Abstract
Selenium, zinc and chromium are essential micronutrients. Their alterations have been associated with HIV disease progression, metabolic complications and mortality.
This is a cross-sectional study in children with perinatally acquired HIV (PHIV, n=57), HIV exposed uninfected (HEU, n=59) and HIV unexposed uninfected (HIV-, n=56) children aged 2-10 years old, age and sex matched, enrolled in Uganda. PHIV were on stable antiretroviral therapy (ART) with undetectable viral load. We measured plasma concentrations of selenium, zinc and chromium as well as markers of systemic inflammation, monocyte activation, and gut integrity.
Among PHIV children, 93% had viral load ≤ 20 copies/mL, median CD4 was 37% and 77% were receiving a non-nucleotide reserve transcriptase regimen. Median age of all participants was 8 years and 55% were girls. Median selenium concentrations were higher in PHIV compared to the HEU and HIV- groups (p <0.001), 46% of children overall had low zinc status (p=0.18 between groups). Higher selenium, but not chromium or zinc, was associated with lower IL6, sTNFRI and II and higher beta d glucan, a marker of fungal translocation, zonulin, a marker of gut permeability, oxidized LDL and insulin resistance (p≤0.01).
In this cohort of PHIV on ART in Uganda, there is a high prevalence of low zinc status overall. Higher plasma selenium concentrations were associated with lower systemic inflammation and higher gut integrity markers. Although our findings do not support the use of micronutrient supplementation broadly for PHIV in Uganda, further studies are warranted to assess the role of selenium supplements in attenuating heightened inflammation.
Keywords: micronutrients, Children with HIV, HIV-exposed uninfected infants, gut integrity, inflammation, translocation
INTRODUCTION
Nutrient deficiencies are associated with immune dysfunction and rapid disease progression in HIV(1, 2). Micronutrients are important and may have a role in HIV infection and its associated morbidities. Several cross-sectional studies have reported a significant association between low selenium levels and HIV-infection(3–6). Chromium is a nutrient that promotes the action of insulin for the use of sugars and fats, its deficiency has been reported to cause insulin resistance, hyperglycemia and hyperlipidemia(7). There are conflicting results in adults with HIV (ALHIV) as to whether chromium supplementation improves insulin resistance(8, 9). Zinc is another essential micronutrient that has been linked with HIV disease progression, independent of baseline CD4 cell count and age- and calorie-adjusted dietary intake(10).
Although HIV has transitioned to a chronic manageable disease, a number of inflammation-associated complications specifically cardiovascular and metabolic diseases are increasing. This is especially concerning for children with perinatally acquired HIV (PHIV) who face a lifetime exposure to HIV and antiretroviral therapy (ART) and are at risk of cardiometabolic complications as they age. Understanding why inflammation persists in HIV despite viral suppression and how it causes non-AIDS comorbidities, particularly in resource-limited settings, is paramount to mitigating complications associated with premature aging in HIV. Specifically, the relationship between essential micronutrients, inflammation and metabolic complications warrants further investigation in PHIVs.
Due to the success of prevention of maternal to child transmission, new HIV infections among infants have decreased significantly, while the number of HIV-exposed uninfected infants (HEU) has steadily increased(11). Numerous studies have reported adverse health outcomes in HEU children including higher mortality (12). We have previously shown that HEU infants have increased inflammation compared to unexposed infants(13).
In this study, we focused on selenium, chromium and zinc, micronutrients known to be associated with cardiometabolic complications and measured their levels in PHIVs who are virally suppressed on stable ART compared to HIV exposed uninfected (HEU) and unexposed and uninfected children (HIV-) in Uganda. Furthermore, we explored the association of these micronutrients with metabolic complications as well as with markers of inflammation, monocyte activation, and intestinal damage. Although there have been extensive investigations on the role of micronutrient in the pediatric population in lower income settings, our study is unique in that we focused on healthy children not currently admitted to a health facility, without co-infections, other than HIV, who are not viremic and well controlled on ART. In addition, we used well matched control groups both HIV exposed uninfected and HIV unexposed uninfected to further investigate the role of HIV and ART exposure on micronutrients and non-AIDS complications.
Methods
Study Design
This is an observational cohort of PHIV, HEU and HIV unexposed uninfected children prospectively enrolled at the Joint Clinical Research Center (JCRC) in Kampala, Uganda between January 2017 and May 2018. The study was approved by the Research Ethics Committee in Uganda, the Ugandan National Council of Science and Technology as well as the IRB of the University Hospitals Cleveland Medical Center, Cleveland, Ohio. Caregivers gave written informed consent; older children also gave informed assent. All participants were 2-10 years of age. PHIV participants were on stable ART for at least 6 months with HIV-1 RNA < 400 copies/mL. PHIV participants were recruited during routine clinic visits. HIV- participants were either HIV- siblings of the PHIV or recruited from the community using community liaison volunteers from the JCRC. All participants lived in Kampala or peri-urban surroundings. History of acute infections (malaria, tuberculosis, helminthiasis, pneumonia, meningitis) in the last 3 months, moderate or severe malnutrition, and diarrhea in the last 3 months were exclusionary. Known diabetes and cardiovascular diseases were also excluded.
