Abstract
Long-term survival after lung transplantation remains profoundly limited by graft rejection. Recent work has shown that bronchus-associated lymphoid tissue (BALT), characterized by the development of peripheral nodal addressin (PNAd)-expressing high endothelial venules and enriched in B and Foxp3+ T cells, is important for the maintenance of allograft tolerance. Mechanisms underlying BALT induction in tolerant pulmonary allografts, however, remain poorly understood. Here, we show that the development of PNAd-expressing high endothelial venules within intragraft lymphoid follicles and the recruitment of B cells, but not Foxp3+ cells depends on IL-22. We identify graft-infiltrating gamma-delta (γδ) T cells and Type 3 innate lymphoid cells (ILC3s) as important producers of IL-22. Reconstitution of IL-22 at late time points through retransplantation into wildtype hosts mediates B cell recruitment into lymphoid follicles within the allograft, resulting in a significant increase in their size, but does not induce PNAd expression. Our work has identified cellular and molecular requirements for the induction of BALT in pulmonary allografts during tolerance induction and may provide a platform for the development of new therapies for lung transplant patients.
1. Introduction
Bronchus-associated lymphoid tissue (BALT) is a pulmonary tertiary lymphoid organ (TLO) that plays a critical role in the inflammatory response to a range of lung pathology, including infection, autoimmunity, and allograft rejection (1-3). However, recent evidence has emerged that pulmonary TLOs are not synonymous with the generation of deleterious proinflammatory responses (4-7). To this end, the development of BALT has been associated with the downregulation of immune responses. Tumor-associated TLOs are one such example, which have been found in both human and mouse models of lung cancer (4). Pulmonary tumor-associated TLOs are enriched in Foxp3+ regulatory T cells, which when selectively depleted result in activation of dendritic cells as well as T lymphocytes and tumor regression. Similarly, we have found that BALT is induced in tolerant lung allografts that is enriched in regulatory Foxp3+ T cells (5). Our work has revealed that Foxp3+ T cells residing within BALT of tolerant lung allografts prevent antibody-mediated rejection by inhibiting the local activation of B cells (6).
Mechanisms leading to the induction of BALT, particularly that which is associated with downregulation of immune responses, remain poorly understood. BALT that develops in response to inflammatory stimuli, such as LPS administration or infection, principally depends on IL-17 production by T cells. Here, IL-17 stimulates lymphocyte-attracting chemokines, such as CXCL12 and CXCL13, resulting in B and T cell accumulation, and BALT formation; this process is independent of lymphotoxin signaling, distinguishing it from the mechanisms that mediate the formation of secondary lymphoid organs (SLO) (1, 8, 9). Other studies investigating the requirements for induction of TLOs in lungs and other tissues suggest that LTβ, IL-22, and IL-23, among other factors, may play a role in their development (10-12). The cell populations responsible for TLO induction also vary considerably among organ systems and include Th17 cells, innate lymphoid cells (ILC), gamma-delta (γδ) T cells, and natural killer cells (8) (11, 13).
Here, we take advantage of our previously established orthotopic, vascularized mouse lung transplant model to show that the recruitment of B cells and the development of peripheral nodal addressin (PNAd)-expressing high endothelial venules (HEVs) in tolerant pulmonary allografts is dependent on IL-22 (14). We have found that graft-infiltrating γδ T cells and Type 3 ILCs (ILC3s) are important producers of IL-22 following lung transplantation. Remarkably, the formation of Foxp3+ cell aggregates within tolerant allografts does not depend on IL-22. Retransplantation experiments demonstrate that delayed restoration of IL-22 expression mediates B cell recruitment to the intragraft lymphoid follicles and increases their size, but does not result in the development of PNAd-expressing HEVs. Thus, our findings define a new pathway for the induction of BALT in tolerant lung allografts and suggest new therapeutic avenues.
2. Materials and methods
2.1. Mice
C57BL/6J (B6), Balb/cJ (BALB/c), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+), B6.129P2-Tcrdtm1Mom/J (TCRδ−/−), and B6 Foxp3-DTR (diphtheria toxin receptor) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). RORγt-cre AHRfl/fl mice (referred to as AR mice) and IL-22-deficient mice were obtained from M. Colonna and S. Hultgren (Washington University, St. Louis, MO), respectively (15, 16).
2.2. Lung Transplantation
Left orthotopic vascularized lung transplants were performed as previously described (17). For select experiments mice were treated with costimulatory blockade consisting of MR1 (250 μg intraperitoneally (i.p.)) and CTLA4- Ig (200 μg i.p.) on days 0 and 2, respectively (Bio X Cell, West Lebanon, NH). For select experiments, lung recipients were additionally treated with anti-IL-23 antibody (20 μg i.p., clone N71-1183; BD Biosciences, San Jose, CA) on days 0, 2, 4, 6 and then 3 times weekly thereafter (18). Lung retransplantations were performed as previously described (19). No additional immunosuppressants were given after retransplantation. The mice were sacrificed on day 7 or at least 30 days after primary transplantation or retransplantation as indicated. DT (List Biologic Laboratories, Campbell, CA) (1 μg i.p.) was administered at the time of retransplantation (days 0, 1) to B6 mice receiving grafts that had initially been transplanted into IL-23-neutralized B6 Foxp3-DTR hosts. All procedures were approved by the Institutional Animal Care and Use Committee at Washington University.
2.3. Histology
Longitudinal sections of lung allografts were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). For quantification of lymphoid follicles, two separate and non-adjacent sections were obtained from one paraffin-embedded tissue block. Cell aggregates with more than 100 cells were classified as lymphoid follicles. The number and area occupied by lymphoid follicles was quantified using Image J image processing and analysis software (National Institutes of Health, Bethesda, MD).
