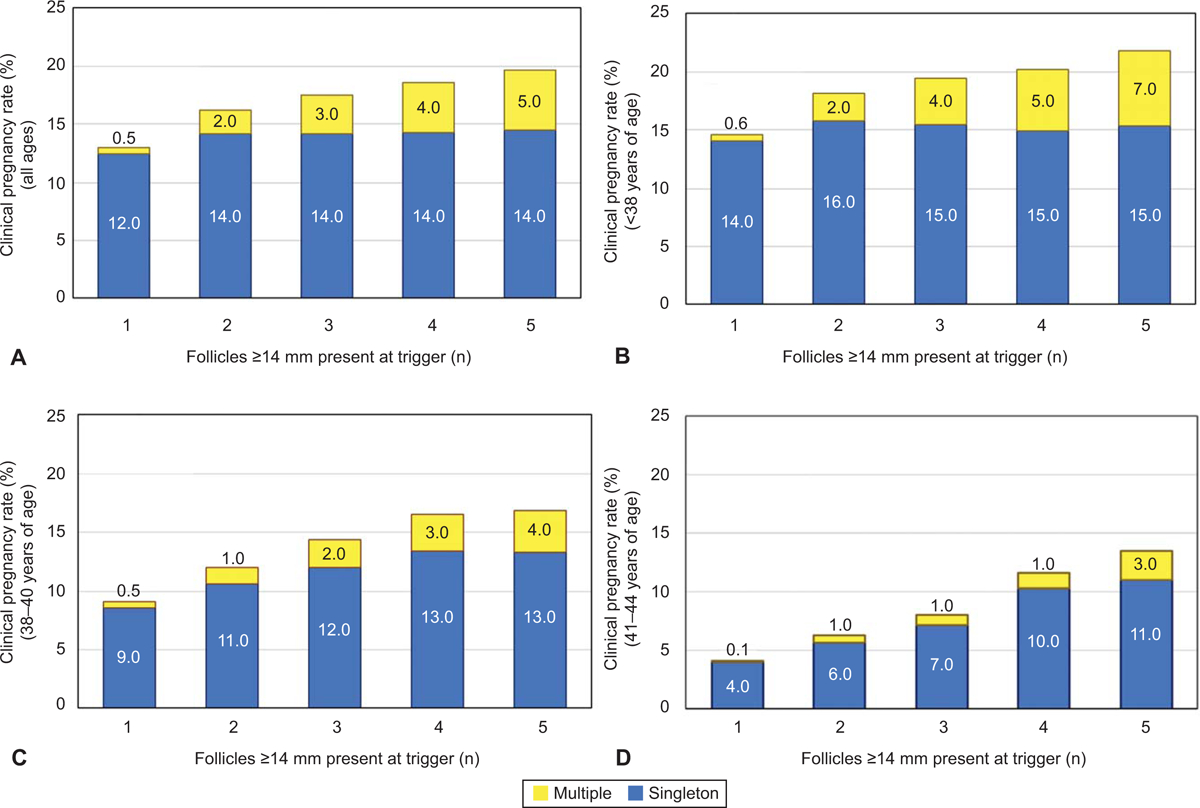
Clinical pregnancy, singleton, and multiple pregnancy rates per intrauterine insemination (IUI) in patients of all ages (A), patients <38 years of age (B), 38–40 years of age (C), and 41–44 years of age (D). The total height of each column represents the total clinical pregnancy rate per IUI based on follicle number and further divided into singleton (blue) and multiples (yellow) per IUI. The yellow columns are also the absolute risk of a multiple gestation per IUI, further categorized by number of follicles (1–5) that are ≥14 mm on the day of ovulation trigger. Generalized estimating equations were used to adjust for multiple cycles per patient.
Baseline characteristics, clinical and multiple pregnancy rates were examined using chi square and Student’s t-test as indicated. To adjust for repeated IUI cycles in the same patient, generalized estimating equations were used to assess the odds of clinical pregnancy and multiple gestation per IUI and multiple gestation per pregnancy and presented as odds ratios with 95% confidence intervals. Duration of infertility (in months) was included in all adjusted models as a clinically relevant covariate. All analysis was completed using STATA (StataCorp LLC, College Station, TX). Statistical significance was considered at P<0.05.
RESULTS:
We identified 24,649 women who had undergone a total of 50,473 IUI cycles from 2004 to 2017. There were 16,837 cycles (33.4%) with 1 mature follicle, 16,598 cycles (32.9%) with 2 mature follicles, 10,534 cycles (20.9%) with 3 mature follicles, 4,805 cycles (9.5%) with 4 mature follicles, and 1,699 cycles (3.3%) with 5 mature follicles (Table 1). The majority of cycles used clomiphene citrate alone (28.4%), and 40.8% of all cycles used clomiphene citrate supplemented by gonadotropins. Letrozole alone was used in 6.1% of all cycles, letrozole plus gonadotropins in 0.4%, and 1.4% were natural cycles without stimulation. Gonadotropins alone were used in 22.9% of cycles. The majority of the patients were diagnosed with unexplained infertility (39.9%), followed by polycystic ovarian syndrome (PCOS) or oligo-ovulation (20.1%), exclusively male factor (9.2%), diminished ovarian reserve (7.9%), utilization of donor sperm (5.6%) (i.e., same-sex couples and single patients), and endometriosis (2.5%). The remainder of the patients (14.8%) were categorized in our data set as “other” (i.e., sexual aversion from pelvic pain, cervical stenosis, or diagnosis not entered). The mean duration of infertility was 16 months (range: 0 to 276 months). There was no difference in clinical pregnancy or multiple gestation based on duration of infertility. Additionally, the year of treatment assessment analysis did not change any outcomes over the study period, indicating the relatively stable outcome results in IUI cycles over the study period.