Study Evaluations
Participants were recruited and seen at the outpatient pediatric clinic at the JCRC. Blood was drawn after an 8-hour fast. Element free tubes were used for the measurement of the micronutrients. The samples were used for measurement of glucose, insulin, lipids, micronutrients and soluble markers of monocyte activation, systemic inflammation and gut integrity. Insulin was measured by ELISA sandwich immunoassay (ALPCO, Salem, New Hampshire, USA) and the derived homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as described(14). Plasma levels of zinc, trivalent chromium and selenium levels were measured using Coupled Plasma/Mass Spectrometry (ICP-MS) at the Cleveland Heart Lab (Cleveland, OH). These specific micronutrients were selected because of their known associations with metabolic and cardiovascular disease risk in the general population(15–18). The reference ranges for zinc are 55-150 μg/dL, for chromium <5μg/L and for selenium 23-190 μg/L (ARUP Laboratories, SLC, UT).
The primary parent or caregiver were given a 12-item questionnaire that incorporated the WHO STEPS instrument (19), which is a standardized but flexible framework for countries to monitor the main non communicable diseases (NCD), and the Demographic and Health Surveys Wealth Index from USAID(20), which assesses a household’s cumulative living standard including food, water and sanitation facilities access. The questionnaire measured overall food insecurity over the past 3 months. A binary variable was created to capture food insecurity (defined as frequency of hunger) compared to no food insecurity (ie: being hungry seldom or never).
Inflammation, monocyte activation and gut integrity markers
Plasma markers of monocyte activation (sCD163 and sCD14), systemic inflammation [soluble TNFα receptor I and II (sTNFRI and II), high sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6)], coagulation (d-dimer), and oxidized lipids (oxidized LDL) were measured by ELISA (R &D Systems, Minneapolis, Minnesota, USA and ALPCO, Salem, New Hampshire, USA and Mercodia, Uppsala, Sweden). The marker of fungal translocation Beta D Glucan (BDG, Mybiosource Inc. CA), lipopolysaccharide-binding protein (LBP, Hycult Biotech Inc. PA), an indirect marker of microbial translocation; zonulin (Promocell Germany), a marker of intestinal permeability and intestinal fatty acid binding protein (I-FABP, R &D Systems, Minneapolis, Minnesota, USA), a marker of intestinal integrity were measured by ELISA. The intra-assay variability ranged between 4-8% and inter-assay variability was less than 10% for all markers. All assays were performed on batched samples, never previously thawed, at Dr. Funderburg’s laboratory at Ohio State University, Columbus, OH. Laboratory personnel were blinded to group assignments and clinical characteristics.11
Statistical Analyses
The primary objective of this analysis was to compare micronutrient levels between PHIV, HEU and HIV- children. The secondary objectives were to determine the association between micronutrients and inflammatory biomarkers, as well as metabolic parameters specifically body mass index (BMI), waist hip ratio, lipids and HOMA.
We hypothesized that PHIV children would have lower micronutrient levels. Second, we hypothesized that in PHIV, low micronutrient levels would be associated with higher levels of inflammation, altered intestinal integrity and metabolic complications.
We performed descriptive analyses on all of the covariates (including age, sex, race, nadir and current CD4, ART duration and type) and on the micronutrients. Kruskal-Wallis tests were used to compare continuous variables and Fisher’s exact test for categorical variables, by HIV exposure/status. Spearman correlation was used to assess correlations with micronutrients and each biomarker as well as metabolic parameters combined as well as by groups. Micronutrients were analyzed as continuous variables and dichotomous variables (deficiency versus not deficient). Statistical significance was defined as p≤ 0.05. Regression analyses were used to determine the association between Selenium and HOMA-IR after adjusting for BMI.
All the statistical analyses were performed using Stata 15 and R 3.4.1
Results
Baseline characteristics
Overall, 172 participants were enrolled in this study and included in this analysis: 57 PHIV, 59 HEU and 56 HIV- children. Detailed patient and maternal characteristics have been previously described(21). Median [Q1, Q3] age was 7.8 years [6.39, 8.84], 55% of participants were female, median BMI was 15.2 kg/m2 [14.38, 15.81] and 24% had food insecurity. There was no difference between baseline demographic characteristics between the groups. As shown in Table 1, HDL cholesterol, triglycerides, HOMA-IR and waist hip ratio were higher in PHIVs (<0.02).