2.4. Immunostaining
For immunohistochemistry, anti-mouse PNAd carbohydrate epitope (clone MECA-79; BD Biosciences, San Jose, CA), anti-Foxp3 monoclonal (clone FJK-16s; eBioscience, Waltham, MA), and anti-CCL21 polyclonal (R&D Systems, Minneapolis, MN) primary antibodies were used. For immunofluorescence, the following primary antibodies were used: anti-mouse CD45R (B220) (clone RA3-6B2, Thermo Fisher Scientific, Waltham, MA), anti-CD3ε monoclonal antibody (clone SP7, Thermo Fisher Scientific), anti-CXCL13 polyclonal antibody (Thermo Fisher Scientific). Abundance of B220+ and CD3+ cells in lymphoid follicles was quantified using Image J and reported as B220 / CD3 ratios.
2.5. Flow cytometry
In order to obtain single-cell suspensions, lungs were digested with type I collagenase (Worthington Biochemical, Lakewood, NJ) and DNase I (Sigma-Aldrich, St. Louis, MO), red blood cell lysis was performed with ACK lysing buffer, and cells were passed through a 40μM wire mesh. For determination of IL-22 expression cell suspensions were stimulated overnight in complete-stimulation media using recombinant mouse IL-23 (50 ng/mL) (R&D Systems, Minneapolis, MN). Subsequently, cells were stimulated with PMA/ionomycin for 4 hours with Brefeldin present for the last 3 hours of stimulation as previously described (20). Surface and intracellular staining were performed with the following antibodies: anti-IL-22 (clone 1H8PWSR) (ThermoFisher/eBioscience, Waltham, MA), CD3ε (clone 145-2C11, BD Biosciences and clone 17A2, Biolegend, San Diego, CA), CD4 (clone RM4-5, Biolegend), CD8 (clone 53-6.7, Biolegend), CD19 (clone 6D5, Biolegend), CD45.1 (clone A20, Biolegend), CD90.2 (clone 53-2.1, Biolegend), CD127 (clone A7R34, Biolegend), RORγt (clone Q31-378, BD Biosciences), TCRγδ (clone GL3, BD Biosciences) and NKp46 (biotinylated polyclonal, R&D Systems). Biotin was detected with fluorochrome-conjugated streptavidin (BD Biosciences). Dead cells were excluded with Zombie Fixable Viability stain (Biolegend). Samples were acquired on an LSR Fortessa X-20 equipped with 5 lasers (355, 405, 488, 561, 640 nm; BD Biosciences) and analyzed using FlowJo Version 10.5 (TreeStar).
2.6. Quantitative PCR
Homogenized lung tissue was lysed with TRIzol (Thermo Fisher Scientific). Total RNA was isolated using QIAGEN RNeasy Mini Kit (QIAGEN), and quantitative PCR was performed as previously described (21). Primer sequences were as follows: IL-22, 5′- ATACATCGTCAACCGCACCTTTA −3′ and 5′- AGCCGGACATCTGTGTTGTTA −3′; IL-23p19, 5’ -GACCCACAAGGACTCAAGGAC −3’ and 5’- ATGGGGCTATCAGGGAGTAGAG −3’; IL-17, 5’- TCAGCGTGTCCAAACACTGAG-3’ and 5’- CGCCAAGGGAGTTAAAGACTT-3’; LTB, 5’-GATGACAGCAAACCGTCGTG-3’ and 5’- CCTGGAAGCATTGGATCTCTG-3’
2.7. Statistics
Gene expression levels and continuous variables from histology specimens (i.e.: follicle number, B220/CD3 ratios) were compared between groups and presented in figures as mean ± standard error of the mean. The nonparametric Mann-Whitney U test was used to determine statistical differences between groups. P values less than 0.05 were considered to be statistically significant, as indicated in the figure legends.
2.8. Data availability
The data that support the findings of this study are available from the corresponding author on request.
3. Results
3.1. Induction of BALT in tolerant lung grafts is dependent on IL-22
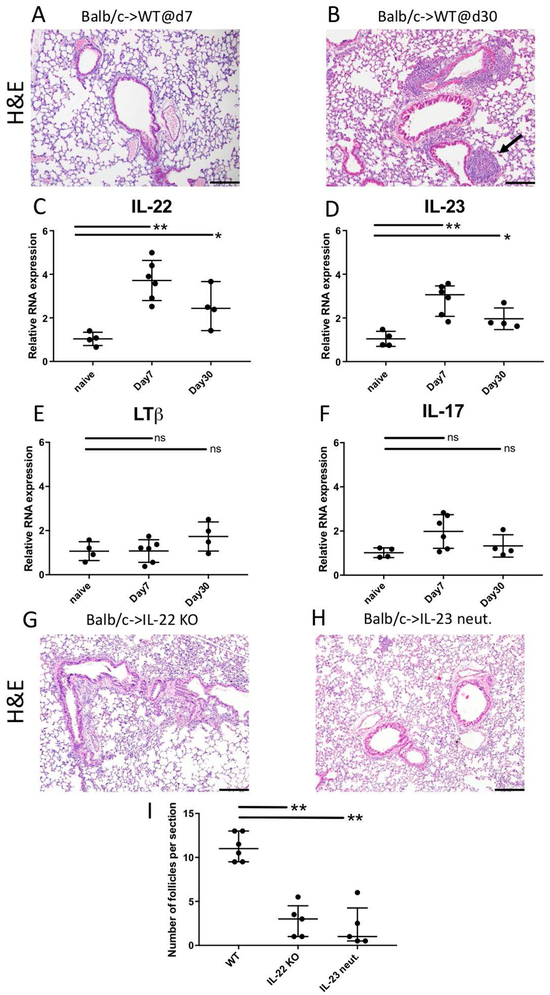
We have previously shown that peri-operative blockade of CD40-CD154 and B7-CD28 costimulatory pathways in recipient mice prevents acute graft rejection 7 days after lung transplantation (Figure 1A). In this model, allografts survive long-term, BALT is consistently induced by 30 days after transplantation and recipients develop donor-specific tolerance (Fig. 1B) (5). To determine the molecular cues responsible for BALT induction, we performed real-time PCR to quantify expression of cytokines and other factors which have been linked to TLO formation in other models. We found that expression levels of IL-22 (Fig. 1C) and IL-23 (Fig. 1D) were significantly elevated in tolerant lung allografts at both 7 and 30 days after transplantation compared to naïve lungs. The expression levels of LTβ (Fig. 1E) and IL-17 (Fig. 1F), however, were not elevated in lung allografts compared to naïve lungs.