Table 1:
Number of patients (n) with singleton or multiple pregnancy, and clinical pregnancy rate by follicle number. Including all ages, < 38 years of age, 38–40 years of age, and greater than 40 years of age. Adjusted odds ratio (aOR, 95% CI) indicate the increasing risk of a multiple pregnancy with increasing follicle number, with one follicle as the reference. Generalized estimating equations were used to adjust for multiple cycles per patient. Denominator in the clinical pregnancy ratio represents the total number of patients in that category who had an IUI performed.
| 1 Follicle | 2 Follicles | 3 Follicles | 4 Follicles | 5 Follicles | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | aOR (95%CI) | n | % | aOR (95%CI) | n | % | aOR (95%CI) | n | % | aOR (95%CI) | n | % | aOR (95%CI) | ||
| All Patients | Singleton | 2094 | 95.8 | 2343 | 87.1 | 1489 | 80.7 | 678 | 76.4 | 243 | 73.0 | |||||
| Multiple | 91 | 4.2 | Referent | 348 | 12.9 | 3.46 (2.72–4.39) | 355 | 19.3 | 5.55 (4.36–7.06) | 210 | 23.6 | 7.21 (5.55–9.37) | 90 | 27.0 | 8.60 (6.23–11.85) | |
| Clinical Pregnancy | 2185/16,837 | 13.0 | Referent | 2691/16598 | 16.2 | 1.30 (1.22–1.38)) | 1844/10534 | 17.5 | 1.42 (1.33–1.52 | 888/4805 | 18.5 | 1.52 (1.39–1.65) | 333/1699 | 19.6 | 1.63 (1.43–1.85) | |
| <38 | Singleton | 1835 | 95.9 | 1989 | 86.8 | 1192 | 79.7 | 499 | 74.3 | 168 | 70.3 | |||||
| Multiple | 78 | 4.1 | Referent | 303 | 13.2 | 3.59 (2.78–4.65) | 303 | 20.3 | 5.99 (4.62–7.77) | 173 | 25.7 | 8.18 (6.15–10.88) | 71 | 29.7 | 9.91 (6.92–14.18) | |
| Clinical Pregnancy | 1913/13077 | 14.6 | Referent | 2292/12612 | 18.2 | 1.30 (1.21–1.39) | 1495/7693 | 19.4 | 1.41 (1.30–1.51) | 672/3341 | 20.1 | 1.46 (1.33–1.61) | 239/1094 | 21.8 | 1.63 (1.40–1.90) | |
| 38–40 | Singleton | 203 | 94.9 | 274 | 88.1 | 229 | 83.6 | 126 | 80.8 | 48 | 78.7 | |||||
| Multiple | 11 | 5.1 | Referent | 37 | 11.9 | 2.49 (1.24–5.00) | 45 | 16.4 | 3.63 (1.83–7.20) | 30 | 19.2 | 4.40 (2.11–9.08) | 13 | 21.3 | 5.00 (2.11–11.83) | |
| Clinical Pregnancy | 214/2373 | 9.0 | Referent | 311/2590 | 12.0 | 1.37 (1.14–1.65) | 274/1901 | 14.4 | 1.70 (1.40–2.05) | 156/947 | 16.5 | 1.98 (1.59–2.48) | 61/361 | 16.9 | 2.03 (1.49–2.77) | |
| >40 | Singleton | 56 | 98.2 | 80 | 90.9 | 68 | 90.7 | 53 | 88.3 | 27 | 81.8 | |||||
| Multiple | 1 | 1.8 | Referent | 8 | 9.1 | 5.60 (0.68–46.04) | 7 | 9.3 | 5.76 (0.69–48.25) | 7 | 11.7 | 7.40 (0.88–62.18) | 6 | 18.2 | 12.46 (1.43–108.77) | |
| Clinical Pregnancy | 57/1385 | 4.1 | Referent | 88/1395 | 6.3 | 1.57 (1.12–2.21) | 75/940 | 8.0 | 2.03 (1.42–2.89) | 60/517 | 11.6 | 3.06 (2.10–4.46) | 33/244 | 13.5 | 3.64 (2.31–5.72) | |
Clinical pregnancy ranged widely from 4.1% to 21.8% per IUI across all age ranges and decreased with increasing age. When evaluating the full cohort, the mean clinical pregnancy rate (CPR) per IUI ranged from 13.0% with 1 mature follicle to 19.6% with 5 mature follicles. The singleton rate per IUI increased by only 1.9% from 12.4% with 1 follicle to 14.3% with 5 follicles, while the odds of multiples increased by a factor of 8.6 (aOR 8.6, 95%CI 6.2–11.8) (Figure 1). With 5 mature follicles present, the per pregnancy twin risk significantly increased from 3.9% to 22.4% (P<0.001) and higher order multiple gestation risk significantly increased from 0.3% to 7.6% (P<0.001) (Figure 2).
