Abstract
The epidemiology of inflammatory bowel disease (IBD) is progressively evolving impacting the type of patients with IBD we will see in clinical practice. In this review, we discuss specific challenges and solutions in the management of (a) obese, (b) older and (c) obstetric (pregnant) patients with IBD. With the global obesity epidemic, almost one in three patients with IBD are obese. Obesity is associated with greater difficulty in achieving remission, higher risk of disease relapse and higher burden and costs of hospitalization in patients with IBD. Obese patients also have inferior response to biologic therapy related to altered pharmacokinetics and obesity-mediated chronic inflammation. Surgical management of obese patients with IBD is also challenging. Similar to obesity, the prevalence of IBD in older patients is rising and it is anticipated that almost one-third of patients with IBD will be older than 60 years within the next decade. Older patients present unique diagnostic and therapeutic dilemmas, and management of these individuals warrants careful consideration of the risks of disease-related vs. treatment-related complications, non-IBD-related extra-intestinal complications (e.g. cardiovascular disease, malignancy), in the context of individual values, preferences, functional status and comorbidities. With evolving therapeutics, medical management of IBD surrounding pregnancy continues to be challenging. Overall, the management of pregnant patients requires a pro-active, multidisciplinary approach, with an emphasis on optimal disease control not just during, but prior to pregnancy. This often involves continuation of highly effective therapies, of which the vast majority are safe during pregnancy and breastfeeding, resulting in a reduction of risk of adverse maternal fetal outcomes.
INTRODUCTION
Prior chapters in this issue of the Journal have addressed different aspects in the management of an adult patient with inflammatory bowel disease (IBD). In this review, we focus on special patient populations, which though not well-represented in clinical trials, are frequently and increasingly encountered in clinical practice. We present the epidemiology, natural history, specific challenges and solutions for the management of (a) obese, (b) older and (c) obstetric (pregnant) patients with IBD.
MANAGEMENT OF IBD IN OBESE PATIENTS
Epidemiology and Pathophysiology
The incidence and prevalence of IBD is rising in parallel with the global obesity epidemic. Approximately 15–40% adult patients with IBD are obese (body mass index [BMI] ≥30 kg/m2) and an additional 20–40% are overweight, with a comparable distribution of obesity in Crohn’s disease (CD) and ulcerative colitis (UC).1–3 Similar trends are observed in pediatric IBD patients.4, 5 Obesity may also be associated with an increased risk of developing CD, but not UC. In the Danish National Birth Cohort of over 75,000 women, pre-pregnancy obesity was associated with a 1.9-fold increase in risk of developing CD (hazard ratio (HR), 1.88; 95% confidence interval [CI] 1.02–3.47), but not UC (HR, 0.77; 95% CI 0.48–1.25).6 On the other hand, a high level of physical activity (recreational or occupational) may be associated with decreased risk of developing IBD.7
Obesity may contribute to the development and perpetuation of IBD through multiple pathways.1, 8 Obesity is recognized as a perpetual state of chronic low-grade inflammation, through systemic and paracrine increase in levels of cytokines, chemokines and adipokines. Hypertrophic adipocytes seen in patients with obesity, particularly those with central/visceral adiposity (as compared to subcutaneous adipose tissue) have a pro-inflammatory gene expression profile and produce large amounts of pro-inflammatory mediators. Additionally, resident immune cells within the hypertrophic fat-tissue in obesity are primed toward a more pro-inflammatory subtype. The locally restricted mesenteric fat accumulation in patients with CD, creeping fat, is independent of overall obesity, and also has systemic pro-inflammatory effects. Metabolically active mesenteric fat increases leptin secretion from adipocytes and resistin secretion from macrophages and leukocytes, that increase levels of pro-inflammatory cytokines such as tumor necrosis factor, interleukin-1 and −6. In addition, both obesity and IBD are associated with increased gut bacterial translocation, reduction in bacterial diversity and dysbiosis.
Impact of Obesity on Clinical Characteristics and Natural History of IBD
Obesity has been variably associated with a milder IBD phenotype (as conventionally reported using Montreal classification) in cross-sectional studies. Pringle and colleagues observed a lower prevalence of penetrating disease complications in obese patients, but comparable prevalence of stricturing and perianal complications, compared with adults with normal BMI.9 However, despite possibly a milder phenotype, obese patients are more likely to have persistent symptoms and higher anxiety, depression, fatigue, pain and inferior social function scores on PROMIS measures, as compared to non-obese patients with IBD.10 In a cross-sectional study using the Nationwide Inpatient Sample, Singh and colleagues observed among 6742 hospitalized patients with UC, obese adults had significantly higher rates of surgery (23% vs. 14%), severe hospitalization (need for surgery or hospital stay >7 days - 35% vs. 26%) and longer hospital stay (mean, 6.0 vs. 5.4 days) as compared to non-obese patients.11
Longitudinal studies suggest that obesity may negatively impact clinical course and healthcare utilization. In a large internet-based cohort study of 7296 patients with IBD (4748 patients with CD, 19.5% obese; 2548 patients with UC, 20.3% obese), Jain and colleagues observed that obesity was independently associated with increased risk of persistent disease activity or relapse in patients with CD (class II or III obesity vs. normal BMI: OR, 1.86; 95% CI, 1.30–2.68) and UC (OR, 2.97; 95% CI, 1.75–5.17).10 Seminerio and colleagues observed inferior IBD-related quality of life and higher frequency of elevated levels of serum C-reactive protein in patients with obesity (particularly class II or III obesity) compared with normal weight patients; however, there was no significant difference in the risk of IBD-related surgery, hospitalization or emergency department use between patients who were obese, overweight or a normal BMI.3 In a propensity score-matched, nationally representative cohort study of 42,285 patients with IBD (12.4% obese), Nguyen and colleagues observed that obese patients with IBD had a higher annual burden and costs of hospitalization, as compared to non-obese patients.12
Besides overall obesity, central/visceral adiposity (not creeping fat) has been more consistently associated with adverse outcomes in patients with IBD. High visceral adipose tissue volume was associated with increased risk of penetrating or stricturing complications (OR, 1.7; 95% CI, 1.1–2.9), hospitalization (OR, 1.9; 95% CI, 1.2–3.4) and shorter time interval to surgery (HR, 1.4; 95% CI, 1.0–2.0), after adjusting for age and BMI in a pediatric cohort of patients with CD.13 High visceral fat area has also been associated with increased risk of recurrence of CD after surgical resection (endoscopic recurrence: HR 8.6, 95% CI 1.6–47.1; and clinical recurrence: HR 2.6, 95% CI 1.0–6.7).14
Impact of Obesity on Medical and Surgical Management
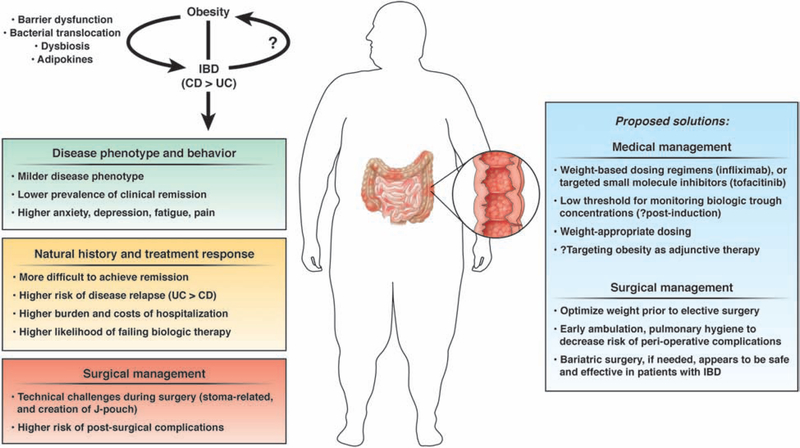
Population pharmacokinetic studies of all biologic agents used in IBD have identified high body weight as a risk factor associated with increased drug clearance, resulting in shorter half-lives and low trough drug concentrations.1 This effect might be related to impaired absorption of subcutaneously administered agents, rapid proteolysis and to a ‘TNF-sink’ phenomenon with higher inflammatory burden due to adipose tissue in obese patients. The practical negative effect of obesity on response to biologic therapies in patients with IBD has been variably observed. Kurnool and colleagues observed that each 1kg/m2 increase in BMI was associated with 4% increase in the risk of treatment failure, 8% increase in the risk of surgery or hospitalization and 6% lower odds of achieving endoscopic remission in a cohort 160 biologic-treated patients with UC, with comparable effects seen with fixed-dose therapies and weight-based agents.15 In contrast, in a post-hoc analysis of clinical trials of infliximab in patients with UC, obesity was not independently associated with lower risk of achieving remission or mucosal healing.16 In a prospective cohort of adalimumab-treated patients with CD, Bultman and colleagues observed that over one-third of patients were dose-escalated to weekly adalimumab within a median 5 months of initiating therapy, and BMI was the only independent predictor of dose escalation.17 Lean body weight also significantly impacts subcutaneous absorption of adalimumab, with high lean body weight being associated with high clearance.18 In a systematic review of 54 cohorts including 19,372 tumor necrosis factor-α (TNFα) antagonist-treated patients with immune-mediated inflammatory diseases (23% obese), Singh and colleagues observed that patients with obesity had 60% higher odds of failing therapy (OR,1.60; 95% CI,1.39–1.83), with a dose-response relationship; each 1kg/m2 increase in BMI was associated with 6.5% higher odds of treatment failure.19 Whether a similar negative effect of obesity on response to targeted small molecule inhibitors, such as tofacitinib, is unclear. Pharmacokinetically, the clearance of tofacitinib is not affected by body weight. Based on these observations, in Figure 1, we propose specific solutions for managing obese patients with IBD, including potentially considering weight-based dosing regimens for obese patients and having a low threshold for monitoring biologic trough concentrations (for example, post-induction). While obesity may negatively both weight-based and fixed-dose therapies, we believe the impact may be more profound with fixed-dose therapies.