Figure 1:
BALT induction in tolerant lung allografts requires IL-22 and IL-23 expression. Histological appearance of Balb/c lung allografts (A) 7 and (B) 30 days after transplantation into B6 wildtype (WT) mice that received peri-operative costimulatory blockade, with arrow depicting BALT at 30 days. Relative RNA expression of (C) IL-22, (D) IL-23, (E) LTβ, and (F) IL-17 from whole lung lysates of naïve Balb/c lungs and transplanted lungs at days 7 and 30. Appearance of lung allografts 30 days after transplantation into (G) IL-22 deficient hosts or (H) wildtype recipients receiving IL-23 neutralization. Recipients were treated with peri-operative costimulatory blockade. Scale bars: 100μM. (I) Quantification of the number of lymphoid follicles per section 30 days after transplantation into various recipients. n≥4 for all conditions. * p<0.05; **p<0.01; ns=not significant.
IL-23 signaling has been shown to trigger IL-22 expression in both humans and mice (22-24). Therefore, we next asked whether IL-22 and IL-23 are required for BALT induction in tolerant lung allografts. To examine this, we transplanted Balb/c lung grafts into IL-22-deficient B6 recipients (Fig. 1G) or wildtype B6 hosts that were treated with an IL-23-neutralizing antibody (Fig. 1H). We found that in both conditions allografts survived long term (>30 days), but developed significantly fewer lymphoid follicles when compared to wildtype conditions (Fig. 1I). When we transplanted Balb/c lungs into non-immunosuppressed B6 recipients grafts were acutely rejected irrespective of IL-22 expression in the recipient (Suppl. Fig. 1).
3.2. γδ T cells and ILC3s are important sources of IL-22 in tolerant lung allografts
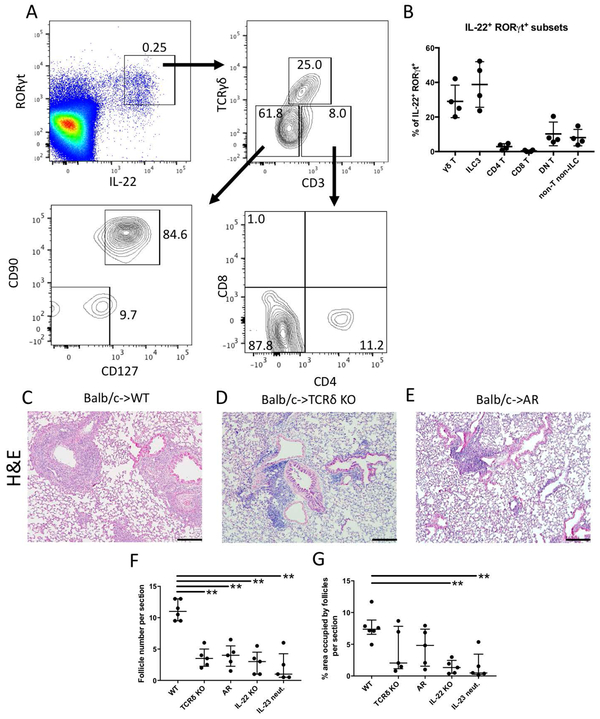
Several cell populations have been found to produce IL-22 in response to IL-23 in both humans and mice. Pulmonary γδ T cells are one such subset, which have largely been found to be proinflammatory and capable of producing IL-17 and IFN-γ in response to infection or sterile inflammation such as ischemia reperfusion injury (24-26). In addition, Type 3 ILCs (ILC3) have also been found to produce IL-22 and are implicated in the regulation of allergic lung disease (27, 28). An analysis of our transplants revealed that both γδ T cells (Suppl. Fig. 2A) and ILC3s (Suppl. Fig. 2B, C) infiltrated lung allografts early during tolerance induction. To determine which cell type was the source of IL-22 in tolerant lung grafts, we harvested Balb/c lungs 7 days after transplantation into wildtype B6 recipients that had received peri-operative co-stimulatory blockade. Flow cytometric analysis revealed that virtually all IL-22+ cells also expressed RAR-related orphan receptor γ (RORγt). The majority of IL-22-expressing cells in tolerant lung grafts consisted of γδ T cells (TCRγδ+CD3+) and ILC3s (TCRγδ−CD3−CD90+CD127+) (Figs. 2A, B). Other RORγt+IL-22+ cells included CD4+ T cells (TCRγδ−CD3+CD4+CD8−), non-T non-ILCs (TCRγδ−CD3−CD90−CD127−), and double-negative T cells that did not express CD4 or CD8 (TCRγδ−CD3+CD4−CD8−), which may include T cells that downregulated their co-receptors during stimulation.
Figure 2:
Graft-infiltrating γδ T cells and ILC3s are important sources of IL-22 after lung transplantation. (A) Flow cytometric characterization of IL-22+ cells types in tolerant Balb/c → B6 CD45.1 lung grafts reveals γδ T cells (RORγt+CD3+TCRγδ+) and ILC3s (RORγt+CD3−TCRγδ−CD127+CD90+) as main producers. Other IL-22+ cells include CD4+ T cells (RORγt+CD3+TCRγδ−CD4+CD8−), double-negative (DN) T cells (RORγt+CD3+TCRγδ−CD4−CD8−), CD8+ T cells (RORγt+CD3+TCRγδ−CD4−CD8+) and non-T non-ILCs (RORγt+CD3−TCRγδ−CD127−CD90−). Plots are gated on live single CD45.1+ cells. (B) Quantification of RORγt+IL-22+ cell subpopulations in tolerant lung grafts based on the gating plots described above. Histological appearance of lung allografts 30 days after transplantation into (C) wildtype (WT), (D) γδ T cell-deficient (TCRδ KO) recipients and (E) AR mice (RORγt-cre Ahrfl/fl) that received peri-operative co-stimulatory blockade. Quantification of the (F) number of lymphoid follicles and (G) percentage of total area occupied by lymphoid follicles per section 30 days after transplantation into various recipients. n≥4 for all conditions. **p<0.01. IL-22 KO: IL-22-deficient; IL-23 neut: IL-23 neutralization. Scale bars: 100μM.