Figure 2:
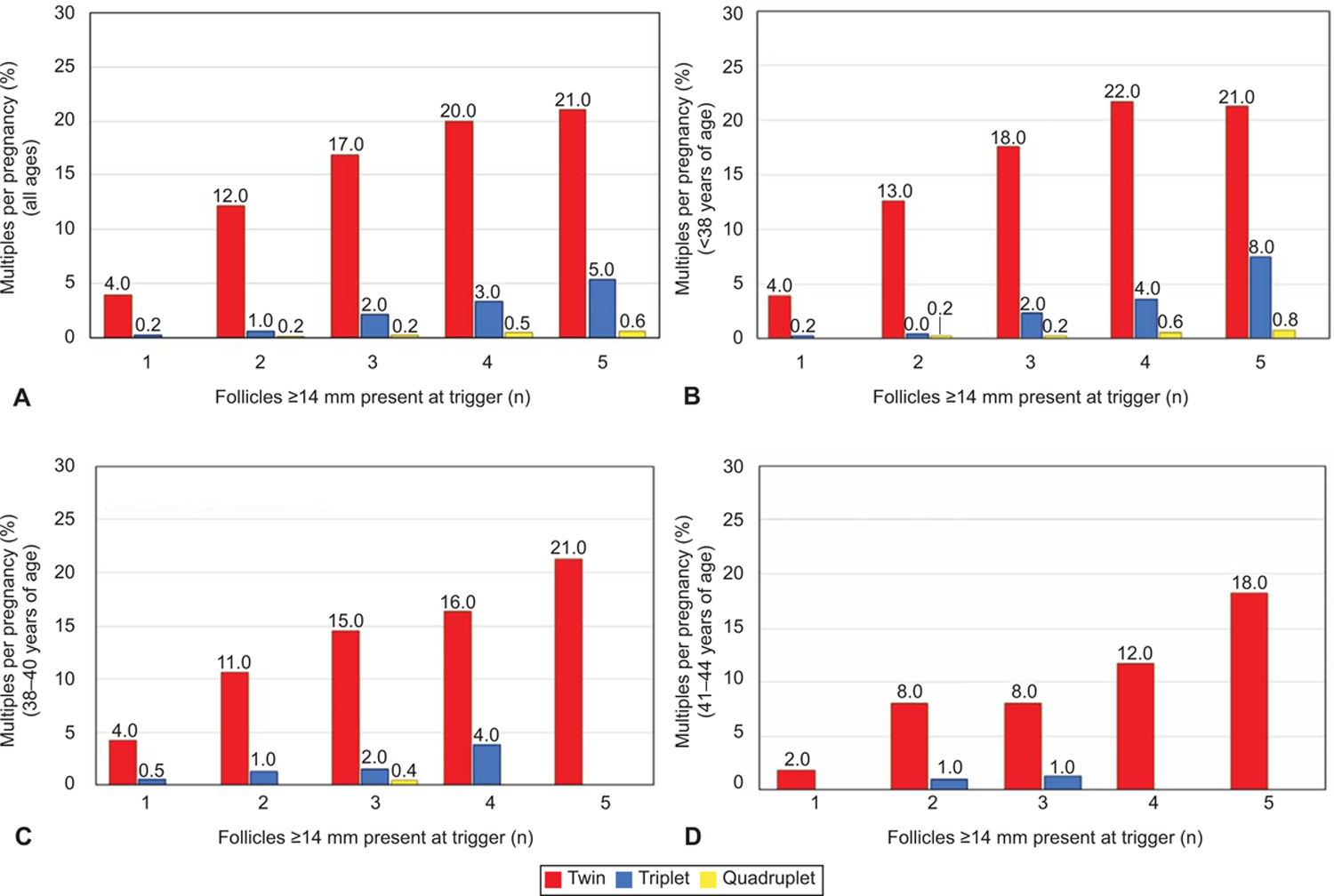
Relative risk of multiples: multiples per pregnancy by follicle number at ovulation trigger in patients of all ages (A), patients <38 years of age (B), 38–40 years of age (C), and 41–44 years of age (D). There were no quadruplets in women 38 years of age and older. Generalized estimating equations were used to adjust for multiple cycles per patient.
In women of all ages, the odds of pregnancy increased modestly with the presence of each additional mature follicle: 2 follicles compared 1 (aOR 1.3, CI 1.2–1.4. P<0.001), 3 follicles compared to 1 (aOR 1.4, CI 1.3–1.5. P<0.001), 4 follicles compared to 1 (aOR 1.5, CI 1.4–1.7. P<0.001), and 5 follicles compared to 1 (aOR 1.6, CI 1.4–1.9. P < 0.001). However, a greater significantly increased risk of multiples was seen with the presence of each additional mature follicle: 2 follicles compared to 1 (aOR 3.5, 95% CI 2.7–4.4. P<0.001), 3 follicles compared to 1 (aOR 5.6, 95% CI 4.4–7.1. P<0.001), 4 follicles compared to 1(aOR 7.2, 95% CI 5.6–9.4, P<0.001) and 5 follicles compared to 1 (aOR 8.6, 95% CI 6.2–11.8, P<0.001).
In women <38 years of age, when the number of mature follicles increased from 1 to 5, the clinical pregnancy rates per IUI increased from 14.6% to 21.9% (aOR 1.6, 95%CI 1.4–1.9), with a significant increase in multiples per IUI from 0.6% to 6.5% (aOR 9.9, 95% CI 6.9–14.2) (Figure 1). There was little increase in singleton pregnancies per IUI (14.1–16.4%) regardless of mature follicle number. With 5 mature follicles, the per pregnancy twin and higher order multiple gestation risk significantly increased (3.9% to 23.3%, P<0.01 and 0.2% to 10.6%, P<0.01, respectively) (Figure 2). In women <38 years of age with > 3 mature follicles, over one quarter of all pregnancies resulted in a multiple gestation.