Table 1:
Baseline characteristics
| Variables | PHIV | HEU | HIV- | p-value |
|---|---|---|---|---|
| n=57 | n=59 | n=56 | ||
|
Cardiovascular and metabolic variables | ||||
| Weight- for- age- z score | −0.63 [−1.48, −0.06] | −0.46 [−0.99, 0.04] | −0.31 [−0.85, 0.10] | 0.41 |
| Height-for-age-z-score | −0.85[−1.53,0.39] | −0.22 [−1.16, 0.48] | −0.25 [−1.11, 0.14] | 0.13 |
| BMI (kg/m2) | 14.93 [14.48, 15.91] | 14.92 [14.06, 15.60] | 15.17 [14.57, 16.25] | 0.08 |
| Waist (cm) | 57 [55, 59] | 56 [53, 59] | 57 [53, 58] | 0.37 |
| Hip (cm) | 60 [58, 65] | 62 [58, 67] | 64 [61, 67] | 0.05 |
| Waist:Hip Ratio | 0.92 [0.89, 0.98] | 0.91 [0.87, 0.93] | 0.89 [0.85, 0.92] | <0.01 |
| Systolic Blood Pressure (mmHg) | 98 [95, 105] | 101 [93, 106] | 105 [99, 109] | 0.04 |
| Diastolic Blood Pressure (mmHg) | 63 [58, 68] | 63 [58, 67] | 64 [60, 67] | 0.53 |
| Cholesterol (md/dL) | 152 [130, 168] | 143 [129, 165] | 143 [128, 160] | 0.42 |
| HDL (mg/dL) | 50 [39, 57] | 43 [36, 51] | 42 [37, 48] | 0.01 |
| Non-HDL (mg/dL) | 96 [83, 122] | 98 [81, 118] | 99 [88, 113] | 0.84 |
| Cholesterol:HDL ratio | 3.00 [2.70, 3.70] | 3.30 [2.85, 3.85] | 3.50 [3.10, 3.80] | 0.10 |
| LDL (mg/dL) | 80 [69, 102] | 79 [68, 106] | 86 [73, 101] | 0.54 |
| VLDL (mg/dL) | 17 [13, 23] | 14 [11, 22] | 13 [10, 17] | 0.02 |
| Triglycerides (mg/dL) | 83 [64, 113] | 72 [56, 109] | 65 [51, 86] | 0.02 |
| Insulin (μIU/mL) | 5 [3, 7] | 3 [2, 4] | 3 [2, 4] | 0.00 |
| HOMA-IR | 0.94 [0.62, 1.51] | 0.57 [0.42, 1.06] | 0.68 [0.41, 1.08] | 0.02 |
|
Micronutrients | ||||
| Selenium (μg/L) | 106 [94, 15] | 81 [71, 97] | 100 [75, 21] | <0.001 |
| Zinc (μg/dL) | 74 [64, 82] | 78 [71, 87] | 76 [68, 85] | 0.18 |
| Chromiun (μg/L) | < 1 [1, 1] | <1 [1, 1] | <1 [1, 1] | 0.01 |
Median [Interquartile range]
Bold values represent p< 0.05
HDL: high density lipoprotein, HEU: Children exposed uninfected to HIV, HIV-: Children unexposed and uninfected to HIV, HOMA-IR: Homeostatic assessment insulin resistance, LDL: low-density lipoprotein, NRTI: nucleotide reverse transcriptase inhibitor, PHIV: Children with perinatally acquired HIV, PI: protease inhibitor, NNRTI: non-nucleotide reverse transcriptase inhibitor, VLDL: very low density lipoprotein.
Among PHIV, median CD4 cell count % was 37% [27, 41], viral load was 20 copies/mL. Median ART duration was 6 years [5,6 ], 23% were on a protease-inhibitor based regimen (lopinavir/ritonavir) and the remainder on a non-nucleotide reverse transcriptase inhibitor regimen (28% on efavirenz and 51% on nevirapine).
Micronutrient levels
As shown in Table 1, median selenium concentrations were higher in the PHIV group compared to the HEU and HIV- groups (p <0.001). In addition, selenium deficiency, as defined by plasma concentrations <85 μg/L, was most prevalent in the HEU group and lowest in the PHIV group (Figure 1).