Figure 1:
Challenges and proposed solutions in the management of obese patients with IBD. While obesity may negatively both weight-based and fixed-dose therapies, we believe the impact may be more profound with fixed-dose therapies. Whether a similar negative effect of obesity on response to targeted small molecule inhibitors, such as tofacitinib, is unclear. Pharmacokinetically, the clearance of tofacitinib is not affected by body weight. Weight-appropriate dosing refers to dosing infliximab based on actual body weight.
Intra-abdominal surgeries in patients with obesity are both technically challenging and are usually associated with higher rates of post-operative complications than surgeries in patients with a normal BMI. In a systematic review, Makino and colleagues observed longer operative times, an increased likelihood of conversion to open procedures, more comorbidities, a higher risk of postoperative complications (in particular wound infection) and a longer length of hospital stay in obese patients undergoing colorectal resection compared to individuals who were not obese.20 Two aspects of surgery that might be particularly challenging in patients with IBD merit special mention. First, obesity makes creating a stoma challenging due to stomal retraction, higher rates of complications such as parastomal hernia, mucocutaneous separation and stoma prolapse. Second, the mesentery of patients with obesity tends to be foreshortened by the mesenteric fat, making it more challenging to create a J-pouch in patients with UC. Obesity increases risk of short-term postoperative complications in patients undergoing ileal pouch-anal anastomosis, although long-term outcomes might be comparable to those in patients without obesity in experienced centers.21
In light of this negative impact of obesity on the natural history and treatment response in patients with IBD, it is conceivable that treating obesity might improve outcomes in patients with IBD. While there are no interventional studies of intentional weight loss in IBD, trials of diet and/or lifestyle-induced weight loss in other autoimmune diseases including psoriasis suggest improvement in clinical outcomes with intentional weight loss, with beneficial effects being seen with as little as 5% weight loss.22 While dietary interventions for weight loss have limited efficacy, and endoscopic bariatric interventions may be too invasive, and are currently contraindicated in patients with IBD, pharmacological therapy for weight loss may be an attractive option in obese patients with IBD. for A phase 2 clinical trial an FDA-approved weight loss medication, phentermine-topiramate in obese biologic-treated patients with UC is ongoing.
MANAGEMENT OF IBD IN OLDER PATIENTS
Epidemiology and Pathophysiology
Though the majority of patients with IBD are diagnosed as young adults, 10–15% of new IBD diagnoses have been reported to occur in individuals older than 60 years, with incidence rates as high as 18.9 per 100,000.23 In a systematic synthesis of 68 population-based cohort studies, incidence of elderly-onset CD (>60y age) and UC was 4.5 and 11.6 per 100,000 person-years, respectively.24 Besides rising incidence of new-onset of IBD in older patients (elderly-onset IBD), the prevalence of IBD in older patients is also anticipated to continue rising. Within the next decade, it is expected that over 1/3rd of patients with IBD will be older patients.25
Whether the pathogenesis of IBD in older patients is the same as for young-onset IBD is unclear. The aging immune system is associated with a relative systemic immunodeficiency (immunosenescence of aging), with decline in functionality of both innate and adaptive immunity, and increased susceptibility to immune-mediated diseases.26, 27 The gastrointestinal tract also changes with aging as a result of dietary shifts among older people, alterations in gastrointestinal motility and gastric pH due to mucosal atrophy, increased intestinal permeability and changes in the gut microbiota that might influence host—inflammatory responses. Bacterial composition in older people shows greater proportions of facultative anaerobes and obligate anaerobes such as Bacteroides, and decreased proportions of Firmicutes and Bifidobacteria, similar to changes associated with IBD.28 Besides age-associated immunological and microbial changes, chronic smoking can contribute to microvascular thrombosis and ischemia with aging and may also contribute to the rising incidence of IBD in older patients.
Impact of Older Age on Clinical Characteristics and Natural History of IBD
Several conditions that are more prevalent in older patients can mimic IBD. These include complicated diverticular disease presenting either as perforation, bleeding or segmental colitis associated with diverticular disease, ischemic colitis, medication-induced colitis (such as non-steroidal anti-inflammatory drugs), microscopic colitis, radiation colitis and infectious diarrhea. This often leads to higher rates of misdiagnosis and delay in diagnosis of IBD in older patients as compared to younger patients. Hence, the diagnosis of IBD in older patients requires an appreciation and suspicion for IBD in patients presenting with gastrointestinal symptoms, and a thorough work-up including colonoscopy with ileal examination and abdominal imaging using enterography protocols.
Several cohort studies have reported differences in disease phenotype and behavior in older and younger patients with IBD. In a systematic review of 43 studies comprising 8274 elderly-onset and 34,641 younger-onset IBD subjects, Ananthakrishnan and colleagues observed that, compared with younger-onset patients, elderly-onset CD patients were more likely to have colonic disease (OR, 2.56, 95% CI, 1.88–3.48) and inflammatory behavior (OR, 1.19; 95% CI, 1.07–1.33), and less likely to have penetrating disease or perianal involvement.29 More elderly-onset UC patients had left-sided colitis. Extra-intestinal manifestations are less frequent in elderly-onset IBD (<5% patients), as compared to non-elderly onset IBD.
Despite a suggestion of a relatively milder phenotype in elderly-onset IBD, rates of surgery, hospitalization and progression to disease-related complications are similar to adult-onset IBD (Table 1). In population-based cohort studies, cumulative median 1-, 5- and 10-year risk of bowel surgery in elderly-onset CD is 13.5% (range, 9.5–18), 24% (range, 15–29) and 31.5% (range, 18–33), respectively, with a higher risk of surgery at diagnosis or within 1st year of diagnosis in elderly-onset vs. adult-onset CD.30–34
Table 1.
