Abstract
Bariatric surgery improves markers of kidney health in severe obesity, yet it is unclear if kidney disease outcomes differ according to age at surgery. Therefore, we examined health effects of Roux-en-Y gastric bypass between 161 adolescents and 396 adults participating in two related but distinct studies. Primary outcomes were elevated urine albumin-to-creatinine ratio (UACR) of 30mg/g or more and hyperfiltration (an estimated glomerular filtration rate of 135 ml/min/1.73m2 or more). Analyses were stratified by the presence of pre-operative type 2 diabetes. Adolescents with pre-operative type 2 diabetes had a significantly increased prevalence of elevated UACR prior to surgery compared to adults (22.5 vs. 9.0%). Resolution of elevated UACR following surgery differed between adolescents and adults with type 2 diabetes, with adolescents experiencing a significantly earlier improvement following surgery. Adolescents without pre-operative type 2 diabetes demonstrated a significantly increased prevalence of UACR prior to surgery compared to adults (9.4 vs. 4.5%), with no improvement occurring in either group post-operatively. Adolescents with pre-operative type 2 diabetes had a significantly increased prevalence of hyperfiltration that remained throughout the study period, whereas hyperfiltration prevalence was similar among those without type 2 diabetes. Thus, adolescents with pre-operative type 2 diabetes experienced earlier attenuation of elevated UACR compared to adults with pre-operative type 2 diabetes in response to gastric bypass.
Trial Registration:
Keywords: type 2 diabetes, elevated albumin excretion, hyperfiltration, diabetic kidney disease, metabolic bariatric surgery, Roux-en-Y gastric bypass
Introduction:
Diabetic kidney disease (DKD) and obesity-related nephropathy are major causes of kidney failure, morbidity and mortality in the US (1–4). Current medical treatments are only partially protective against DKD and obesity-related nephropathy (5, 6). Bariatric surgery on the other hand may confer kidney protection in severely obese adolescents and adults with and without type 2 diabetes (7, 8). In fact, our group recently demonstrated that compared to bariatric surgery, medical treatment of severely obese youth with type 2 diabetes was associated with substantially higher odds of kidney disease over 5-years of follow-up (9). Although bariatric surgery is most commonly performed in later adulthood, data indicate that the longstanding effects of severe obesity from adolescence to adulthood increase the likelihood of complications including hypertension, nephropathy and cardiovascular disease (10). Additionally, a recent analysis suggests that adolescents were more likely than adults to experience remission of type 2 diabetes in response to Roux-en-Y gastric bypass (gastric bypass) (11). It remains unclear whether age at bariatric surgery impacts kidney outcomes in severely obese adolescents and adults with and without type 2 diabetes.
Accordingly, in the present analysis, we examined kidney outcomes of gastric bypass in a cohort of adolescents with severe obesity and compared them to outcomes in a cohort of adults who had been obese since adolescence. We hypothesized that gastric bypass in severely obese adolescents would be associated with earlier and greater attenuation of markers of kidney disease than the same operation performed in adults with longstanding obesity.
Results:
Baseline comparison between adolescents and adults
A total of 242 adolescents were enrolled in the Teen–LABS study, 161 (67%) of whom underwent Roux-en-Y gastric bypass surgery and were included in the current analysis. In the LABS study, 1738 (71%) underwent Roux-en-Y gastric bypass surgery, of whom 396 reported history of obesity dating back to age 18 or earlier and were selected for comparison with the adolescents in the present analysis. Fourteen percent of the adolescent participants and 31% of adults had type 2 diabetes pre-operatively (p<0.001). Due to the significant difference in prevalence of pre-operative type 2 diabetes between adolescents and adults, and also the greater risk of kidney disease in severely obese people with diabetes compared to those without diabetes, all further analyses were stratified by pre-operative type 2 diabetes status.
Participants with pre-operative type 2 diabetes
The distribution of sex, race and ethnicity were similar among adolescent and adult participants with pre-operative type 2 diabetes (Table 1). The pre-operative weight, BMI and HbA1c were also similar among adolescents and adults (Table 1). At baseline, adolescent participants had lower triglycerides (121 [98, 177] vs. 157 [110, 218] mg/dl, p=0.03) and lower prevalence of hypertension (57 vs. 80%, p=0.006). In contrast adolescents had higher baseline insulin concentrations (43.0 vs 23.5 uU/mL, p=0.002).
Table 1.