Figure 1:
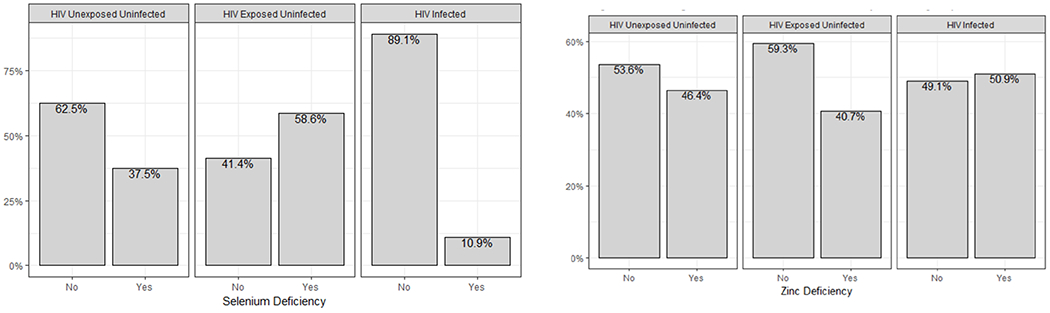
Percent of participants with Selenium and Zinc Deficiency between groups
Median zinc levels were not different between the arms (p=0.18). In the PHIV group, 51% had zinc deficiency, as defined by plasma concentrations <75 μg/dL (Figure 1).
Median chromium concentrations were <1 μg/L; 1 PHIV child and 6 HEU children had chromium levels > 1 μg/L, ranging between 1.1-1.7 μg/L (p<0.01 between groups).
Micronutrients and metabolic complications
Univariable analyses were performed for each micronutrient, metabolic and anthropometric measures for all participants and then by group. Higher selenium correlated with higher levels of HOMA-IR for all participants combined (ρ=0.22, p<0.01) and for PHIVs (ρ=0.25, p=0.05).
In multivariable analyses, selenium still remained associated with HOMA-IR after adjusting for BMI (β=0.007, p=<0.01) for all participants but not for PHIVs (β=0.013, p=0.09).
Selenium, zinc and chromium did not correlate with BMI, waist hip ratio or any lipid fractionation in all participant combined or by groups (p≥0.1).
We did further subgroup analyses for those participants with micronutrient deficiencies and did not find any correlation between micronutrient and metabolic measures for all participants combined or by group (p≥0.07).
Micronutrients and inflammation and intestinal alteration
We performed additional univariate analyses to assess the relationship between micronutrients and biomarkers of inflammation, immune activation, intestinal integrity and microbial translocation. As shown in Figure 2, selenium levels correlated with several markers of inflammation and intestinal permeability for all participants. After excluding statistical outliers, the correlations between selenium and biomarkers remained significant for all (p≤0.01) except sCD163 and D-dimer (p≥0.32). Higher zinc levels correlated with lower zonulin (ρ=−0.21, p<0.01). Chromium levels did not correlate with any biomarkers (≥0.1).
Figure 2:
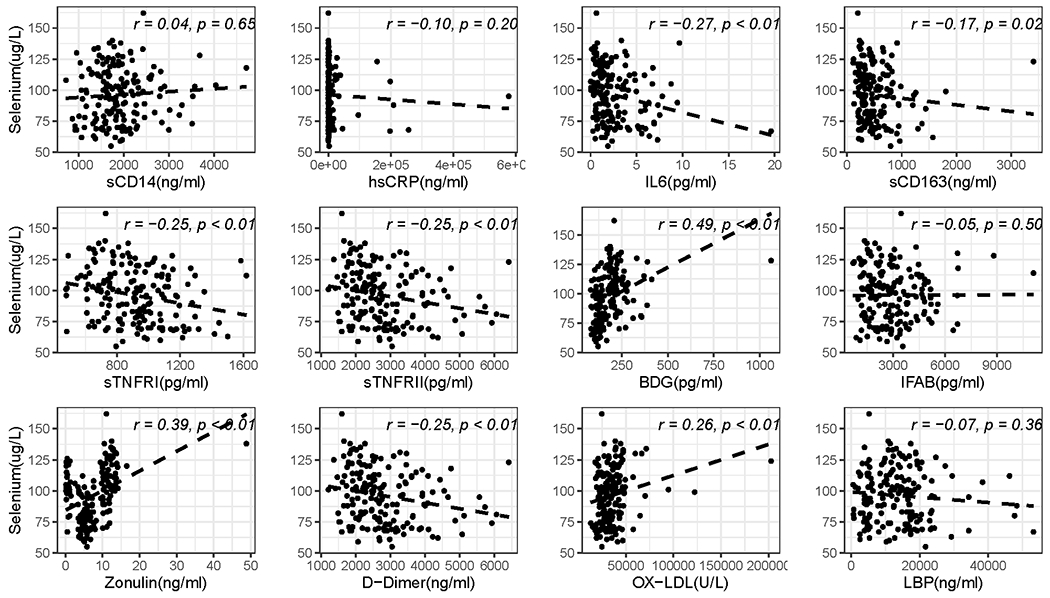
Scatter plot of the correlation between Selenium and biomarkers
Discussion
Prevalence of metabolic disorders is on the rise worldwide, and we were interested in exploring whether micronutrients are associated with any metabolic complications or ongoing inflammation and alteration in intestinal integrity in PHIV in Uganda. We observed that there was no association between low micronutrient levels and metabolic complications, however, selenium may play a role in modulating inflammation.