Cumulative risk of surgery, hospitalization, disease progression and medication exposure in elderly-onset IBD, based on population-based cohort studies [numbers represent range, derived from individual studies]
| Elderly- onset IBD |
Cumulative risk |
Surgery | Hospitalization | Disease Progression |
Medication exposure | |||
|---|---|---|---|---|---|---|---|---|
| 5-ASA | Corticosteroids | Anti metabolites |
Biologic agents |
|||||
| Crohn’s disease | 1y | 10–24% | 39–42% | - | 35–68% | 29–39% | 10–25% | 3–9% |
| 5y | 15–29% | 49–55% | 0–20% | 43–77% | 38–58% | 18–40% | 5–15% | |
| 10y | 18–33% | 55% | 30% | 80% | 47% | 28–48% | 9–23% | |
| Ulcerative colitis | 1y | 2–6% | 12% | - | 65–70% | 21–43% | 3–10% | 0–1% |
| 5y | 2–14% | 25% | 7–12% | 78–80% | 34–60% | 10–17% | 0–3% | |
| 10y | 6–19% | 30% | 9–21% | 84% | 47–70% | 15–22% | 2–5% | |
Similarly, in patients with elderly-onset UC, cumulative median 1-, 5- and 10-year risk of colectomy is 4% (range, 0.5–6%), 7.5% (range, 1.9–14%) and 8% (range, 6–18.5%), respectively. In a nationally representative longitudinal study of hospitalized adults using the Nationwide Readmissions Database 2013, Nguyen and colleagues observed that older patients with IBD have higher annual burden of hospitalizations, higher in-hospital mortality, require more invasive procedures and blood transfusions and have significantly higher healthcare costs, as compared to younger patients with IBD.35 Serious infections and cardiovascular complications are leading causes of hospitalization in these older patients. Overall, all-cause mortality may also be higher in elderly-onset IBD as compared to the age-matched general population, and IBD-specific mortality may be higher in elderly-onset CD vs. adult-onset CD (33.1 vs. 5.6 per 10,000pyr, p<0.01), and comparable for elderly-onset UC vs. adult-onset UC (2.89 vs. 1.33 per 10,000pyr, p=0.25).36 While the overall risk of malignancy is higher in elderly-onset IBD as compared to adult-onset IBD, this risk may not be specifically increased as compared to age-matched general population, including the risk of colorectal cancer.37
Impact of Older Age on Medical and Surgical Management
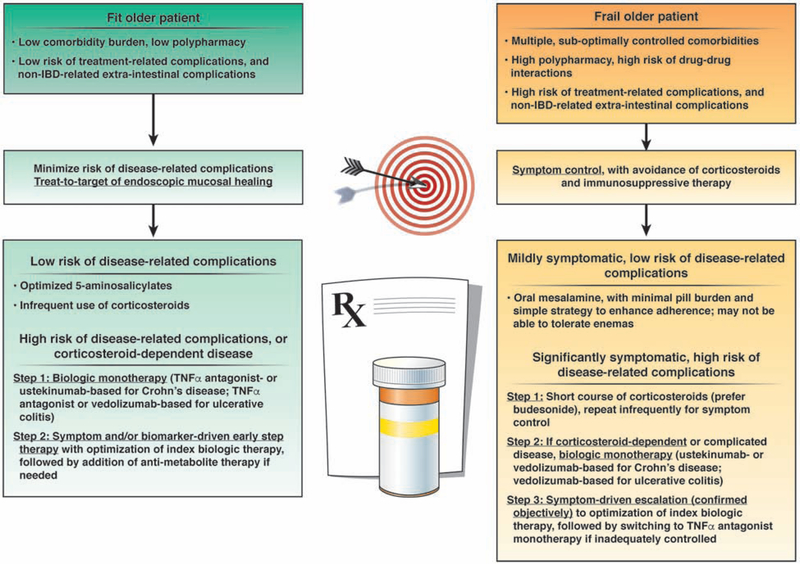
Management of IBD in older patients is an art that weighs and balances the risks of disease-related vs. treatment-related complications and extra-intestinal complications (e.g., cardiovascular disease, malignancy, etc.), in the context of patients’ values and preferences, functional status and comorbidities. While a target of mucosal healing is generally well-defined and consistent in younger patients at high-risk of disease complications, treatment targets and goals need to be flexible and dynamic in older patients, since older patients are more susceptible to treatment-and extra-intestinal complications. Figure 2 provides an evidence-derived algorithm for the management of older patients with IBD.
Figure 2:
Approach to management of older patients with IBD. Corticosteroid-dependence implies need for >1–2 corticosteroid courses per year. Since there is only modest correlation between symptoms and presence of active inflammation in patients with CD, particularly small bowel disease, we suggest objective confirmation of inflammation in symptomatic patients, with either serum or stool biomarkers or if needed, on endoscopy or active inflammation on imaging.
There is considerable practice variability in managing older patients with IBD, with a preponderance of long-term corticosteroid use and limited use of steroid-sparing therapies.38, 39 This may be driven by a physician’s perception of milder disease course (which is untrue as noted above), familiarity and “perceived safety” of corticosteroids, and perceived risks associated with immunosuppressive therapy in older patients. However, current data does not support this practice. In an analysis of 3522 elderly-onset IBD, Brassard and colleagues observed that exposure to corticosteroids is associated with a 2.3 times higher risk of serious infections.40 Besides a higher risk of serious infections, older patients may also be more susceptible to and easily compromised by short-term side effects of corticosteroids such as insomnia, mood instability and delirium, and may have significant impact due to long-term effects such as osteoporosis and pathologic fractures, hyperglycemia and cataracts. In a retrospective cohort study among Medicaid and Medicare beneficiaries with IBD from 2001 to 2013 (mean age, 52y), Lewis and colleagues observed a higher risk of death, major adverse cardiovascular events and hip fracture, with chronic corticosteroid use, as compared to patients treated with TNFα antagonists, without a significant difference in risk of serious infections.41
With age-related waning immunity, frailty and comorbidities, older patients are more susceptible to serious infections and malignancy with immunosuppressive therapy. Though the effectiveness of thiopurines may be comparable in older vs. younger patients with IBD, older patients may not tolerate thiopurines as well, have a higher risk of serious and opportunistic infections, and be at higher absolute risk of lymphoma and non-melanoma skin cancer.42 In the prospective study of 19,486 patients with IBD, Beaugerie and colleagues observed that among thiopurine-treated patients, risk of lymphoproliferative diseases in patients >65y may be 15-times higher than adults <50y (IR, 5.41 vs. 0.37 per 1000PY).43 In a pooled analysis, exposure to thiopurines was associated with a 4.8-times higher risk of lymphoma in patients >50y age as compared to age-matched general population, with an absolute risk of one lymphoma per 354-thiopurine-treated patients per year.44 Likewise, risk of side effects in biologic-treated patients increases with age. In a meta-analysis of 14 cohort studies across all immune-mediated inflammatory diseases, exposure to biologics was associated with 2.3 times higher odds of serious infections in older patients vs. younger patients; among older patients, exposure to biologics (vs. non-exposure) was associated with 3.6-times higher odds of serious infections.45 In a French population-based cohort study, exposure to TNFα antagonist monotherapy was associated with a numerically higher risk of lymphoma in older patients as compared to untreated older IBD patients.46 There is limited data on safety of non-TNF-targeted biologic agents in older patients. By virtue of their mechanism of action, vedolizumab and ustekinumab may be less immunosuppressive, and presumably safer in older patients. However, in a retrospective cohort study of 234 biologic-treated older patients with IBD, Adar and colleagues observed no significant difference in risk of serious infections between TNFα antagonist-treated patients vs. vedolizumab-treated patients (1y: 20% vs. 17%, p=0.54).47 While combination therapy with TNFα antagonist and anti-metabolites is generally avoided in older patients due to risk of treatment-related complications, selective use of an algorithmic treatment step-up strategy in older patients with suboptimal disease control may be safe and effective to decrease treatment disutility and avoid persistence on chronic corticosteroids, particularly since risk of immunogenicity may be higher in older patients.48 In a post-hoc analysis of Randomized Evaluation of an Algorithm for Crohn’s Treatment (REACT) cluster randomized trial, Singh and colleagues observed that a strategy of algorithmic early combined immunosuppression strategy was equally effective in older vs. younger patients, and was more effective than conventional management in maintaining corticosteroid-free clinical remission and delaying risk of CD-related surgery, hospitalization and serious disease-related complications.49 As anticipated, a greater percentage of older patients died, but the proportions of deaths was not higher in patients in the early combined immunosuppression group compared to the conventional management group.