To determine if γδ T cells are required for BALT formation, we transplanted Balb/c lungs into B6 TCRδ−/− hosts that were treated with peri-operative costimulatory blockade (29). Previous studies have employed conditional knockout mice in which aryl hydrocarbon receptor (AHR) deficiency is restricted to RORγt-expressing cells (AR mice) to examine the functional roles of ILC3s in inflammation (15). Therefore, to evaluate the role of ILC3s in the induction of BALT, we transplanted Balb/c allografts into AR recipients under peri-operative costimulatory blockade. Compared to wildtype recipients (Fig. 2C), γδ T cell-deficient (Fig. 2D) and AR (Fig. 2E) recipients had substantially fewer lymphoid follicles per section (Fig. 2F), resembling IL-22 deficient (Fig. 1G) or IL-23 neutralized (Fig. 1H) hosts. Lymphoid follicles after transplantation into control AHRfl/fl recipients resembled wildtype conditions (data not shown). To examine the size of induced BALT, the area occupied by lymphoid follicles per section was measured. We found that grafts transplanted into IL-22-deficient or IL-23-neutralized recipients had a lower mean area occupied by lymphoid follicles compared to wildtype hosts. Interestingly, while γδ T cell-deficient and AR recipients developed fewer lymphoid follicles than wildtype recipients, the mean area occupied by lymphoid follicles per section was not significantly different (Fig. 2G).
3.3. IL-22 is required for PNAd expression and B cell, but not Foxp3+ cell, recruitment into lymphoid follicles
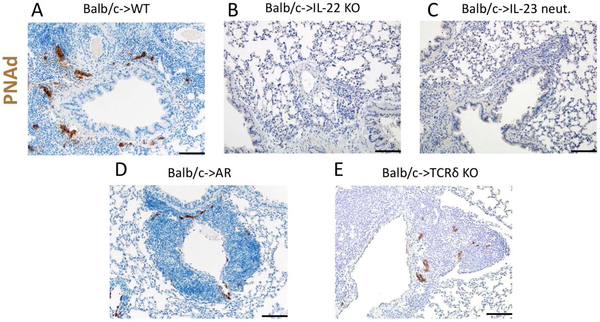
A hallmark of TLOs is the neogenesis of PNAd-expressing HEVs. PNAd facilitates the recruitment of lymphocytes into TLOs including BALT (30). Consistent with our previous report, tolerant lung grafts express abundant PNAd within BALT after transplantation into wildtype recipients (Fig. 3A). By contrast, we did not detect PNAd within the few, small lymphoid follicles that form within lung allografts after transplantation into IL-22-deficient (Fig. 3B) or IL-23-neutralized (Fig. 3C) recipients. PNAd staining was detected in lymphoid follicles in lung allografts after transplantation into AR hosts (Fig. 3D) or γδ T cell-deficient (Fig. 3E), albeit less abundantly compared to wildtype recipients (Fig. 3A).
Figure 3:
Expression of peripheral node addressin in lymphoid follicles is dependent on IL-22. Histological appearance and representative staining for PNAd (brown) in Balb/c lung allografts 30 days after transplantation into (A) wildtype (WT), (B) IL-22-deficient, (C) IL-23-neutralized, (D) AR (RORγt-cre Ahrfl/fl) mice, and (E) γδ T cell-deficient (TCRδ−/−) recipients. Recipient mice were treated with peri-operative costimulatory blockade. Scale bars: 50 μm.
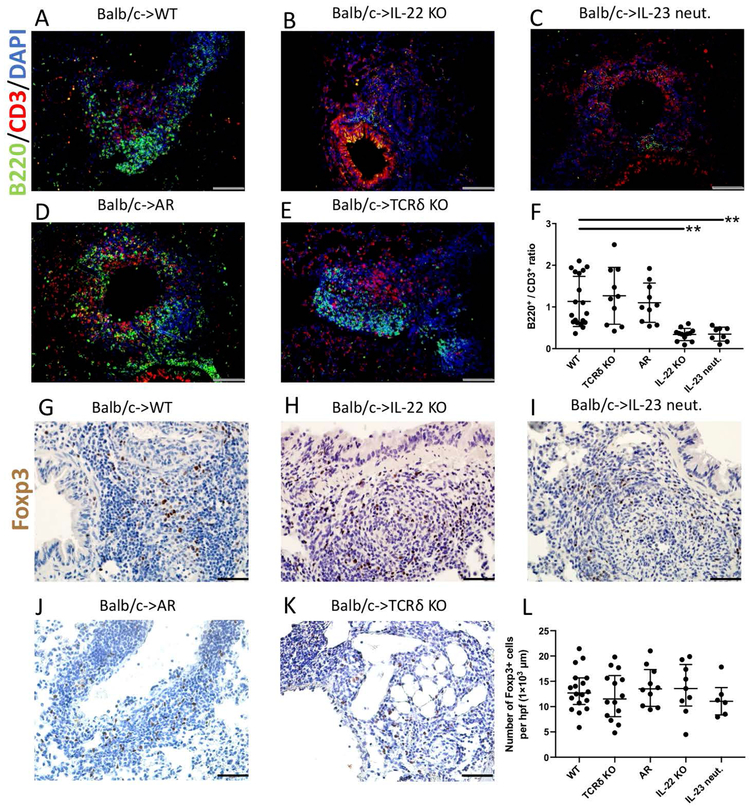
In accordance with our previous report, we observed that both B and T cells accumulated within the BALT of tolerant lung allografts after transplantation into wildtype recipients (Fig. 4A) (6). In stark contrast, while T cells were present within the small lymphoid follicles that formed in pulmonary grafts after transplantation into IL-22-deficient (Fig. 4B) or IL-23-neutralized hosts (Fig. 4C), very few B cells were detected resulting in significantly decreased B cell / T cell ratios in these mice (Fig. 4F). In AR or γδ T cell-deficient recipients (Fig. 4D & E), B cell / T cell ratios were comparable to those seen in wildtype hosts (Fig. 4F). In models of respiratory viral infection, CXCL13 expression by pulmonary fibroblasts has been shown to result in B cell recruitment and ectopic germinal center formation (31). Immunostaining revealed CXCL13 expression in spindle shaped cells within lymphoid follicles that were induced in lung allografts after transplantation into wildtype (Suppl. Fig. 3A), AR mice (Suppl. Fig. 3B) or γδ T cell-deficient recipients (Suppl. Fig. 3C), while staining was less pronounced after engraftment into IL-22-deficient (Suppl. Fig. 3D) or IL-23-neutralized (Suppl. Fig. 3E) recipients. CCL21 expression was comparable between grafts transplanted into wildtype and IL-22 deficient recipients (Suppl. Fig. 4).