There is compelling evidence that micronutrient deficiencies are associated with HIV disease progression and co-morbidities(9, 22–24) and are common in children in resource limited settings(25). ART does not appear to fully resolve micronutrient deficiencies in ALHIV(26, 27).
Plasma selenium, zinc and chromium in this study were measured as an assessment of the micronutrient status in study participants. Micronutrient plasma concentrations can be affected by many factors including inflammation(28, 29), however plasma micronutrient concentrations is the most widely used method to determine micronutrient status. Other methods to assess micronutrient status also include limitations. The total selenium content of the plasma measures several components including the protein and non protein-bound selenium. The measurement of total plasma selenium captures selenium status because in non-deficient individuals, selenoproteins are maximally expressed and may not reflect selenium status in these individuals(29). Plasma zinc concentrations can also be affected by diurnal variation as well as hypoalbuminemia(30). Urinary zinc concentration could be a valuable indicator of zinc status when measured in relation to urinary creatinine, however this appears to be true only for participants with moderate zinc status at baseline(30). Lastly, chromium levels in most human samples are <1 μg/L similarly to our findings. Other techniques that have the sensitivity to detect levels below this limit of detection include neutron activation analysis and graphite furnace atomic absorption spectrometry which are not readily available(31). In order to minimize contamination, we collected our samples using element free supplies.
We found that PHIV do not have low selenium and chromium status compared to age and sex matched HEU and uninfected children who reside in a similar peri-urban area in Uganda. We found the overall prevalence of low zinc levels was high at 46% with no differences between groups. This is comparable to what has been cited for zinc deficiency in the general population of children in a similar age group in Uganda(32–34). In addition, a cross sectional study comparing zinc deficiency in PHIV in Uganda by ART status suggests that ART does not completely eliminate zinc deficiency (33). The etiologies are likely multifactorial and include food insecurity, availability and lack of food fortification (35, 36). Surprisingly, HEU children had a high prevalence of low selenium concentrations. One hypothesis is this may be secondary to the low levels of breastfeeding or early weaning and replacement with complementary feeding with low selenium content(37). This may affect the child’s ability to reach and maintain an adequate selenium status.
Micronutrient deficiencies have been associated with cardiometabolic complications in the general population. Chromium, in the form of naturally occurring dinicotinic acid -gluthatione complex, is vital for carbohydrate metabolism as it modulates the action of insulin(17). Selenium and selenoproteins play a role in the antioxidant defense system, and subsequent oxidative modification of lipids, preventing platelets from aggregating and reducing cardiovascular disease risk (18). Zinc also plays a critical role in oxidative stress and normal cellular structure and function, and its homeostasis is also associated with cardiovascular diseases and insulin resistance(15, 38). The role of micronutrients in cardiometabolic complications in HIV, however, is less clear. In ALHIV, low chromium is associated with parameters of metabolic syndrome(22) and low selenium with the development of cardiomyopathy(39). We hypothesized that low micronutrient status in PHIV could lead to oxidative stress inducing inflammation and contributing to the development of metabolic complications. We investigated the relationships among micronutrient concentrations and anthropometric measures, insulin resistance, and lipids. Higher selenium level correlated with HOMA in PHIV. We did not find any other correlation between low micronutrient status and metabolic measures. Our findings could be the result of several factors 1)our patient population: in PHIVs, well controlled on ART, micronutrients may not contribute to metabolic complications; 2)our sample size: due to the small number of participants with micronutrient deficiencies, our subgroup analyses to investigate the role of micronutrient deficiencies and metabolic complications are likely not well powered to detect a correlation 3) our study design: the cross sectional design precludes us from inferring causality and further longitudinal studies are warranted to truly investigate the role of micronutrients on the development of metabolic complications as PHIVs are exposed to a lifetime of ART and HIV.
Micronutrients may play a role in reducing mitochondrial dysfunction, oxidative stress, and may have important consequences on the chronic inflammation seen in HIV(40). Our group has found that in ALHIV with viral suppression on ART, selenium is associated with T cell activation(41) and that zinc supplementation, in a pilot study, can modulate biomarkers associated with clinical comorbidities(42). To our knowledge, only one study has assessed the role of micronutrients and inflammation on HIV in resource limited settings. The PEARLS trial is a multi-country trial in ART-naïve ALHIV and findings suggest that micronutrients and inflammations levels are associated with CD4 recovery post ART initiation(2, 17). Our findings extend this knowledge and suggests that selenium may play a role in the ongoing inflammation seen in PHIV despite ART.