Older patients have a higher risk of immediate postoperative complications and longer hospital stay after intestinal surgery, particularly frail patients with poor functional status.50 In addition, they may be more susceptible to fecal incontinence after colonic resections, and ileal pouch-anal anastomosis are generally avoided in older patients.51
Besides the choice of medical therapy in older patients with moderate to severe IBD, specific challenges and considerations in the medical and surgical management of IBD in older patients are summarized in Table 2.
Table 2.
Special challenges, considerations and proposed solutions in the management of older patients with IBD
| Risk | Solution | |
|---|---|---|
| Polypharmacy | • High pill burden which impacts adherence • Higher risk of drug-drug interaction (e.g., thiopurines and allopurinol) which increases risk of treatment-related complications |
• Minimize pill burden, and simplify treatment regimens • Pharmacy-led drug reconciliation and work closely with primary care provider |
| Comorbidities | • Contraindications to specific agents in patients with cancer, advanced heart failure, etc. • Higher risk of treatment-related complications with corticosteroids and immunosuppressive agents • Poses challenges for defining treatment target in patients at high risk of non-IBD-related extra-intestinal complications |
• Avoid TNFα antagonist in patients with active malignancy or advanced heart failure • Vaccination and measures to minimize infections • Early and continual engagement in shared decisionmaking and defining treatment targets |
| Compromised physical reserve and functional status | • Impacts patients’ ability to tolerate a flare (urgency and incontinence, rectal bleeding), increasing risk of hospitalization • Higher risk of malnutrition, dehydration and electrolyte abnormalities • Impacts patients’ ability to tolerate invasive procedures |
• Early recognition and nuanced intervention for flares, to avoid progression • Early focus on nutrition, hydration and maintaining physical activity • Prefer non-invasive interventions for monitoring |
| Pharmacokinetics and drug metabolism | • Increase in body fat and decrease in lean muscle mass, total body water, extracellular body fluid and plasma, may modify pharmacokinetics of biologic agents • Higher immunogenicity of TNFα antagonists |
• Closely work with pharmacologists to understand patient-specific aspects • Low threshold for measuring biologic trough concentrations due to higher risk of immunogenicity |
| Surgical management issues | • Higher risk of incontinence and nocturnal bowel movements with ileal pouch anal anastomosis • Ability to manage ostomy due to degenerative joint disease • Higher risk of peri-and post-operative morbidity and mortality |
• Pre-operative: Work-up of anal sphincter function, physical and mental status, ability to manage ostomy, and patient education • Peri-operative: optimization of disease control and nutritional status, avoidance of corticosteroids, control comorbidities • Post-operative: Early ambulation and physical therapy, prophylaxis for thromboembolism, minimize risk of infections (early removal of indwelling urinary catheters, pulmonary hygiene) |
MANAGEMENT OF IBD IN PREGNANT PATIENTS
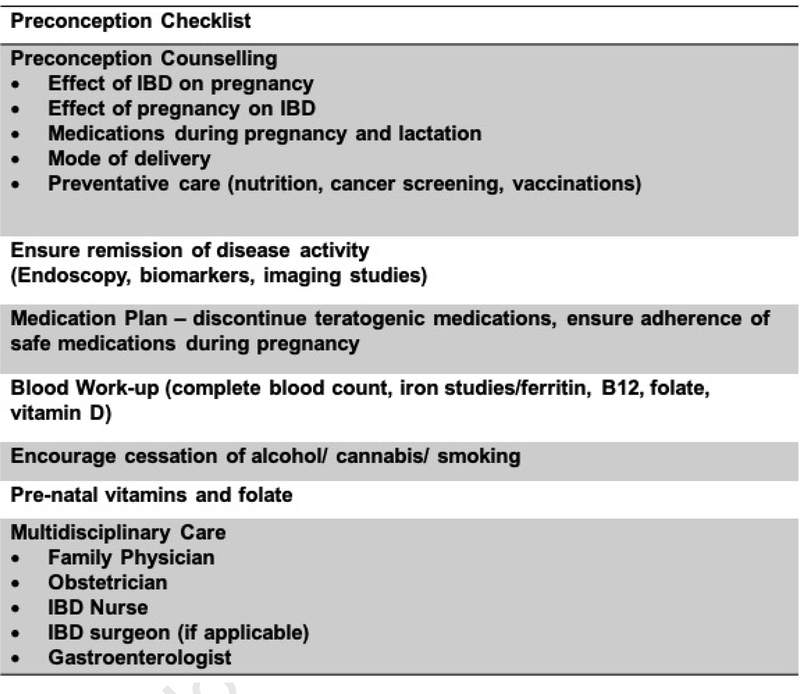
CD has a slight female predominance in the Western world while UC appears to affect females and males equally.52 With the recent increase in pediatric-onset IBD and a peak incidence of the disease occurring between 18 and 35 years of age, management of IBD prior to and during pregnancy will become an increasingly common scenario.52 Accordingly, over 25% of female patients conceive for the first time after their diagnosis.53 Therefore, it is imperative for the practitioner to be conversant with preconception, intra-partum and post-partum counselling. This can be divided into 5 main areas: 1. The effect of IBD on pregnancy, 2. The effect of pregnancy on IBD, 3. The effect of medications during pregnancy and breastfeeding, 4. Mode of delivery, and, 5. Multidisciplinary and preventive care during pregnancy (including nutrition, cervical cancer screening and vaccinations). Figure 3 shows a pre-conception check when planning pregnancy in patients with IBD.
Figure 3:
Preconception checklist when contemplating pregnancy in patients with IBD
Effect of Pregnancy on IBD
Pregnancy can influence the disease course, particularly in patients with UC where intra-partum and post-partum flares appear more common. In contrast, pregnancy has minimal effect on the disease course in women with CD.54, 55 The influence of hormones on cytokine polarisation and subsequent disease activity has been increasingly acknowledged.56
Effect of IBD on Fertility and Pregnancy
Fertility
Voluntary childlessness remains a major contributor to family planning. Women and men with IBD are often concerned about the ability to undertake a pregnancy and parent in light of their own disease state, the fear of heritability, and undue concerns regarding the safety of medications during pregnancy and breastfeeding.57 Women with CD have only marginally lower involuntary fertility rates compared to the general female population, though the rates of subfertility increase with active disease and surgical intervention.58 Ban and colleagues observed that fertility is not decreased in women with UC with an intact colon;58 however, IPAA is associated with a 4-fold reduction in involuntary fertility.59 Surgery in patients with IBD is also associated with a higher risk of miscarriage, use of assisted reproductive therapies, caesarean section delivery and low birth weight infant.60 This highlights the need for aggressive disease control in this cohort to reduce disease flares, and minimize the risk of surgery, and maintain a close dialogue with surgeons to optimise the timing and type of surgery in case surgery is needed.