The chance of pregnancy increased modestly with the presence of each additional mature follicle: 2 mature follicles compared to 1 (aOR 1.3, CI 1.2–1.4. P <0.001), 3 mature follicles compared to 1 (aOR 1.4, CI 1.3–1.5. P <0.001), 4 mature follicles compared to 1 (aOR 1.5, CI 1.3–1.6. P <0.001), and 5 mature follicles compared to 1 (aOR 1.6, CI 1.4–1.9. P <0.001). However, a significantly increased risk of multiples was noted with the presence of each additional mature follicle: 2 follicles compared to 1 (aOR 3.6, 95% CI 2.8–4.6, P<0.001), 3 follicles compared to 1 (aOR 6.0, 95% CI 4.6–7.8, P<0.001), 4 follicles compared to 1 (aOR 8.2, 95% CI 6.2–10.9, P<0.001) and 5 follicles compared to 1 (aOR 9.9, 95% CI 6.9–14.2, P<0.001).
In women 38–40 years of age, increasing mature follicles from 1 to 5 increased the clinical pregnancy rate from 9.5% to 16.9% (aOR 2.0, 95%CI 1.5–2.8) with a marked increase in multiples from 0.5% to 3.6% (aOR 5.0, 95%CI 2.1–11.8) . There was an increase in singleton pregnancies per IUI (8.6–13.3%) with increasing mature follicle number. However, with 5 mature follicles, the per pregnancy twin risk increased significantly (4.3% to 21.3%, P<0.01). There was no significant difference in risk of higher order multiples.
The chance of pregnancy increased modestly with the presence of each additional mature follicle: 2 follicles compared 1 (aOR 1.4, CI 1.1–1.7, P<0.001), 3 follicles compared to 1 (aOR 1.7, CI 1.4–2.1, P<0.001), 4 follicles compared to 1 (aOR 2.0, CI 1.6–2.5, P<0.001), and 5 follicles compared to 1 (aOR 2.0, CI 1.5–2.8, P<0.001). A significant increase in multiples was noted with the presence of additional mature follicles: with 2 follicles compared to 1 (aOR 2.5, 95% CI 1.2–5.0, P<0.001), 3 follicles compared to 1 (aOR 3.6, 95% CI 1.8–7.2, P<0.001), 4 follicles compared to 1 (aOR 4.4, 95% CI 2.1–9.1, P<0.001) and 5 follicles compared to 1 (aOR 5.0, 95% CI 2.1–11.8, P<0.001).
In patients over 40 years of age, increasing the follicle number increased clinical pregnancy without increasing the risk of multiple gestation. Increasing the follicle count from 1 to 5 increased the clinical pregnancy per IUI rate by a factor of 3.6 from 4.1% to 13.5% (aOR 3.6, 95%CI 2.3–5.7). With 5 mature follicles, the per IUI twin and higher order multiple risk increased from 0.1% to 2.5% (aOR 12.5, 95%CI 1.4–108.7) . In women over the age of 40, up to 4 follicles tripled the likelihood of pregnancy (aOR 3.1, 95%CI 2.1–4.5) while maintaining a less than 12% risk of multiple gestation per pregnancy, and a 1.0% absolute risk of multiples. With limited numbers, there was no significant difference in risk of higher order multiples.
Overall, the chance of pregnancy increased at a greater magnitude with the presence of each additional mature follicle, compared to younger age groups: 2 follicles compared 1 (aOR 1.6, CI 1.1–2.2), 3 follicles compared to 1 (aOR 2.0, 95%CI 1.4–2.9), 4 follicles compared to 1 (aOR 3.1, CI 2.1–4.5), and 5 follicles compared to 1 (aOR 3.6, CI 2.3–5.7). In contrast to the younger age groups, a significant increase in multiples was not noted until the presence of 5 follicles compared to 1 (aOR 12.5, 95%CI 1.4–108.8).
The diagnosis comprising the largest portion of patients in our data set was unexplained infertility (39.9%, n = 20,153 cycles). Three-quarters of these IUI cycles used either clomiphene alone or gonadotropins plus clomiphene, 19.5% used gonadotropins alone, and 5.2% used either IUI alone or letrozole plus IUI. When limiting to women with unexplained infertility, all age groups revealed a similar trend to the overall cohort with increased chance of clinical pregnancy and multiple pregnancy with each additional follicle. The only notable difference was seen in women < 38 years of age with unexplained infertility: the singleton clinical pregnancy rate per IUI with 1 follicle present was 11.8% (compared to 14.1% in the entire cohort of women < 38 years of age). Trends were otherwise similar in both singleton and multiple pregnancies. In all age groups with unexplained infertility, increasing the number of mature follicles from 1 to 5 had a similar increase in clinical pregnancy: 2 follicles compared to 1 (aOR 1.5, CI 1.4–1.7), 3 follicles compared to 1 (aOR 1.8, CI 1.6–2.0), 4 follicles compared to 1 a(OR 1.8, CI 1.5–2.0), and 5 follicles compared to 1 (aOR 2.0, CI 1.6–2.4). However, all age groups also revealed a similar increased risk of multiples with each increasing mature follicle number: with 2 follicles compared to 1 (aOR 3.7, 95% CI 2.4–5.7), 3 follicles compared to 1 (aOR 5.4, 95% CI 3.5–8.3), 4 follicles compared to 1 (aOR 7.0, 95% CI 4.4–11.1) and 5 follicles compared to 1 (aOR 7.1, 95% CI 4.1–12.3).