Figure 4:
IL-22 is required for B cell, but not Foxp3+ cell recruitment into lymphoid follicles. Immunofluorescent staining of B cells (green), T cells (red), and DAPI (blue) in Balb/c lung allografts 30 days after transplantation into (A) wildtype (WT), (B) IL-22-deficient, (C) IL-23-neutralized, (D) AR mice (RORγt-cre Ahrfl/fl) and (E) γδ T cell-deficient (TCRδ−/−) recipients. Scale bars: 100 μm (F) Quantification of B220 / CD3 positive cell ratio per lymphoid follicle 30 days after transplantation into the indicated recipients. Each dot represents one lymphoid follicle (n≥2 mice per group). Immunostaining for Foxp3 (brown) in Balb/c lung allografts 30 days after transplantation into (G) wildtype, (H) IL-22 deficient, (I) IL-23 neutralized, (J) AR mice, and (K) γδ T cell-deficient recipients. (L) Quantification of Foxp3 cells per lymphoid follicle 30 days after transplantation of Balb/c lungs into the indicated recipients. Each dot represents one lymphoid follicle (n≥2 mice per group). Recipients were treated with peri-operative co-stimulatory blockade for all conditions. Scale bars: 50 μm.
Having recently demonstrated that Foxp3+ regulatory T cells that aggregate within BALT of lung allografts are critical in maintaining a tolerant state, we next assessed lymphoid follicles in our experimental groups for the presence of Foxp3+ cells (6). We observed that Foxp3+ cells accumulated within lymphoid follicles that were induced in pulmonary allografts after transplantation into wildtype (Fig. 4G), IL-22-deficient (Fig. 4H), IL-23-neutralized (Fig. 4I), AR (Fig. 4J) and γδ T cell-deficient hosts (Fig. 4K) with no significant differences in their density between these groups (Fig. 4L).
3.4. Reconstitution of IL-22 restores B cells recruitment to lymphoid follicles
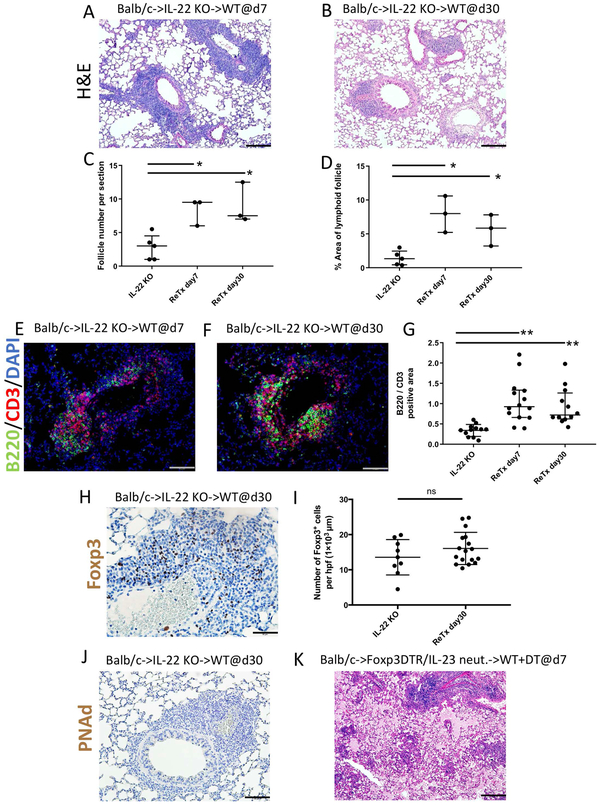
We next wanted to examine whether reconstituting IL-22 at later time points was able to reconstitute the development of BALT within lung allografts. To accomplish this, we took advantage of a lung retransplantation model that was recently developed by our laboratory (5). We have previously reported that tolerant Balb/c lung allografts are not rejected when retransplanted into non-immunosuppressed allogeneic recipients demonstrating that immunoregulation is established locally within the graft (6). Therefore, we next transplanted Balb/c lungs into B6 IL-22-deficient recipients that were treated with peri-operative co-stimulatory blockade and retransplanted these grafts into non-immunosuppressed B6 wildtype mice at least 30 days after the initial transplantation. We examined these grafts 7 and 30 days after retransplantation. At both time points we noted a significant increase in the number of and area occupied by lymphoid follicles (Figs. 5A-D). Furthermore, following retransplantation into wildtype mice we observed that B cells aggregated in the lymphoid follicles resulting in significant increases in B cell / T cell ratios. (Figs. 5E-G). In addition, immunostaining revealed increased expression of CXCL13 within the lymphoid follicles after retransplantation (Suppl. Fig. 3F), which was comparable to our observations 30 days after transplantation of Balb/c lungs into wildtype B6 mice (Suppl. Fig. 3A). Furthermore, the density of Foxp3+ cells 30 days after re-transplantation was comparable to Balb/c lungs 30 days after engraftment into B6 IL-22-deficient hosts (Figs. 5H-I). Of note, however, we did not detect PNAd staining within lymphoid follicles at 30 days (Fig. 5J) after retransplantation. To examine whether Foxp3 cells are important in maintaining a tolerant state in conditions where IL-23/22 signaling is deficient and organized BALT does not form, we transplanted Balb/c lungs into B6 Foxp3-DTR mice that were treated with peri-operative costimulatory blockade and IL-23 neutralizing antibodies. At least 30 days later these tolerant lung grafts were retransplanted into non-immunosuppressed B6 wildtype mice that were treated with DT to deplete graft-resident Foxp3 cells, as previously described. Seven days after retransplantation all grafts were not ventilated and had histological features characteristic of antibody-mediated rejection, such as fibrinous deposits within the alveoli and hyaline membrane formation (Fig. 5K).