Micronutrients are essential components of intestinal health and can affect the composition and function of intestinal microbiota (43, 44). Specifically, the benefits of zinc on the prophylaxis and treatment of diarrhea are well documented (45). Selenoproteins are detected in the intestine and are known to affect the intestinal microbiota(46). As such, we hypothesized that micronutrient deficiencies would be associated with altered intestinal barrier function and microbial translocation. Surprisingly, we found that high selenium status correlated with high BDG, a marker of fungal translocation, and high zonulin, a marker intestinal permeability. BDG is associated with inflammation and immune activation in ALHIV(47). Zonulin is a human protein that regulates intestinal permeability by modulating intercellular tight junctions in the gut and increases permeability and macromolecule absorption(48). Interestingly, high zonulin is associated with lower mortality in ALHIV who were virally suppressed(49). We did not measure zonulin using monoclonal antibody and caution should be used when interpreting zonulin assays using ELISA commercial assays(50). We hypothesize that the relationship between micronutrient, intestinal health and microbiota may be complex in PHIV in resource limited settings, this may be due to other medications (all PHIVs were on cotrimoxazole which can affect intestinal microbiota), chronic helminthiasis, unsanitary water source or dietary patterns.
Our study has important limitations. First, our findings may not be generalizable as we included PHIV from a peri-urban area of Uganda who are stable on ART and virally suppressed. Second, no direct microbial translocation measures were performed, we cannot confirm whether micronutrients are associated with translocation of bacterial products. Third, we do not have detailed dietary assessments. Nevertheless, our study includes a detailed evaluation of inflammation, monocyte activation and intestinal integrity biomarkers. Additionally, the age and sex matched HEU and HIV- groups from the same peri-urban area in Uganda allows for adequate comparison and significantly adds to the literature.
Conclusion
Plasma selenium and chromium were sufficient in PHIV, however, similarly to prior studies, we found a high prevalence of low plasma zinc levels in all Ugandan children without diarrhea. In addition, our study demonstrates consistent correlations between selenium and inflammation, monocyte activation and intestinal integrity. Counter to our hypothesis, plasma micronutrient levels were not associated with metabolic measures in PHIV. Further longitudinal studies are warranted to consider the role of micronutrients in sustained inflammation in PHIV in resource-limited settings.
What is known:
Chronic inflammation is a hallmark of HIV, despite viral suppression
Inflammation is associated with cardiometabolic complications
Nutrient deficiencies persist in controlled HIV, however their roles in HIV-associated inflammation and non-infectious complications are unknown
What is new:
In children with HIV, selenium may play a role in modulating inflammation and gut integrity.
Low micronutrient levels do not appear to be associated with metabolic complications in children with controlled HIV.
Acknowledgments
The authors would like to thank the patients who participated in this research.
Sources of support: This work was supported by Rainbow Babies and Children’s Hospital internal grants to SDF, the Eunice Kennedy Shriver National Institute of Child Health [K23HD088295-01A1 to SDF] and from the National Institute of Diabetes and Digestive and Kidney Diseases [R21DK118757 to GM].
Conflict of Interest
GAM served as a consultant for Gilead, GSK/Viiv, and Merck, and has received research funding from Gilead, Merck, GSK/Viiv, Roche, Astellas, Tetraphase, and BMS. NF serves as a consultant for Gilead. All other authors had no conflict of interest.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings were submitted for a research presentation (poster or oral) at ID week meeting, in San Francisco, Ca in October 2019. The decision is currently pending.