Additional analyses were done on patients with polycystic ovarian syndrome (PCOS) or oligo-ovulation (20.1%, n = 10,089 cycles). Of these IUI cycles, 36.7% used clomiphene citrate alone, 30.0% used gonadotropins alone, 23.0% used gonadotropins plus clomiphene, 8.5% used letrozole alone, and the remainder used letrozole plus gonadotropins. When limiting to women with ovulatory disorders, all age groups revealed a similar trend to the overall cohort with increased chance of clinical pregnancy and multiple pregnancy with each additional follicle. In all age groups with ovulatory disorders, increasing the number of mature follicles from 1 to 5 had a similar increase in clinical pregnancy: 2 follicles compared to 1 (aOR 1.3, CI 1.1–1.4), 3 follicles compared to 1 (aOR 1.5, CI 1.3–1.7), 4 follicles compared to 1 (aOR 1.8, CI 1.5–2.1), and 5 follicles compared to 1 (aOR 1.6, CI 1.2–2.1). However, all age groups also revealed a significantly increased risk of multiples with each increasing mature follicle number: with 2 follicles compared to 1 (aOR 3.9, 95% CI 2.5–6.0), 3 follicles compared to 1 (aOR 8.2, 95% CI 5.2–12.7), 4 follicles compared to 1 (aOR 12.5, 95% CI 7.7–20.5) and 5 follicles compared to 1 (aOR 14.3, 95% CI 7.7–26.7).
The tradeoff between the relative increase in clinical pregnancy rate and increasing frequency of multiples per pregnancy was evaluated graphically. In women < 38 years, 2 mature follicles increased the odds of pregnancy by 30% (aOR 1.3, 95%CI 1.2–1.4) compared to a single follicle. However, beyond 2 follicles, the absolute increase in clinical pregnancy was negligible (4%) with a 4.9–7.2 fold increase in multiple pregnancy with 3 or more follicles. In women 38–40 years of age, the relative increase in pregnancy rate was much higher than patients in the younger group. However, the risk of multiples per pregnancy also steadily increased with increasing follicle number. Above 3 follicles, the relative increase in pregnancy was 15% with nearly a 20% risk of multiples per pregnancy. There was no benefit in relative increase in pregnancy rates when more than 4 follicles were present, yet multiple pregnancy risk increased further to 30%. In women over 40 years of age, the odds of pregnancy nearly tripled by pushing to 4 or more follicles (aOR 3.1, 95%CI 2.1–4.5) while maintaining a less than 12% risk of multiples per pregnancy until a 5th follicle was present. With 5 follicles present, the risk of multiples increased to 18% per pregnancy (Figure 2). The number of prior IUI’s per patient ranged from 0 to 12, with a mean of 2.2. Additionally, the risk of multiples persisted regardless of whether it was the patient’s first or last cycle recorded during this time frame.
DISCUSSION:
This large retrospective study reveals that caution should be used in proceeding with IUI when more than 2 mature follicles are seen in women under the age of 40 due to the substantially increased risk of multiple gestation without an improved chance of singleton clinical pregnancy. Deciding whether to cancel or proceed based upon follicular response can present a clinical quandary. Despite the known increased risk of multiples with increasing mature follicle number, previous literature does not differentiate an age specific risk of multiples based on follicle number. In contrast to in vitro fertilization where multiple pregnancy may be controlled by the number of embryos transferred, in IUI with ovarian stimulation, health care providers have little control over reducing the occurrence of multiples during stimulation outside of cancelling the treatment cycle or converting to IVF. A lack of clear guidance when several mature follicles develop can yield a difficult clinical decision for the patient and health care provider, and a resulting multiple gestation poses a potentially dangerous maternal and/or neonatal outcome. Singleton pregnancy per IUI is increasingly being considered a health care quality measure due to the morbidities and mortalities that can be associated with multiples. Furthermore, this retrospective cohort study provides a valuable resource for clinicians to help minimize the risk of multiples in IUI with ovarian stimulation cycles, and to counsel patients based upon age and mature follicle number.
This study reports multiples per IUI, but it also reports the multiples per pregnancy. The reporting of twin and higher order gestations per pregnancy rather than twin, triplets, or quadruplets per IUI better emphasizes the substantial risk for multiples. For example, if a health care provider is counseling a 35 year old patient with 3 mature follicles that she has a 3% absolute risk of multiples per IUI, this risk is much more clearly conveyed if it is stated that the relative risk of a multiple gestation (percent chance of a multiple gestation if she becomes pregnant) is at least 20%. Therefore, reporting multiples per pregnancy helps to express the risk. Heat maps (Figure 3 example from our data) may facilitate counseling of patient’s chances of clinical pregnancy, absolute risk of multiple gestation, and relative risk of multiple gestation and allow for personalized medical care. It is important to consider, that although this is a large retrospective study, the data were extrapolated from a single site which may limit the generalizability of these data to the varying success rates achieved at practices.