Pregnancy
Active disease during pregnancy is associated with higher risks of adverse outcomes including preterm birth, low birth weight, miscarriage and stillbirth.55, 61,62 Preterm birth, defined as delivery prior to 37 weeks gestation, is particularly concerning, as complications of preterm delivery remain the leading cause of early childhood mortality, and significant morbidity including severe infection, neurodevelopmental delay, chronic lung disease and sensory impairment, which can lead to lifelong disability.63,64, 65 In a Swedish case-control study, Broms and colleagues observed that significant IBD disease activity during pregnancy increased the odds of preterm birth more than 2-fold (OR, 2.20; 95% CI, 1.37–3.53), and more concerningly, almost 5-fold if there was ongoing disease activity throughout pregnancy, i.e., in both early and late pregnancy (OR, 4.78; 95% CI, 2.10–10.9); 61 conversely, patients with mild disease activity during pregnancy were not at increased risk of preterm birth.61
Assessment of disease activity is paramount and outcomes may be further improved with the use of biomarkers and imaging. A recent systematic review assessed the utility of fecal and laboratory tests in the assessment of the IBD patient during pregnancy.66 Only fecal calprotectin correlated with disease activity during all gestational periods, while hemoglobin, albumin or CRP were not useful during pregnancy. Further, bowel sonography may be a useful adjunct in the assessment of the asymptomatic pregnant woman with CD in allowing detection of subclinical inflammation, while in symptomatic women, it can stratify CD activity.67 Current studies of the safety of lower GI endoscopy during pregnancy are confounded by indication and disease activity.68, 69 However, endoscopic evaluation of IBD during pregnancy should be performed if it will change clinical management (resulting in the initiation or change in medical or surgical therapy).
Both the Toronto Consensus Statements for the Management of IBD in Pregnancy and the AGA’s IBD in Pregnancy Clinical Care Pathway recommend continuation of maintenance medical therapy throughout pregnancy (with the exception of methotrexate and more recently, tofacitinib) to optimize maternal-fetal outcomes.70, 71
Patients’ biggest concerns are about the safety and potential teratogenicity in using medications, when trying to conceive, during pregnancy and while breastfeeding. This may lead to lower medical adherence, with studies observing that 32% women believe IBD medications were ‘bad’ for the fetus believe, 48% report stopping a prescribed IBD medication while pregnant or attempting to conceive, and 25% report that they would rather tolerate the symptoms of IBD, rather than expose the fetus to IBD medications.72,73 While 84% women with IBD were concerned about the impact of IBD medications on pregnancy, only 19% were concerned about the impact of disease activity on pregnancy, which is the bigger risk factor for adverse outcomes.74 Informed and appropriate counselling for patients is therefore imperative alongside appropriate education for the treating physicians regarding the impact and safety of IBD medications during pregnancy. A cross sectional survey demonstrated that less than half of physicians felt comfortable managing pregnant IBD patients. Further, there was significant variation in prescribing patterns through pregnancy.75, 76 Due to these misconceptions about IBD medications, women may completely stop taking their medications during pregnancy or take a lower dosage of medication than prescribed, resulting in adverse maternal and fetal outcomes.70, 71 Comprehensive preconception counselling is independently associated with a greater than five-fold improvement in adherence to IBD medications and a significant 49% reduction in disease relapse during pregnancy.77 A multitude of resources are available for physicians and patients contemplating pregnancy, the most comprehensive of which is the IBD Parenthood Project (http://www.ibdparenthoodproject.gastro.org/) which is associated with the recently published IBD in Pregnancy Clinical Care Pathway.71
Effect of medications during pregnancy and breastfeeding
It should be reinforced that patients should be more concerned about the effects of active disease during pregnancy than the effect of medications, which can keep disease under control. The vast majority of approved IBD therapies, with the exception of methotrexate and tofacitinib are considered safe during conception, pregnancy and breastfeeding.
5-aminosalicylates:
Mesalamine is considered safe during pregnancy and breastfeeding, though preference should be given to non di-butyl-phthalate containing products (all 5-aminosalicylate formulations in the United States are free of di-butyl-phthalate).70
Corticosteroids:
While early data suggested an increased risk of cleft lip and palate, recent data has not demonstrated this to be a significant concern. However, corticosteroids can increase the risks of gestational diabetes, preterm birth and low birth weight, and their use should be limited to treating acute flares during pregnancy.78, 79
Thiopurines:
Despite the historic classification of thiopurines as an FDA category D drug, azathioprine has been widely used in the treatment of inflammatory disorders and in the setting of organ transplantation during pregnancy and lactation, without significant concerns. Earlier concerns on the risks of neonatal anemia,80 and immunosuppression81 have since been replaced by reassuring new data as summarised by the Toronto Consensus Statements for the Management of IBD in Pregnancy.70 Whilst maintenance thiopurines should be continued in those with established disease remission, intrapartum thiopurine initiation should be avoided due to the risk of potential idiosyncratic and severe adverse reactions, including pancreatitis.
Biologic agents and targeted small molecules:.
TNFα antagonists:
With the exception of certolizumab pegol which lacks the neonatal fragment crystallizable (Fc) receptor (FcRn) which is responsible for transfer of protective immunoglobulins from mother to baby, other TNFα antagonists (and non-TNF-targeted biologics) all have a IgG backbone and are actively transferred across the placenta during the second and third trimesters. There is minimal transfer in the first trimester, the period of organogenesis, and these agents have not been associated with an increased risk of congenital abnormalities or fetal death.82,83, 84 Transplacental transfer of biologic therapies begins in the second trimester. Despite this leading to in utero exposure to the fetus, it is recommended that therapy be continued throughout the pregnancy in most mothers. This reduces the risk of IBD relapse and does not increase the risks of maternal or infant complications.70, 85 In the large multi-center TEDDY study, Chaparro and colleagues established that pre-term birth, but not TNFα antagonist monotherapy, is associated with increased risk of neonatal infection.64 Similarly, using the French national health insurance database, Luu and colleagues did not observe any increase in risk of serious infections in infants exposed to TNFα antagonists during pregnancy.85 Regardless, in risk averse patients second trimester cessation of biologic agents may be considered on a case-by-case basis in patients in sustained remission to limit neonatal exposure, after thorough discussion.86 In contrast to biologic monotherapy, addition of thiopurines to TNFα antagonists during pregnancy may increase the risk of infections in infancy, with an almost 3-fold higher risk as compared to TNFα antagonist monotherapy.87 A balanced discussion regarding the relative benefits and risks of TNFα antagonist monotherapy vs. combination therapy during pregnancy, and continuation of combination therapy into the third trimester is therefore warranted.70
Because of the steady transfer of IgG-based biologic agents in the latter trimesters, infants born to biologic-exposed mothers have circulating levels of the biologic agents in the first year of life, which exceeds that of the mother at time of birth.87–89 This has implications for the provision of live vaccinations in the infant in the first year of life and will be further discussed in the preventive care section. The minimal amounts of drug that are transferred to breast milk are proteolyzed by the infants digestive system with no reported short-term (risk of infection) or long-term adverse effects (milestone achievement).90 Therefore the decision to breastfeed should not be dependent on whether a mother is on biologic therapy.70
Non-TNF-targeted biologic agents:
Early data suggests that vedolizumab is safe to use during pregnancy.91–93 While data on ustekinumab is limited to case reports, no major safety signals have been observed with maternal exposure to ustekinumab in the dermatology, rheumatology or gastroenterology literature.94 At present, due to limited prospective and controlled registry data, but with no clear evidence of a negative impact on pregnancy outcomes, detailed discussions and individualized decisions should be made balancing the benefits of ongoing disease control of the mother and the potential risks to the neonate.70
Tofacitinib:
Though human studies are very limited, very high doses of tofacitinib in rats has been demonstrated to cause teratogenicity and fetal death,95 Hence, current manufacturer recommendations are to use effective contraception during tofacitinib treatment, and for 4 to 6 weeks after the last dose. Breastfeeding is not recommended.