REFERENCES
- 1.Mayer KH, Ivers LC, Cullen KA, Freedberg KA, Coates J, Webb P, et al. HIV/AIDS, Undernutrition, and Food Insecurity. Clinical Infectious Diseases. 2009;49(7):1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Pee S, Semba RD. Role of Nutrition in HIV Infection: Review of Evidence for more Effective Programming in Resource-Limited Settings. Food and Nutrition Bulletin. 2010;31(4_suppl4):S313–S44. [PubMed] [Google Scholar]
- 3.Beck KW, Schramel P, Hedl A, Jaeger H, Kaboth W. Serum trace element levels in HIV-infected subjects. Biological trace element research. 1990;25(2):89–96. [DOI] [PubMed] [Google Scholar]
- 4.Delmas-Beauvieux MC, Peuchant E, Couchouron A, Constans J, Sergeant C, Simonoff M, et al. The enzymatic antioxidant system in blood and glutathione status in human immunodeficiency virus (HIV)-infected patients: effects of supplementation with selenium or beta-carotene. The American journal of clinical nutrition. 1996;64(1):101–7. [DOI] [PubMed] [Google Scholar]
- 5.Tohill BC, Heilig CM, Klein RS, Rompalo A, Cu-Uvin S, Piwoz EG, et al. Nutritional biomarkers associated with gynecological conditions among US women with or at risk of HIV infection. The American journal of clinical nutrition. 2007;85(5):1327–34. [DOI] [PubMed] [Google Scholar]
- 6.Allard JP, Aghdassi E, Chau J, Salit I, Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. The American journal of clinical nutrition. 1998;67(1):143–7. [DOI] [PubMed] [Google Scholar]
- 7.Panchal SK, Wanyonyi S, Brown L. Selenium, Vanadium, and Chromium as Micronutrients to Improve Metabolic Syndrome. Current hypertension reports. 2017;19(3):10. [DOI] [PubMed] [Google Scholar]
- 8.Aghdassi E, Arendt BM, Salit IE, Mohammed SS, Jalali P, Bondar H, et al. In patients with HIV-infection, chromium supplementation improves insulin resistance and other metabolic abnormalities: a randomized, double-blind, placebo controlled trial. Current HIV research. 2010;8(2):113–20. [DOI] [PubMed] [Google Scholar]
- 9.Stein SA, Mc Nurlan M, Phillips BT, Messina C, Mynarcik D, Gelato M. Chromium Therapy for Insulin Resistance Associated with HIV-Disease. Journal of AIDS & clinical research. 2013;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falutz J, Tsoukas C, Gold P. Zinc as a cofactor in human immunodeficiency virus-induced immunosuppression. Jama. 1988;259(19):2850–1. [DOI] [PubMed] [Google Scholar]
- 11.UNAIDS. 2017. [Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
- 12.Brennan AT, Bonawitz R, Gill CJ, Thea DM, Kleinman M, Useem J, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS (London, England). 2016;30(15):2351–60. [DOI] [PubMed] [Google Scholar]
- 13.Dirajlal-Fargo S, Mussi-Pinhata MM, Weinberg A, Yu Q, Cohen R, Harris DR, et al. HIV-exposed-uninfected infants have increased inflammation and monocyte activation. AIDS (London, England). 2019;33(5):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 15.Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition (Burbank, Los Angeles County, Calif). 2010;26(11-12):1050–7. [DOI] [PubMed] [Google Scholar]
- 16.Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules (Basel, Switzerland). 2013;18(3):3292–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris BW, Blumsohn A, Mac Neil S, Gray TA. The trace element chromium--a role in glucose homeostasis. The American journal of clinical nutrition. 1992;55(5):989–91. [DOI] [PubMed] [Google Scholar]
- 18.Rayman MP. Selenium and human health. Lancet (London, England). 2012;379(9822):1256–68. [DOI] [PubMed] [Google Scholar]
- 19.Organization TWH. WHO STEPS Instrument. 2018.
- 20.USAID. Wealth Index.
- 21.Dirajlal-Fargo S, El-Kamari V, Weiner L, Shan L, Sattar A, Kulkarni M, et al. Altered intestinal permeability and fungal translocation in Ugandan children with HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghdassi E, Salit IE, Fung L, Sreetharan L, Walmsley S, Allard JP. Is chromium an important element in HIV-positive patients with metabolic abnormalities? An hypothesis generating pilot study. Journal of the American College of Nutrition. 2006;25(1):56–63. [DOI] [PubMed] [Google Scholar]
- 23.Di Bella S, Grilli E, Cataldo MA, Petrosillo N. Selenium deficiency and HIV infection. Infectious disease reports. 2010;2(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhal N, Austin J. A clinical review of micronutrients in HIV infection. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill : 2002). 2002;1(2):63–75. [DOI] [PubMed] [Google Scholar]
- 25.Fawzi W, Duggan C. Micronutrients and Child Health: Studies in International Nutrition and HIV Infection. Nutrition Reviews. 2001;59(11):358–69. [DOI] [PubMed] [Google Scholar]
- 26.Look MP, Riezler R, Berthold HK, Stabler SP, Schliefer K, Allen RH, et al. Decrease of elevated N,N-dimethylglycine and N-methylglycine in human immunodeficiency virus infection during short-term highly active antiretroviral therapy. Metabolism: clinical and experimental. 2001;50(11):1275–81. [DOI] [PubMed] [Google Scholar]