Figure 3:
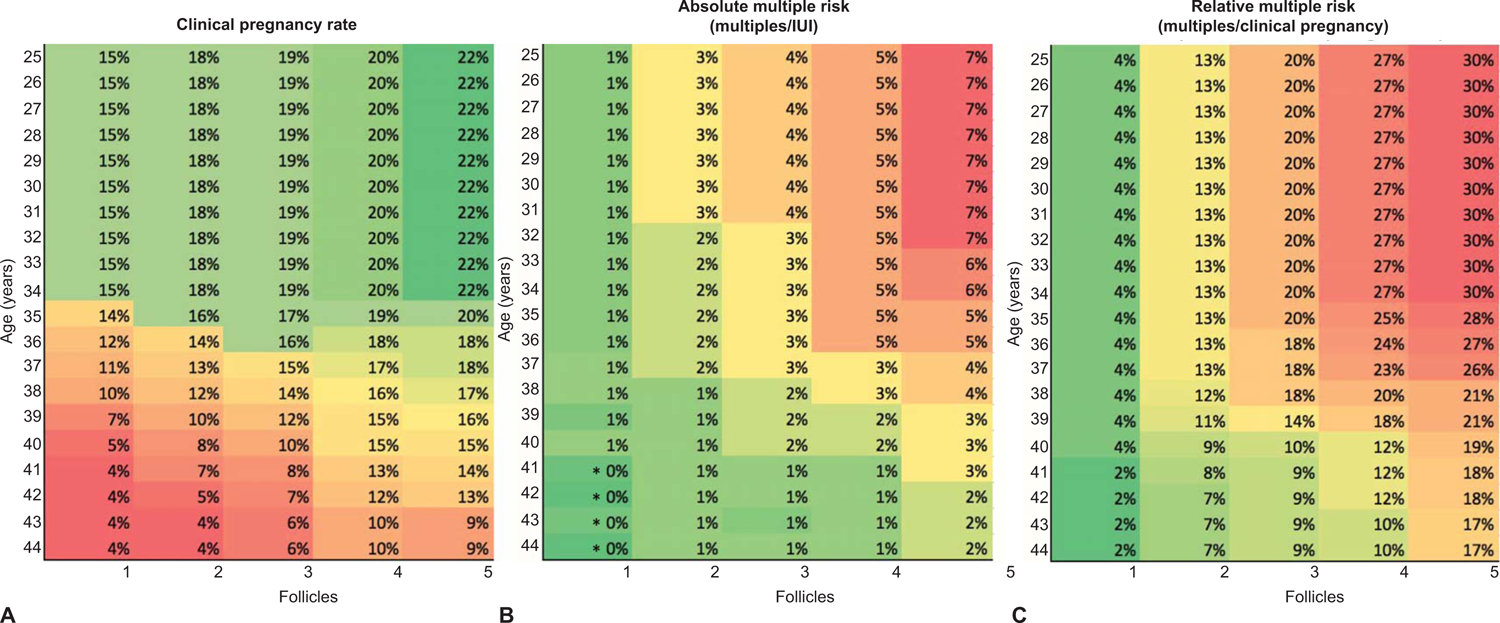
Heat maps to guide clinical decisions and counseling patients on the risks versus benefits of their pregnancy outcome. Clinical pregnancy rate (A), absolute multiple risk: multiples/intrauterine insemination (IUI) (B), and relative multiple risk: multiples/clinical pregnancy (C). The red region indicates low pregnancy success or high multiples risk, yellow indicates moderate pregnancy success or multiples risk, and green indicates highest success in acquiring a pregnancy or lowest multiples risk. Follicle number across the bottom of the graphs are ≥14 mm in size. This tool can counsel the patient, based on her age and number of follicles present, what her overall clinical pregnancy rate is, absolute multiple risk (multiples/IUI), and if she became pregnant, what her risk of multiples would be (relative risk). *Percentages are rounded to the closest whole number and represent mean outcomes from the study. 95% CI of the actual risk are not shown.
Our results reveal a relatively low risk of multiples even with higher numbers of follicles in women 41–44 years of age. The effects of aging on oocyte quality likely account for this discrepancy seen in the older patients. Clinical pregnancy rates per IUI cycle increase with increasing mature follicle number, but only modestly in younger patients. Singleton pregnancy rates per IUI cycle change very little with increasing mature follicles, especially for patients under age 38. Women 38–40 years of age have a slight increase in singleton pregnancy rates per IUI with increasing mature follicle number, but the risk of multiples is above 17% when over 2 follicles are present, and increases to 21% per pregnancy with 5 follicles present.
We observed little evidence to suggest any increase in the probability of achieving a singleton pregnancy with more than two follicles present, unless over 40 years of age. Higher quality oocytes in younger women may increase the risk of multiple gestation when multiple follicles are present. The risk of multiples is high (>13% per pregnancy) for women up to 40 years with more than one follicle, and very high (>27% per pregnancy) among women under 38 years with four or more follicles. The relative risk of triplets is as high as 3% to 10% among patients under 38 years with 3 to 5 follicles, respectively, and 2% to 4% in patients 38–40 years as well. It is crucial that patients are aware of these risks when undergoing ovulation induction to avoid the morbidity and/or mortalities and financial burden associated with multiple gestation pregnancies.
Strengths of this study include its high volume of clinical data and categorization of multiple pregnancy risk by age and follicle number. With over 50,000 cycles for analysis, there was adequate power to provide robust estimates for the risks in most subgroup analyses and these numbers may have utility in counseling patients. The accompanying figures also may serve as tools that can be used to show patients visual estimates of their risks and benefits. An additional strength of our study was that our analysis included all follicles 14mm and larger. Although larger follicles are typically considered in clinical management when deciding when to administer an HCG trigger to induce ovulation or recommend timed intercourse, the accompanying smaller follicles present should not be discounted and can lead to an increased risk of a multiple pregnancy as well. Prior studies have shown that pregnancies can occur in cycles with follicles of < 15 mm22,26. In contrast, we acknowledge as weakness that we were unable to analyze additionally the contribution of follicles <14mm to the likelihood of pregnancy and multiple gestations.