Mode of delivery
Rates of cesarean delivery in women with IBD may be up to two-fold higher than the general population.96 This appears to be at odds to the defined indications outlined in the 2016 Toronto Consensus Statements for the Management of IBD which provided a strong recommendation that the decision regarding cesarean delivery should be based on obstetric considerations and not the diagnosis of IBD alone.70, 97 While there are emergency and obstetric indications, and not withstanding personal preference for cesarean delivery, cesarean delivery should constitute the minority of deliveries in women with IBD. Exceptions may be made in two specific scenarios: the presence of active perianal disease98 or perineal lacerations,99 and patients who have undergone an ileal pouch anal anastomosis in order to reduce the risk of anal sphincter injury which may lead to fecal incontinence.70 Women who undergo a caesarean delivery should receive post-operative pharmacologic thromboembolism prophylaxis.70
Multidisciplinary and preventive care
Given the complexities of IBD management during pregnancy, shared care between the family physician, IBD specialist and an obstetrician with familiarity in dealing with potential high-risk pregnancies is essential.71 This ensures that both preventive care measures and care during the preconception period and pregnancy are optimised.
The need for repeat encounters with the health system during pregnancy provides the opportunity for preventive health care. Despite this, Mao and colleagues observed suboptimal rates of health care maintenance in women with IBD.100 Discussions should include nutrition, cervical cancer screening and safety of maternal and infant vaccinations. A prenatal vitamin is recommended, along with routine assessment and relevant supplementation of vitamins D, B12 and iron. All women should receive routine Papanicolaou smears and vaccination status should be reviewed.
Neonatal vaccinations are essential, to prevent a number of serious and potentially deadly infections. Non-live vaccinations should be administered according to local recommended immunization schedules. In utero exposure to a biologic therapy was not found to affect antibody titer concentrations against common vaccinations.101 Current guidelines recommend avoiding any live vaccinations (Bacillus Calmette-Guerin [BCG], rotavirus, oral polio) for at least 6 months unless serum levels in the infant are undetectable,70, 102, 103 though Moens and colleagues reported that exposure to the rotavirus vaccine was not been associated with an increase in adverse events in infants exposed to vedolizumab.92 The report of a fatal case of disseminated BCG infection following vaccination in an infant with in utero exposure to infliximab continues to cause concern.104 Future data incorporating an assessment of immune function and drug levels in exposed infants is required.
Ultimately, the management of pregnant patients with IBD requires a pro-active, multidisciplinary approach, with an emphasis on optimal disease control not just during, but prior to pregnancy. This often involves continuation of highly effective therapies, of which the vast majority are safe during pregnancy and breastfeeding, resulting in a reduction of risk of adverse maternal fetal outcomes.
Table 3.
Safety of medications for inflammatory bowel diseases during pregnancy and lactation.
| 5-aminosalicylates | Safe | Safe | • Avoid di-butyl-phthalate containing products • Ensure folate supplementation with sulfasalazine |
| Thiopurines (azathioprine and 6- mercaptopurine) | Safe | Safe | • Previous concerns about neonatal anaemia and immunosuppression and not demonstrated in recent studies |
| Methotrexate | Teratogen- Avoid in pregnancy | Unsafe-excreted in breast milk | • Cease 6 months prior to pregnancy |
| Corticosteroids (Prednisone, Budesonide) | Safe | Safe | • Increased risk of gestational diabetes and preterm birth. • Use for short periods |
| Infliximab | Safe | Safe | |
| Adalimumab | Safe | Safe | |
| Golimumab | Safe | Safe | |
| Certolizumab pegol | Safe | Safe | • Medication does not cross transplacental barrier |
| Vedolizumab | Limited Data - likely to be safe | Limited Data -likely to be safe | |
| Ustekinumab | Limited Data - likely to be safe | Limited Data- likely to be safe | |
| Tofacitinib | Limited Data- Teratogen in animal models-Avoid until further data available | Limited Data | • Stop 4–6 weeks prior to pregnancy |
Acknowledgments
Funding: Research reported in this publication was supported NIDDK K23DK117058, ACG Junior Faculty Development Award and Crohn’s and Colitis Foundation Career Development Award #404614 to Siddharth Singh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures:
Siddharth Singh has received research grant support from AbbVie; has served as a consultant for AbbVie, Takeda and AMAG Pharmaceuticals, and has received honorarium from Pfizer for ad-hoc grant review
Sherman Picardo has received speaker fees from Takeda and Pfizer.
Cynthia H. Seow has served as a speaker and consultant to Abbvie, Janssen, Takeda, Pfizer, Shire and Ferring.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol 2016;30:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flores A, Burstein E, Cipher DJ, et al. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci 2015;60:2436–45. [DOI] [PubMed] [Google Scholar]
- 3.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2015;21:2857–63. [DOI] [PubMed] [Google Scholar]
- 4.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis 2011;17:2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kugathasan S, Nebel J, Skelton JA, et al. Body mass index in children with newly diagnosed inflammatory bowel disease: observations from two multicenter North American inception cohorts. J Pediatrics 2007;151:523–7. [DOI] [PubMed] [Google Scholar]
- 6.Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014;43:843–55. [DOI] [PubMed] [Google Scholar]
- 7.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses’ Health Study cohorts. BMJ 2013;347:f6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol 2014;5:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pringle PL, Stewart KO, Peloquin JM, et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn’s Disease. Inflamm Bowel Dis 2015;21:2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Nguyen NH, Proudfoot JA, et al. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am J Gastroenterol 2019;114:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Khera R, Sandborn WJ. Obesity is Associated with Worse Outcomes in Hospitalized Patients with Inflammatory Bowel Diseases: A Nationwide Study, 2016. [Google Scholar]
- 12.Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. Obesity Is Independently Associated With Higher Annual Burden and Costs of Hospitalization in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2019;17:709–718 e7. [DOI] [PubMed] [Google Scholar]
- 13.Uko V, Vortia E, Achkar J-P, et al. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn’s disease. Inflamm Bowel Dis 2014;20:2286–91. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhu W, Gong J, et al. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn’s disease. Colorectal Dis 2015;17:225–34. [DOI] [PubMed] [Google Scholar]
- 15.Kurnool S, Nguyen NH, Proudfoot J, et al. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther 2018;47:1472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Proudfoot J, Xu R, et al. Obesity and Response to Infliximab in Patients with Inflammatory Bowel Diseases: Pooled Analysis of Individual Participant Data from Clinical Trials. Am J Gastroenterol 2018;113:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bultman E, de Haar C, van Liere-Baron A, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn’s disease patients. Aliment Pharmacol Ther 2012;35:335–41. [DOI] [PubMed] [Google Scholar]