- 27.Rousseau MC, Molines C, Moreau J, Delmont J. Influence of highly active antiretroviral therapy on micronutrient profiles in HIV-infected patients. Annals of nutrition & metabolism. 2000;44(5-6):212–6. [DOI] [PubMed] [Google Scholar]
- 28.Wieringa FT, Dijkhuizen MA, Fiorentino M, Laillou A, Berger J. Determination of zinc status in humans: which indicator should we use? Nutrients. 2015;7(5):3252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Combs GF Jr., Watts JC, Jackson MI, Johnson LK, Zeng H, Scheett AJ, et al. Determinants of selenium status in healthy adults. Nutrition journal. 2011;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. The American journal of clinical nutrition. 2009;89(6):2040s–51s. [DOI] [PubMed] [Google Scholar]
- 31.Veillon C, Patterson KY. Analytical issues in nutritional chromium research. The Journal of Trace Elements in Experimental Medicine. 1999;12(2):99–109. [Google Scholar]
- 32.Bitarakwate E, Mworozi E, Kekitiinwa A. Serum zinc status of children with persistent diarrhoea admitted to the diarrhoea management unit of Mulago Hospital, Uganda. African health sciences. 2003;3(2):54–60. [PMC free article] [PubMed] [Google Scholar]
- 33.Ndeezi G, Tumwine JK, Bolann BJ, Ndugwa CM, Tylleskar T. Zinc status in HIV infected Ugandan children aged 1-5 years: a cross sectional baseline survey. BMC pediatrics. 2010;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bimenya GS, Lutalo-Bosa AJ, Nzaro E. Serum zinc levels in normal children (HbAA) and sickle cell children (HbSS) in and around Kampala. East African medical journal. 1980;57(12):825–7. [PubMed] [Google Scholar]
- 35.Arinola GO, Morenikeji OA, Akinwande KS, Alade AO, Olateru-Olagbegi O, Alabi PE, et al. Serum Micronutrients in Helminth-infected Pregnant Women and Children: Suggestions for Differential Supplementation During Anti-helminthic Treatment. Annals of global health. 2015;81(5):705–10. [DOI] [PubMed] [Google Scholar]
- 36.Development-USAID USAfI. FANTA-2. The Analysis of the Nutrition Situation in Uganda Food and NutritionTechnical Assistance II Project (FANTA-2), . Washington, DC: 2010. [Google Scholar]
- 37.Litov RE, Combs GF Jr. Selenium in pediatric nutrition. Pediatrics. 1991;87(3):339–51. [PubMed] [Google Scholar]
- 38.Huang L, Teng T, Zhao J, Bian B, Yao W, Yu X, et al. The Relationship Between Serum Zinc Levels, Cardiac Markers and the Risk of Acute Myocardial Infarction by Zinc Quartiles. Heart, lung & circulation. 2018;27(1):66–72. [DOI] [PubMed] [Google Scholar]
- 39.Twagirumukiza M, Nkeramihigo E, Seminega B, Gasakure E, Boccara F, Barbaro G. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Current HIV research. 2007;5(1):129–37. [DOI] [PubMed] [Google Scholar]
- 40.Drain PK, Kupka R, Mugusi F, Fawzi WW. Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy. The American journal of clinical nutrition. 2007;85(2):333–45. [DOI] [PubMed] [Google Scholar]
- 41.Hileman CO, Dirajlal-Fargo S, Lam SK, Kumar J, Lacher C, Combs GF Jr., et al. Plasma Selenium Concentrations Are Sufficient and Associated with Protease Inhibitor Use in Treated HIV-Infected Adults. The Journal of nutrition. 2015;145(10):2293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahera Dirajlal-Fargo JY, Manjusha Kulkarni, Abdus Sattar, Nicholas Funderburg, McComsey Grace A., editor Zinc supplementation and inflammation in treated HIV. Conference on Retroviruses and Opportunistic Infections; 2019; Seattle, WA2019. [Google Scholar]
- 43.Basu TK, Donaldson D. Intestinal absorption in health and disease: micronutrients. Best practice & research Clinical gastroenterology. 2003;17(6):957–79. [DOI] [PubMed] [Google Scholar]
- 44.Mach N, Clark A. Micronutrient Deficiencies and the Human Gut Microbiota. Trends in microbiology. 2017;25(8):607–10. [DOI] [PubMed] [Google Scholar]
- 45.Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012;26(2-3):66–9. [DOI] [PubMed] [Google Scholar]
- 46.Kasaikina MV, Kravtsova MA, Lee BC, Seravalli J, Peterson DA, Walter J, et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. The FASEB Journal. 2011;25(7):2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiner LD, Retuerto M, Hager CL, El Kamari V, Shan L, Sattar A, et al. Fungal Translocation Is Associated with Immune Activation and Systemic Inflammation in Treated HIV. AIDS research and human retroviruses. 2019;35(5):461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. Journal of cell science. 2000;113 Pt 24:4435–40. [DOI] [PubMed] [Google Scholar]
- 49.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. The Journal of infectious diseases. 2014;210(8):1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PloS one. 2019;14(1):e0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]