Clinical paradigms in managing ovarian stimulation historically have been directed toward inducing mono-follicular development in anovulatory patients versus trying to induce multi-follicular development in patients with unexplained infertility. Subgroup analyses in this study demonstrated that multifollicular development resulted in higher odds of clinical pregnancy in patients with unexplained infertility, but also resulted in a higher odds of multiple gestation in anovulatory patients. While these results support the historical paradigm, it should be noted that the 95%CI of most of these estimates overlapped, precluding a definitive conclusion. However, in both groups the increased odds of multiple gestation were greater than the increased odds of clinical pregnancy. This suggests that caution should be employed when considering IUI in all patients with more than two follicles, regardless of the diagnosis, if the goal is to achieve a singleton pregnancy. Another clinical paradigm is that the duration of infertility justifies the stimulation of more mature follicles. When evaluating duration of infertility as a continuous variable and as a categorical variable (<12, 12–23, 24–35, and ≥36 months) and adjusting for patient age, duration of infertility was not associated with either clinical pregnancy or multiple gestation. These data suggest caution should be used in aggressive ovarian stimulation based on duration on infertility.
One weakness of our study is that it does not categorize outcomes based on the patient’s diagnosis, aside from the subanalyses performed of patients with unexplained infertility and ovulatory dysfunction. Additionally, multiple studies in the van Rumste et al meta-analysis included patients only diagnosed with unexplained infertility29–31or unexplained infertility with “mild male factor infertility”32–34. Another weakness is that infertility was grouped according to broad diagnoses categories, which may represent a heterogenous group of patients with a wide range of prognosis for fecundity and multiple gestation. Uterine factor and tubal factor infertility are two examples of broad diagnoses categories that would have a wide range of disease states. We did not subdivide infertility groups into smaller specific etiologies, as the number of categories would become very large and power would be lost to detect meaningful differences. Further limitations may include the use of IUI in anovulatory patients, where ovulation induction alone increases pregnancy. The first line ovulation induction agent for PCOS changed during this study period from clomiphene citrate to letrozole based published literature35. However, the risk of multiple gestation remained similar across the timeline of this study, suggesting the number of follicles that develop infers the risk of multiple gestation. A high number of follicles, regardless of the medication used to stimulate them, inferred a greater risk of multiple gestation.
Lastly, based on our data set, we were unable to evaluate outcomes further than when clinical pregnancy was routinely documented (approximately 7 weeks estimated gestational age). It is still possible that patients may miscarry past this point, despite having fetal cardiac activity at approximately 7 weeks estimated gestational age. This is particularly more plausible in advanced maternal age patients, as one of the most common etiologies of early pregnancy loss is advanced maternal age36. Pregnancy loss ranges from 20–40% from ages 35–40, and increases as high as 80% at age 4536,37. Although these limitations should be discussed when counseling the patient, we feel that the risks presented in our data set our certainly not negligible.
Supplementary Material
Acknowledgments
This research was supported by the Division of Intramural Research: Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda MD.
Footnotes
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U. S. Government.
Presented at the ACOG Armed Forces District meeting in Honolulu, HI, September 25th, 2018 and the American Society of Reproductive Medicine conference in October 8th, 2018 in Denver, CO.
Financial Disclosure
Micah Hill served on the advisory board of and received personal fees from Ohana Biosciences. Kevin S. Richter disclosed that he was a paid consultant for EMD Serono. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES:
- 1.D’Alton ME, Mercer BM. Antepartum management of twin gestation: ultrasound. Clin Obstet Gynecol 1990;33:42–51. [DOI] [PubMed] [Google Scholar]
- 2.Adams DM, Sholl JS, Haney EI, Russell TL, Silver RK. Perinatal outcome associated with outpatient management of triplet pregnancy. Am J Obstet Gynecol 1998;178:843–7. [PubMed] [Google Scholar]
- 3.Multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Practice Bulletin No. 169. American College of Obstetricians and Gynecologists. Obstet Gynecol 2016;128:e131–46. [DOI] [PubMed] [Google Scholar]
- 4.Henderson CE, Scarpelli S, LaRosa D, Divon MY. Assessing the risk of gestational diabetes in twin gestation. J Natl Med Assoc 1995;87:757–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, MacDorman MF, Mathews TJ. Triplet births: trends and outcomes, 1971–94. Vital Health Stat 21 1997:1–20. [PubMed] [Google Scholar]
- 6.Martin JA, Park MM, Sutton PD. Births: preliminary data for 2001. Natl Vital Stat Rep 2002;50:1–20. [PubMed] [Google Scholar]
- 7.Ombelet W, De Sutter P, Van der Elst J, Martens G. Multiple gestation and infertility treatment: registration, reflection and reaction--the Belgian project. Hum Reprod Update 2005;11:3–14. [DOI] [PubMed] [Google Scholar]
- 8.Wein P, Warwick MM, Beischer NA. Gestational diabetes in twin pregnancy: prevalence and long-term implications. Aust N Z J Obstet Gynaecol 1992;32:325–7. [DOI] [PubMed] [Google Scholar]