Figure 5:
Late reconstitution of IL-22 expression mediates B cell recruitment to lymphoid follicles. Histological appearance of Balb/c lung grafts initially transplanted into IL-22 deficient recipients that received peri-operative costimulatory blockade and then retransplanted into non-immunosuppressed wildtype secondary recipients 30 days later. Images show lung allografts harvested (A) 7 and (B) 30 days after retransplantation. n=3. Scale bars =100 μm. Quantification of lymphoid follicle (C) number and (D) percent of total area per section in IL-22-deficient recipients 30 days after primary transplantation, as well as 7 and 30 days after retransplantation into wildtype secondary hosts. Histological appearance of B cell (green), T cell (red), and DAPI (blue) immunofluorescent staining from Balb/c lung grafts (E) 7 and (F) 30 days after retransplantation (scale bars =50 μm) and (G) quantification of B220 / CD3 cell ratio per lymphoid follicle among the various conditions. Each dot represents one lymphoid follicle (n≥2 mice per group). Immunostaining for Foxp3 (brown) (H) 30 days after retransplantation and (I) quantification of Foxp3 cells per lymphoid follicle at 30 days among various conditions. Scale bars =50 μm. Each dot represents one lymphoid follicle (n≥2 mice per group). Immunostaining for PNAd (brown) (J) 30 days after retransplantation. Scale bars =50 μm. (K) Histological appearance of Balb/c lung grafts initially transplanted into IL-23 neutralized B6 Foxp3-DTR recipients that received peri-operative costimulatory blockade and then retransplanted into non-immunosuppressed wildtype B6 recipients 30 days later that received DT at the time of retransplantation. Images are shown 7 days after retransplantation (H&E) (n=4). Scale bars =100 μm. ns= not significant; *p<0.05; **p<0.01.
4. Discussion
Our study demonstrates that IL-22 plays an important role in the induction of BALT within tolerant lung allografts. Here, we have identified γδ T cells and ILC3s as important producers of IL-22. We have shown that IL-22 is critical for the recruitment of B cells to lymphoid follicles as well as development of PNAd-expressing HEVs within these structures. Interestingly, Foxp3+ cells accumulate within the few, small lymphoid follicles that form in pulmonary allografts when recipient mice lack IL-22, and recipient IL-22 expression is not required to induce tolerance after lung transplantation. Restoring IL-22 at late time points through retransplantation of lung allografts into wildtype hosts leads to a significant enlargement of the follicles with reconstitution of B cells, but does not result in the formation of PNAd+ HEVs.
TLOs that are induced in transplanted grafts have been mostly associated with deleterious immune responses (32). For example, TLOs have been observed in cardiac allografts that have evidence of acute or chronic rejection (33). In aortic allografts TLOs were suggested to be the site of local generation of humoral immune responses that result in rejection (34). Some reports exist that BALT is associated with chronic lung allograft rejection (3). By contrast, we and others have observed intragraft Foxp3-rich TLOs that play an important role in the maintenance of a tolerant state (5, 6, 35). For example, the development of such structures is associated with improved graft function within murine renal allografts (35). While we have previously reported that BALT is not induced in acutely rejected lungs, our laboratory has shown that Foxp3-rich BALT is induced in tolerant murine pulmonary allografts (5, 36). Perhaps more importantly, we have recently reported that depletion of Foxp3+ cells from tolerant lung allografts results in activation of B cells by T cells within the graft and triggers antibody-mediated rejection (6). Foxp3-rich TLOs have also been recently described in tumor models where they suppress immune responses that are directed against the tumor (4). At the present time it remains unknown why tertiary lymphoid organs promote deleterious immune responses in some grafts while they apparently protect others from rejection. We speculate that the inflammatory state at the time when tertiary lymphoid organs are induced determines their cellular composition. To this end, their induction in a tolerogenic setting, such as following treatment with co-stimulatory blockade in our experiments, may favor the enrichment of regulatory immune cells within these structures. Future studies will need to examine whether inducing cellular and soluble mediators play a role in determining whether “pro-inflammatory” or “tolerogenic” tertiary lymphoid organs form within allografts.
Only a few studies have addressed the cellular and molecular requirements that mediate the induction of TLOs after transplantation. It has been shown in skin allografts that overexpression of lymphotoxin-α results in the neogenesis of lymphoid follicles that harbor PNAd+ vessels (37). Similarly, lymphotoxin β receptor signaling has been shown to promote the development of TLOs in other disease models, such as atherosclerosis or autoimmunity (10, 38). In cardiac allografts inhibiting lymphotoxin β receptor signaling abrogates the development of B cell-enriched TLOs and attenuates humoral immune responses (39). Graft-infiltrating Th17 cells have also been implicated in promoting lymphoid neogenesis in a study examining chronically rejected human renal allografts (40); this observation is somewhat reminiscent of reports in murine models where the induction of TLOs also depends on IL-17. For example, the development of BALT following intranasal administration of LPS to neonatal mice required IL-17 production by T cells (8). Interestingly, in this study BALT was induced in RORγt- or Id2-deficient mice indicating that lymphoid tissue inducer cells did not play an important role in this process.