- 18.Vande Casteele N, Baert F, Bian S, et al. Subcutaneous Absorption Contributes to Observed Interindividual Variability in Adalimumab Serum Concentrations in Crohn’s Disease: A Prospective Multicentre Study. J Crohns Colitis 2019;13:1248–1256. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-alpha agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One 2018;13:e0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino T, Shukla PJ, Rubino F, et al. The Impact of Obesity on Perioperative Outcomes After Laparoscopic Colorectal Resection. Ann Surg 2012;255:228–236. [DOI] [PubMed] [Google Scholar]
- 21.McKenna NP, Mathis KL, Khasawneh MA, et al. Obese Patients Undergoing Ileal Pouch-Anal Anastomosis: Short-and Long-term Surgical Outcomes. Inflamm Bowel Dis 2017;23:2142–2146. [DOI] [PubMed] [Google Scholar]
- 22.Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond) 2015;39:1197–202. [DOI] [PubMed] [Google Scholar]
- 23.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther 2014;39:459–77. [DOI] [PubMed] [Google Scholar]
- 24.Singh SUF, Loftus EV, Dulai PS, Ghosh S, Sandborn WJ, Ng SC, Kaplan GG. Worldwide Incidence of Older-Onset Inflammatory Bowel Diseases in the 21st Century: A Systematic Review of Population-Based Studies. Gastroenterology 2019; 156:S394–S395. [Google Scholar]
- 25.Coward S, Clement F, Benchimol EI, et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019;156:1345–1353 e4. [DOI] [PubMed] [Google Scholar]
- 26.Ha CY, Katz S. Clinical implications of ageing for the management of IBD. Nat Rev Gastroenterol Hepatol 2014;11:128–38. [DOI] [PubMed] [Google Scholar]
- 27.Britton E, McLaughlin JT. Ageing and the gut. Proc Nutr Soc 2013;72:173–7. [DOI] [PubMed] [Google Scholar]
- 28.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science 2015;350:1214–5. [DOI] [PubMed] [Google Scholar]
- 29.Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic Review and Meta-analysis: Phenotype and Clinical Outcomes of Older-onset Inflammatory Bowel Disease. J Crohns Colitis 2016;10:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everhov A, Halfvarson J, Myrelid P, et al. Incidence and Treatment of Patients Diagnosed With Inflammatory Bowel Diseases at 60 Years or Older in Sweden. Gastroenterology 2018;154:518–528.e15. [DOI] [PubMed] [Google Scholar]
- 31.Alexakis C, Saxena S, Chhaya V, et al. Do Thiopurines Reduce the Risk of Surgery in Elderly Onset Inflammatory Bowel Disease? A 20-Year National Population-Based Cohort Study. Inflamm Bowel Dis 2017;23:672–680. [DOI] [PubMed] [Google Scholar]
- 32.Jeuring SF, van den Heuvel TR, Zeegers MP, et al. Epidemiology and Long-term Outcome of Inflammatory Bowel Disease Diagnosed at Elderly Age-An Increasing Distinct Entity? Inflamm Bowel Dis 2016;22:1425–34. [DOI] [PubMed] [Google Scholar]
- 33.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 34.Lakatos PL, David G, Pandur T, et al. IBD in the elderly population: results from a population-based study in Western Hungary, 1977–2008. J Crohns Colitis 2011;5:5–13. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen NH, Ohno-Machado L, Sandborn WJ, et al. Infections and Cardiovascular Complications are Common Causes for Hospitalization in Older Patients with Inflammatory Bowel Diseases. Inflamm Bowel Dis 2018;24:916–923. [DOI] [PubMed] [Google Scholar]
- 36.Olen O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 37.Cheddani H, Dauchet L, Fumery M, et al. Cancer in Elderly Onset Inflammatory Bowel Disease: A Population-Based Study. Am J Gastroenterol 2016;111:1428–1436. [DOI] [PubMed] [Google Scholar]
- 38.Govani SM, Wiitala WL, Stidham RW, et al. Age Disparities in the Use of Steroid-sparing Therapy for Inflammatory Bowel Disease. Inflamm Bowel Dis 2016;22:1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benchimol EI, Cook SF, Erichsen R, et al. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J Crohns Colitis 2013;7:878–89. [DOI] [PubMed] [Google Scholar]
- 40.Brassard P, Bitton A, Suissa A, et al. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol 2014;109:1795–802; quiz 1803. [DOI] [PubMed] [Google Scholar]
- 41.Lewis JD, Scott FI, Brensinger CM, et al. Increased Mortality Rates With Prolonged Corticosteroid Therapy When Compared With Antitumor Necrosis Factor-a-Directed Therapy for Inflammatory Bowel Disease. Am J Gastroenterol 2018;113:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaparro M, Ordas I, Cabre E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 2013;19:1404–10. [DOI] [PubMed] [Google Scholar]
- 43.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617–25. [DOI] [PubMed] [Google Scholar]
- 44.Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol 2015;13:847–58 e4; quiz e48–50. [DOI] [PubMed] [Google Scholar]
- 45.Borren NZ, Ananthakrishnan AN. Safety of Biologic Therapy in Older Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association Between Use of Thiopurines or T umor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA 2017;318:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor alpha antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther 2019;49:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul S, Roblin X. Letter: immunogenicity of anti-TNF in elderly IBD patients. Aliment Pharmacol Ther 2019;50:336. [DOI] [PubMed] [Google Scholar]
- 49.Singh S, Stitt LW, Zou G, et al. Early combined immunosuppression may be effective and safe in older patients with Crohn’s disease: post hoc analysis of REACT. Aliment Pharmacol Ther 2019;49:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis 2009;15:182–9. [DOI] [PubMed] [Google Scholar]
- 51.McKenna NP, Mathis KL, Pemberton JH, et al. The Impact of Age at Time of Ileal Pouch Anal Anastomosis on Short and Long-Term Outcomes in Adults. Inflamm Bowel Dis 2018;24:1857–1865. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan GG, Bernstein CN, Coward S, et al. The Impact of Inflammatory Bowel Disease in Canada 2018: Epidemiology. J Can Assoc Gastroenterol 2019;2:S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20:1761–9. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen N, Bortoli A, Duricova D, et al. The course of inflammatory bowel disease during pregnancy and postpartum: a prospective European ECCO-EpiCom Study of 209 pregnant women. Aliment Pharmacol Ther 2013;38:501–12. [DOI] [PubMed] [Google Scholar]
- 55.de Lima-Karagiannis A, Zelinkova-Detkova Z, van der Woude CJ. The Effects of Active IBD During Pregnancy in the Era of Novel IBD Therapies. Am J Gastroenterol 2016;111:1305–12. [DOI] [PubMed] [Google Scholar]
- 56.van der Giessen J, Huang VW, van der Woude CJ, et al. Modulatory Effects of Pregnancy on Inflammatory Bowel Disease. Clin Transl Gastroenterol 2019;10:e00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mountifield R, Bampton P, Prosser R, et al. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm Bowel Dis 2009;15:720–5. [DOI] [PubMed] [Google Scholar]
- 58.Ban L, Tata LJ, Humes DJ, et al. Decreased fertility rates in 9639 women diagnosed with inflammatory bowel disease: a United Kingdom population-based cohort study. Aliment Pharmacol Ther 2015;42:855–66. [DOI] [PubMed] [Google Scholar]
- 59.Rajaratnam SG, Eglinton TW, Hider P, et al. Impact of ileal pouch-anal anastomosis on female fertility: meta-analysis and systematic review. Int J Colorectal Dis 2011;26:1365–74. [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Crowe M, Seow CH, et al. The impact of surgical therapies for inflammatory bowel disease on female fertility. Cochrane Database Syst Rev 2019;7:CD012711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broms G, Granath F, Linder M, et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm Bowel Dis 2014;20:1091–8. [DOI] [PubMed] [Google Scholar]
- 62.Kammerlander H, Nielsen J, Kjeldsen J, et al. The Effect of Disease Activity on Birth Outcomes in a Nationwide Cohort of Women with Moderate to Severe Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1011–1018. [DOI] [PubMed] [Google Scholar]