- 9.Torok O, Lapinski R, Salafia CM, Bernasko J, Berkowitz RL. Multifetal pregnancy reduction is not associated with an increased risk of intrauterine growth restriction, except for very-high-order multiples. Am J Obstet Gynecol 1998;179:221–5. [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of the American Society for Reproductive M. Multiple pregnancy associated with infertility therapy. Fertil Steril 2006;86:S106–10. [DOI] [PubMed] [Google Scholar]
- 11.WM C, MK E, Saiyi P, Qiuying Y, Wu WS. SHORT COMMUNICATION: Adverse maternal outcomes in multifetal pregnancies. BJOG: An International Journal of Obstetrics & Gynaecology 2004;111:1294–6. [DOI] [PubMed] [Google Scholar]
- 12.Multiple gestation pregnancy. The ESHRE Capri Workshop Group. Hum Reprod 2000;15:1856–64. [PubMed] [Google Scholar]
- 13.Adashi EY, Barri PN, Berkowitz R, et al. Infertility therapy-associated multiple pregnancies (births): an ongoing epidemic. Reprod Biomed Online 2003;7:515–42. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease C, Prevention. Contribution of assisted reproductive technology and ovulation-inducing drugs to triplet and higher-order multiple births--United States, 1980–1997. MMWR Morb Mortal Wkly Rep 2000;49:535–8. [PubMed] [Google Scholar]
- 15.Cook JL, Geran L, Rotermann M. Multiple births associated with assisted human reproduction in Canada . J Obstet Gynaecol Can 2011;33:609–16. [DOI] [PubMed] [Google Scholar]
- 16.Dickey RP. The relative contribution of assisted reproductive technologies and ovulation induction to multiple births in the United States 5 years after the Society for Assisted Reproductive Technology/American Society for Reproductive Medicine recommendation to limit the number of embryos transferred. Fertil Steril 2007;88:1554–61. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni AD, Jamieson DJ, Jones HW Jr., et al. Fertility treatments and multiple births in the United States. N Engl J Med 2013;369:2218–25. [DOI] [PubMed] [Google Scholar]
- 18.Martin JA, Hamilton BE, Osterman MJ. Three decades of twin births in the United States, 1980–2009. NCHS Data Brief 2012:1–8. [PubMed] [Google Scholar]
- 19.Schieve LA, Devine O, Boyle CA, Petrini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to US singleton and multiple births. Am J Epidemiol 2009;170:1396–407. [DOI] [PubMed] [Google Scholar]
- 20.Fong SA, Palta V, Oh C, Cho MM, Loughlin JS, McGovern PG. Multiple pregnancy after gonadotropin-intrauterine insemination: an unavoidable event? ISRN Obstet Gynecol 2011;2011:465483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karuppaswamy J, Smedley M, Carter L. Intra-uterine insemination: pregnancy rate in relation to number, size of pre-ovulatory follicles and day of insemination. J Indian Med Assoc 2009;107:141–3, 7. [PubMed] [Google Scholar]
- 22.Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Relationship of follicle numbers and estradiol levels to multiple implantation in 3,608 intrauterine insemination cycles. Fertil Steril 2001;75:69–78. [DOI] [PubMed] [Google Scholar]
- 23.Moosavifar N, Aliakbarzadeh M, Shakeri M. Association of the number of follicles of various sizes and treatment protocol with multiple pregnancies following ovulation induction and intrauterine insemination. J Pak Med Assoc 2008;58:18–22. [PubMed] [Google Scholar]
- 24.van Rumste MM, Custers IM, van der Veen F, van Wely M, Evers JL, Mol BW. The influence of the number of follicles on pregnancy rates in intrauterine insemination with ovarian stimulation: a meta-analysis. Hum Reprod Update 2008;14:563–70. [DOI] [PubMed] [Google Scholar]
- 25.Rubin RS, Richter KS, Naeemi F, Shipley S, Shin P. Redefining and clarifying the relationship between total motile sperm counts (TMSC) and intrauterine insemination (IUI) pregnancy rates. Fertility and Sterility 2015;104:e9. [DOI] [PubMed] [Google Scholar]
- 26.Richmond JR, Deshpande N, Lyall H, Yates RW, Fleming R. Follicular diameters in conception cycles with and without multiple pregnancy after stimulated ovulation induction. Hum Reprod 2005;20:756–60. [DOI] [PubMed] [Google Scholar]
- 27.SART National Summary Report. 2017.
- 28.Guzick DS, Sullivan MW, Adamson GD, et al. Efficacy of treatment for unexplained infertility. Fertil Steril 1998;70:207–13. [DOI] [PubMed] [Google Scholar]
- 29.Iberico G, Vioque J, Ariza N, et al. Analysis of factors influencing pregnancy rates in homologous intrauterine insemination. Fertil Steril 2004;81:1308–13. [DOI] [PubMed] [Google Scholar]
- 30.Steures P, van der Steeg JW, Hompes PG, et al. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;368:216–21. [DOI] [PubMed] [Google Scholar]
- 31.van Rumste MM, den Hartog JE, Dumoulin JC, Evers JL, Land JA. Is controlled ovarian stimulation in intrauterine insemination an acceptable therapy in couples with unexplained non-conception in the perspective of multiple pregnancies? Hum Reprod 2006;21:701–4. [DOI] [PubMed] [Google Scholar]
- 32.Goverde AJ, Lambalk CB, McDonnell J, Schats R, Homburg R, Vermeiden JP. Further considerations on natural or mild hyperstimulation cycles for intrauterine insemination treatment: effects on pregnancy and multiple pregnancy rates. Hum Reprod 2005;20:3141–6. [DOI] [PubMed] [Google Scholar]
- 33.Khalil MR, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. Homologous intrauterine insemination. An evaluation of prognostic factors based on a review of 2473 cycles. Acta Obstet Gynecol Scand 2001;80:74–81. [DOI] [PubMed] [Google Scholar]
- 34.Sikandar R, Virk S, Lakhani S, Sahab H, Rizvi J. Intrauterine insemination with controlled ovarian hyperstimulation in the treatment of subfertility. J Coll Physicians Surg Pak 2005;15:782–5. [PubMed] [Google Scholar]
- 35.ACOG Committee Opinion No. 738: Aromatase Inhibitors in Gynecologic Practice. Obstet Gynecol 2018;131:e194–e9. [DOI] [PubMed] [Google Scholar]
- 36.Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103–11. [DOI] [PubMed] [Google Scholar]
- 37.Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