Our study represents the first study to show the role of IL-22 in TLO formation after organ transplantation. Our findings extend the observations of few reports that have implicated IL-22 in the development of lymphoid follicles at peripheral sites. In a virus-induced model of murine salivary gland inflammation, TLO induction depends on IL-22 (11). Similar to this study, we identified γδ T cells and ILC3s as the major producers of IL-22 in tolerant lung allografts (11). These cells play an important role in BALT induction in our model as evidenced by the reduced number of lymphoid follicles after engraftment into γδ T cell-deficient or AR mice. However, compensatory mechanisms of IL-22 production appear to exist in the absence of γδ T cells or ILC3s, as evidenced by B cell recruitment and the development of PNAd-expressing HEVs in TCRδ−/− hosts or AR recipients. As we did not observe increases in the expression of IL-17 transcripts in tolerant lung allografts, we did not formally examine a role for IL-17 in the induction of BALT in the current study. Our experiments indicate that, in the absence of IL-22, other cytokines such as IL-17 cannot mediate the induction of organized BALT in tolerant lung allografts.
We have uncovered that IL-22 is critical for B cell recruitment to the lymphoid follicles as well as for the development of PNAd+ vessels, but not for the expression of CCL21. Similarly, in a murine model of virus-induced autoimmune inflammation of salivary glands, expression of IL-22 is critical for the local induction of the B cell chemokines CXCL12 and CXCL13 (11) . Consequently, B cell accumulation in TLOs that formed within salivary glands was impaired in the absence of IL-22, which resulted in reductions in autoantibody production. Numerous studies have shown that IL-23, a proinflammatory cytokine that can be produced by dendritic cells, drives the synthesis of IL-22 (41, 42). Therefore, it is not surprising that neutralization of IL-23 and absence of IL-22 in recipient mice yielded similar phenotypes. To this end, a previous study investigating M. tuberculosis-induced BALT demonstrated that IL-23 played an important role in the expression of B cell chemokines and B cell aggregation (12).
It is the prevailing view that lymphocyte homing into BALT and other TLOs depends on HEVs, where PNAd is required for T and B cell extravasation (30). We have found that IL-22 is required for the development of PNAd-expressing vessels within lymphoid aggregates in tolerant lung allografts. Interestingly, late reconstitution of IL-22 through retransplantation of IL-22- deficient grafts into wildtype hosts triggers B cell recruitment, but fails to induce PNAd+ vessels. Previous studies have suggested that lymphotoxin signaling may be critical for the development of PNAd+ vessels during induction of TLOs, and recent reports have suggested that during intestinal inflammation lymphotoxin β receptor signaling in dendritic cells triggers the production of IL-23, which in turn induces the production of IL-22 by RORγt+ cells (42-44). Thus, it is possible that in certain inflammatory models IL-22 can play a role in the development of PNAd+ vessels downstream of lymphotoxin signaling. Other signals such as activation of pattern recognition receptors and signaling via MyD88 can trigger the production of IL-23 (45). Future studies need to determine which signals result in the increase in IL-23 expression in lung allografts during tolerance induction.
We have recently shown that graft-resident Foxp3+ cells are critical for maintenance of tolerance after pulmonary transplantation (6). Interestingly, in this study we found that transplantation into IL-22-deficient or IL-23-neutralized recipients did not affect Foxp3+ cell accumulation in lung allografts. In fact, their density was comparable to our findings in wildtype recipients, providing an explanation for the survival of grafts after transplantation into IL-22-deficient or IL-23-neutralized mice. Although the Foxp3+ cells do not accumulate within organized BALT when recipients lack IL-22/IL-23 signaling, our retransplant experiments show that the tolerance of such lungs depends on the presence of Foxp3+ cells as their selective depletion from the graft triggers rejection. These observations extend the notion that immune circuits that are established locally within the lung graft play an important role in the regulation of tolerance. Lung grafts are unique as they are constantly exposed to the external environment. Since external stimuli are known to impact the function of pulmonary immune cells including impairment of regulatory T cell function, our findings may provide a mechanistic link between exposure to respiratory pathogens as well as air pollutants and lung allograft rejection (46-49). Notably, Foxp3+ cells accumulated in lung allografts that were transplanted into IL-22-deficient or IL-23-neutralized mice despite the absence of PNAd+-expressing HEVs suggesting that they can home to BALT via alternative routes. Accordingly, a study of nasal-associated lymphoid tissue (NALT), a form of mucosal TLO, in mice that lack PNAd expression revealed that while B cells and CD4+CD25− T cells have less accumulation within NALT, regulatory T cell (CD4+CD25+) accumulation is unaffected (50). Our findings in IL-22-deficient or IL-23-neutralized lung grafts are reminiscent of Foxp3-rich organized lymphoid structures that have been observed in stable kidney allografts, which also lack PNAd expression (51). Furthermore, a recent study has revealed that two distinct routes exist for lymphocyte homing into BALT - one from the blood via PNAd-expressing HEVs and one via the lung parenchyma that is independent of HEVs (52).
In conclusion, our findings provide a novel pathway for the induction of TLOs in allografts. We have shown that IL-22 is required for B cell accumulation and development of PNAd-expressing HEVs within BALT that is induced in tolerant lung transplants. We further show that reconstitution of IL-22 at late time points promotes B cell recruitment and enlargement of the lymphoid follicles, but does not result in the development of PNAd-expressing HEVs (6). The formation of Foxp3 aggregates in lung allografts that we have shown to be critical for maintaining a tolerant state, does not depend on IL-22 or PNAd-expressing HEVs. Given that chronic rejection is the leading cause of late-term morbidity and mortality in lung transplant patients, novel therapies that induce tolerance are critically needed (53). Collectively, our findings provide new insights into immunological events that contribute to tolerance after lung transplantation, and these findings need to be taken into consideration when developing new therapies. Our work raises the possibility that outcomes after lung transplantation may be improved by pharmacological or gene therapy interventions that facilitate the induction of Foxp3-rich aggregates in pulmonary grafts. To this end, ex vivo lung perfusion provides a platform to treat lung allografts prior to transplantation (54).
Supplementary Material
Acknowledgments
Daniel Kreisel is supported by NIH grants 1P01AI116501, R01 HL094601 and Veterans Administration Merit Review grant 1I01BX002730. Jason M. Gauthier is supported by 1F32HL-143950.