- 63.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaparro M, Verreth A, Lobaton T, et al. Long-Term Safety of In Utero Exposure to Anti-TNFalpha Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am J Gastroenterol 2018;113:396–403. [DOI] [PubMed] [Google Scholar]
- 65.Howson CP, Kinney MV, McDougall L, et al. Born too soon: preterm birth matters. Reprod Health 2013;10 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tandon P, Leung K, Yusuf A, et al. Noninvasive Methods For Assessing Inflammatory Bowel Disease Activity in Pregnancy: A Systematic Review. J Clin Gastroenterol 2019;53:574–581. [DOI] [PubMed] [Google Scholar]
- 67.Leung Y, Shim HH, Wilkens R, et al. The Role of Bowel Ultrasound in Detecting Subclinical Inflammation in Pregnant Women with Crohn’s Disease. J Can Assoc Gastroenterol 2019;2:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Lima A, Zelinkova Z, van der Woude CJ. A prospective study of the safety of lower gastrointestinal endoscopy during pregnancy in patients with inflammatory bowel disease. J Crohns Colitis 2015;9:519–24. [DOI] [PubMed] [Google Scholar]
- 69.Ludvigsson JF, Lebwohl B, Ekbom A, et al. Outcomes of Pregnancies for Women Undergoing Endoscopy While They Were Pregnant: A Nationwide Cohort Study. Gastroenterology 2017;152:554–563 e9. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016;150:734–757 e1. [DOI] [PubMed] [Google Scholar]
- 71.Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019;156:1508–1524. [DOI] [PubMed] [Google Scholar]
- 72.Gallinger ZR, Rumman A, Nguyen GC. Perceptions and Attitudes Towards Medication Adherence during Pregnancy in Inflammatory Bowel Disease. J Crohns Colitis 2016;10:892–7. [DOI] [PubMed] [Google Scholar]
- 73.Selinger CP, Eaden J, Selby W, et al. Inflammatory bowel disease and pregnancy: lack of knowledge is associated with negative views. J Crohns Colitis 2013;7:e206–13. [DOI] [PubMed] [Google Scholar]
- 74.Mountifield RE, Prosser R, Bampton P, et al. Pregnancy and IBD treatment: this challenging interplay from a patients’ perspective. J Crohns Colitis 2010;4:176–82. [DOI] [PubMed] [Google Scholar]
- 75.Lenti MV, Selinger CP. Medication non-adherence in adult patients affected by inflammatory bowel disease: a critical review and update of the determining factors, consequences and possible interventions. Expert Rev Gastroenterol Hepatol 2017;11:215–226. [DOI] [PubMed] [Google Scholar]
- 76.Huang VW, Chang HJ, Kroeker KI, et al. Management of Inflammatory Bowel Disease during Pregnancy and Breastfeeding Varies Widely: A Need for Further Education. Can J Gastroenterol Hepatol 2016;2016:6193275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Lima A, Zelinkova Z, Mulders AG, et al. Preconception Care Reduces Relapse of Inflammatory Bowel Disease During Pregnancy. Clin Gastroenterol Hepatol 2016;14:1285–1292 e1. [DOI] [PubMed] [Google Scholar]
- 78.Leung YP, Kaplan GG, Coward S, et al. Intrapartum corticosteroid use significantly increases the risk of gestational diabetes in women with inflammatory bowel disease. J Crohns Colitis 2015;9:223–30. [DOI] [PubMed] [Google Scholar]
- 79.Bandoli G, Palmsten K, Forbess Smith CJ, et al. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum Dis Clin North Am 2017;43:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jharap B, de Boer NK, Stokkers P, et al. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut 2014;63:451–7. [DOI] [PubMed] [Google Scholar]
- 81.Thomas C, Monteil-Ganiere C, Mirallie S, et al. A Severe Neonatal Lymphopenia Associated With Administration of Azathioprine to the Mother in a Context of Crohn’s Disease. J Crohns Colitis 2018;12:258–261. [DOI] [PubMed] [Google Scholar]
- 82.Malek A, Sager R, Schneider H. Transport of proteins across the human placenta. Am J Reprod Immunol 1998;40:347–51. [DOI] [PubMed] [Google Scholar]
- 83.Narula N, Al-Dabbagh R, Dhillon A, et al. Anti-TNFalpha therapies are safe during pregnancy in women with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2014;20:1862–9. [DOI] [PubMed] [Google Scholar]
- 84.Shihab Z, Yeomans ND, De Cruz P. Anti-Tumour Necrosis Factor alpha Therapies and Inflammatory Bowel Disease Pregnancy Outcomes: A Meta-analysis. J Crohns Colitis 2016;10:979–88. [DOI] [PubMed] [Google Scholar]
- 85.Luu M, Benzenine E, Doret M, et al. Continuous Anti-TNFalpha Use Throughout Pregnancy: Possible Complications For the Mother But Not for the Fetus. A Retrospective Cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol 2018;113:1669–1677. [DOI] [PubMed] [Google Scholar]
- 86.de Lima A, Zelinkova Z, van der Ent C, et al. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016;65:1261–8. [DOI] [PubMed] [Google Scholar]
- 87.Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of Adalimumab and Infliximab in Mothers and Newborns, and Effects on Infection. Gastroenterology 2016;151:110–9. [DOI] [PubMed] [Google Scholar]
- 88.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:286–92; quiz e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zelinkova Z, de Haar C, de Ridder L, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther 2011;33:1053–8. [DOI] [PubMed] [Google Scholar]
- 90.Matro R, Martin CF, Wolf D, et al. Exposure Concentrations of Infants Breastfed by Women Receiving Biologic Therapies for Inflammatory Bowel Diseases and Effects of Breastfeeding on Infections and Development. Gastroenterology 2018;155:696–704. [DOI] [PubMed] [Google Scholar]
- 91.Bar-Gil Shitrit A, Ben Ya’acov A, Livovsky DM, et al. Exposure to Vedolizumab in IBD Pregnant Women Appears of Low Risk for Mother and Neonate: A First Prospective Comparison Study. Am J Gastroenterol 2019;114:1172–1175. [DOI] [PubMed] [Google Scholar]
- 92.Moens A, van Hoeve K, Humblet E, et al. Outcome of Pregnancies in Female Patients With Inflammatory Bowel Diseases Treated With Vedolizumab. J Crohns Colitis 2019;13:12–18. [DOI] [PubMed] [Google Scholar]
- 93.Mahadevan U, Vermeire S, Lasch K, et al. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:941–950. [DOI] [PubMed] [Google Scholar]
- 94.Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 95.Mahadevan U, Dubinsky MC, Su C, et al. Outcomes of Pregnancies With Maternal/Paternal Exposure in the Tofacitinib Safety Databases for Ulcerative Colitis. Inflamm Bowel Dis 2018;24:2494–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen GC, Boudreau H, Harris ML, et al. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol 2009;7:329–34. [DOI] [PubMed] [Google Scholar]
- 97.Seow CH. Not Too Late to Make Cutting-Edge Decisions: Editorial Response to ‘Predictors of Cesarean Delivery in Pregnant Women with Inflammatory Bowel Disease’. J Can Assoc Gastroenterol 2018;1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Foulon A, Dupas JL, Sabbagh C, et al. Defining the Most Appropriate Delivery Mode in Women with Inflammatory Bowel Disease: A Systematic Review. Inflamm Bowel Dis 2017;23:712–720. [DOI] [PubMed] [Google Scholar]
- 99.Hatch Q, Champagne BJ, Maykel JA, et al. Crohn’s disease and pregnancy: the impact of perianal disease on delivery methods and complications. Dis Colon Rectum 2014;57:174–8. [DOI] [PubMed] [Google Scholar]
- 100.Mao EJ, Sheibani S, Martin C, et al. Preventive Health Care Among Postpartum Women With Inflammatory Bowel Disease: Results From the PIANO Registry. Inflamm Bowel Dis 2019;25:797–802. [DOI] [PubMed] [Google Scholar]
- 101.Beaulieu DB, Ananthakrishnan AN, Martin C, et al. Use of Biologic Therapy by Pregnant Women With Inflammatory Bowel Disease Does Not Affect Infant Response to Vaccines. Clin Gastroenterol Hepatol 2018;16:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107–24. [DOI] [PubMed] [Google Scholar]
- 103.Mahadevan U, Cucchiara S, Hyams JS, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol 2011;106:214–23; quiz 224. [DOI] [PubMed] [Google Scholar]
- 104.Cheent K, Nolan J, Shariq S, et al. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis 2010;4:603–5. [DOI] [PubMed] [Google Scholar]