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Daniel Kreisel received research support from Compass Therapeutics. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–34. [DOI] [PubMed] [Google Scholar]
- 2.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, and Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato M, Hirayama S, Hwang DM, Lara-Guerra H, Wagnetz D, Waddell TK, et al. The role of intrapulmonary de novo lymphoid tissue in obliterative bronchiolitis after lung transplantation. J Immunol. 2009;182(11):7307–16. [DOI] [PubMed] [Google Scholar]
- 4.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43(3):579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Bribriesco AC, Nava RG, Brescia AA, Ibricevic A, Spahn JH, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5(5):544–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Gauthier JM, Higashikubo R, Hsiao HM, Tanaka S, Vuong L, et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest. 2019;129(2):556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang JY, Randall TD, and Silva-Sanchez A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol. 2016;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G, et al. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. 2014;211(4):643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206(1):233–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, et al. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proc Natl Acad Sci U S A. 2015;112(35):11024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187(10):5402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun. 2015;6:8280. [DOI] [PubMed] [Google Scholar]
- 14.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song C, Lee JS, Gilfillan S, Robinette ML, Newberry RD, Stappenbeck TS, et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212(11):1869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treerat P, Prince O, Cruz-Lagunas A, Munoz-Torrico M, Salazar-Lezama MA, Selman M, et al. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol. 2017;10(4):1069–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7(6):1672–9. [DOI] [PubMed] [Google Scholar]
- 18.Kyttaris VC, Kampagianni O, and Tsokos GC. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. Biomed Res Int. 2013;2013:861028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Goldstein DR, Bribriesco AC, Nava RG, Spahn JH, Wang X, et al. Surgical technique for lung retransplantation in the mouse. J Thorac Dis. 2013;5(3):321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180(7):4754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spahn JH, Li W, Bribriesco AC, Liu J, Shen H, Ibricevic A, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol. 2015;194(8):4039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, and Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–41. [DOI] [PubMed] [Google Scholar]
- 25.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5(3):203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsukaguchi K, de Lange B, and Boom WH. Differential regulation of IFN-gamma, TNF-alpha, and IL-10 production by CD4(+) alphabetaTCR+ T cells and vdelta2(+) gammadelta T cells in response to monocytes infected with Mycobacterium tuberculosis-H37Ra. Cell Immunol. 1999;194(1):12–20. [DOI] [PubMed] [Google Scholar]
- 27.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174(5):1054–66. [DOI] [PubMed] [Google Scholar]
- 28.Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One. 2011;6(7):e21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam S, King DP, and Beaman BL. Increase of gammadelta T lymphocytes in murine lungs occurs during recovery from pulmonary infection by Nocardia asteroides. Infect Immun. 2001;69(10):6165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, et al. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197(10):1255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton AE, Innocentin S, Carr EJ, Bradford BM, Lafouresse F, Mabbott NA, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med. 2019;216(3):621–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao HM, Li W, Gelman AE, Krupnick AS, and Kreisel D. The Role of Lymphoid Neogenesis in Allografts. Am J Transplant. 2016;16(4):1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, and Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5(3):510–6. [DOI] [PubMed] [Google Scholar]
- 34.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci U S A. 2005;102(41):14723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown K, Sacks SH, and Wong W. Tertiary lymphoid organs in renal allografts can be associated with donor-specific tolerance rather than rejection. Eur J Immunol. 2011;41(1):89–96. [DOI] [PubMed] [Google Scholar]
- 36.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182(7):3969–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, et al. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7(5):1071–9. [DOI] [PubMed] [Google Scholar]
- 38.Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, and Bolstad AI. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjogren’s syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11(1):R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motallebzadeh R, Rehakova S, Conlon TM, Win TS, Callaghan CJ, Goddard M, et al. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. FASEB J. 2012;26(1):51–62. [DOI] [PubMed] [Google Scholar]
- 40.Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184(9):5344–51. [DOI] [PubMed] [Google Scholar]
- 41.Fatkhullina AR, Peshkova IO, Dzutsev A, Aghayev T, McCulloch JA, Thovarai V, et al. An Interleukin-23-Interleukin-22 Axis Regulates Intestinal Microbial Homeostasis to Protect from Diet-Induced Atherosclerosis. Immunity. 2018;49(5):943–57 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drayton DL, Ying X, Lee J, Lesslauer W, and Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197(9):1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol. 2011;12(10):941–8. [DOI] [PubMed] [Google Scholar]
- 45.Cowardin CA, Kuehne SA, Buonomo EL, Marie CS, Minton NP, and Petri WA Jr., Inflammasome activation contributes to interleukin-23 production in response to Clostridium difficile. MBio. 2015;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18(10):1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhinder S, Chen H, Sato M, Copes R, Evans GJ, Chow CW, et al. Air pollution and the development of posttransplant chronic lung allograft dysfunction. Am J Transplant. 2014;14(12):2749–57. [DOI] [PubMed] [Google Scholar]
- 48.Allyn PR, Duffy EL, Humphries RM, Injean P, Weigt SS, Saggar R, et al. Graft Loss and CLAD-Onset Is Hastened by Viral Pneumonia After Lung Transplantation. Transplantation. 2016;100(11):2424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–52 e10. [DOI] [PubMed] [Google Scholar]
- 50.Ohmichi Y, Hirakawa J, Imai Y, Fukuda M, and Kawashima H. Essential role of peripheral node addressin in lymphocyte homing to nasal-associated lymphoid tissues and allergic immune responses. J Exp Med. 2011;208(5):1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178(4):1635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleige H, Bosnjak B, Permanyer M, Ristenpart J, Bubke A, Willenzon S, et al. Manifold Roles of CCR7 and Its Ligands in the Induction and Maintenance of Bronchus-Associated Lymphoid Tissue. Cell Rep. 2018;23(3):783–95. [DOI] [PubMed] [Google Scholar]
- 53.Balsara KR, Krupnick AS, Bell JM, Khiabani A, Scavuzzo M, Hachem R, et al. A single-center experience of 1500 lung transplant patients. J Thorac Cardiovasc Surg. 2018;156(2):894–905 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1(4):4ra9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.