Baseline clinical characteristics by baseline type 2 diabetes status and study group.
| Baseline Type 2 Diabetes | Without Baseline Type 2 Diabetes | |||||
|---|---|---|---|---|---|---|
| Teen-LABS | LABS-2 | p-value | Teen-LABS | LABS-2 | p-value | |
| Age at Surgery, mean (SD) | 17.1 (1.36) | 40.3 (6.75) | <0.001 | 17.0 (1.56) | 36.4 (6.88) | <0.001 |
| Sex, % (n) | 0.67 | 0.72 | ||||
| Female | 80.0% (24) | 76.4% (107) | 77.9% (102) | 76.2% (189) | ||
| Male | 20.0% (6) | 23.6% (33) | 22.1% (29) | 23.8% (59) | ||
| White | 63.3% (19) | 76.4% (107) | 76.3% (100) | 81.9% (203) | ||
| Black | 30.0% (9) | 16.4% (23) | 14.5% (36) | 19.9% (26) | ||
| Other | 6.7% (2) | 7.1% (10) | 3.6% (9) | 3.8% (5) | ||
| Ethnicity, % (n) | 0.29 | 0.29 | ||||
| Non-Hispanic | 93.3% (28) | 97.1% (136) | 90.1% (118) | 93.2% (231) | ||
| Hispanic | 6.7% (2) | 2.9% (4) | 9.9% (13) | 6.9% (17) | ||
| Weight (kg), mean (SD) | 152.6 (30.64) | 147.9 (27.82) | 0.41 | 150.6 (30.35) | 143.9 (28.25) | 0.034 |
| BMI, mean (SD) | 54.4 (8.92) | 51.7 (8.02) | 0.11 | 53.5 (9.81) | 50.0 (7.40) | <0.001 |
| Lipid-lower medication use, % (n) | 10.0% (3) | 39.4% (54) | 0.002 | 4.6% (6) | 7.3% (18) | 0.31 |
| Anti-hypertension medication use, % (n) | 50.0% (15) | 70.1% (96) | 0.035 | 16.8% (22) | 34.3% (85) | <0.001 |
| RAAS Inhibitor use, % (n) | 36.7% (11) | 40.9% (56) | 0.67 | 8.4% (11) | 11.3% (28) | 0.38 |
| Elevated blood pressure, % (n) | 56.7% (17) | 80.3% (110) | 0.006 | 31.0% (40) | 52.4% (130) | <0.001 |
| Glucose (mg/dl), median (Q1,Q3) | 101 (90, 143) | 107.5 (93, 139) | 0.59 | 91 (84, 100) | 91 (85, 98) | 0.58 |
| Insulin (uU/mL), median (Q1,Q3) | 43.0 (26.8, 67.1) | 23.5 (15.8, 38.2) | 0.002 | 25.2 (15.1, 36.9) | 19.0 (13.3, 28.3) | 0.003 |
| HbA1c (%), mean (SD) | 6.6 (1.90) | 6.9 (1.51) | 0.33 | 5.2 (0.40) | 5.3 (0.40) | 0.11 |
| Hs-CRP (mg/dl), median (Q1,Q3) | 0.91 (0.35, 1.75) | 0.86 (0.54, 1.63) | 0.96 | 0.61 (0.32, 0.94) | 0.79 (0.43, 1.52) | 0.004 |
| LDL cholesterol (mg/dl), mean (SD) | 166 (31.69) | 179.4 (40.69) | 0.09 | 156.6 (28.46) | 182.3 (35.34) | <0.001 |
| HDL cholesterol (mg/dl), mean (SD) | 41.4 (11.64) | 40.8 (9.63) | 0.77 | 36.8 (8.34) | 43.1 (10.31) | <0.001 |
| LDL cholesterol (mg/dl), mean (SD) | 96.5 (27.45) | 102.9 (37.69) | 0.29 | 92.1 (25.1) | 111.8 (29.55) | <0.001 |
| Triglycerides (mg/dl), median (Q1,Q3) | 121 (98, 177) | 157 (110, 218) | 0.033 | 112.5 (81, 169) | 126 (90, 164) | 0.54 |
| Serum creatinine (mg/dl), mean (SD) | 0.65 (0.14) | 0.76 (0.27) | 0.002 | 0.67 (0.12) | 0.76 (0.62) | 0.038 |
Participants without pre-operative type 2 diabetes
The distribution of sex, race and ethnicity were similar among adolescent and adult participants without pre-operative type 2 diabetes. At baseline adolescents had a higher BMI (53.5±9.8 vs. 50.0±7.4 kg/m2, p<0.001), weight (150.6±30.4 vs. 143.9±28.3 kg, p=0.03) and insulin concentrations (25.2 vs. 19.0 uU/mL, p=0.003) than the adult participants (Table 1). Adolescent participants had lower mean total cholesterol (157±28 vs. 182±35 mg/dl, p<0.001), mean HDL cholesterol (37±8 vs. 43±10 mg/dl, p<0.001), mean LDL cholesterol (92±25 vs. 112±30 mg/dl, p<0.001) and prevalence of hypertension (31 vs. 52%, p<0.001).
Effect of gastric bypass on UACR and elevated UACR in adolescents and adults
Participants with pre-operative type 2 diabetes
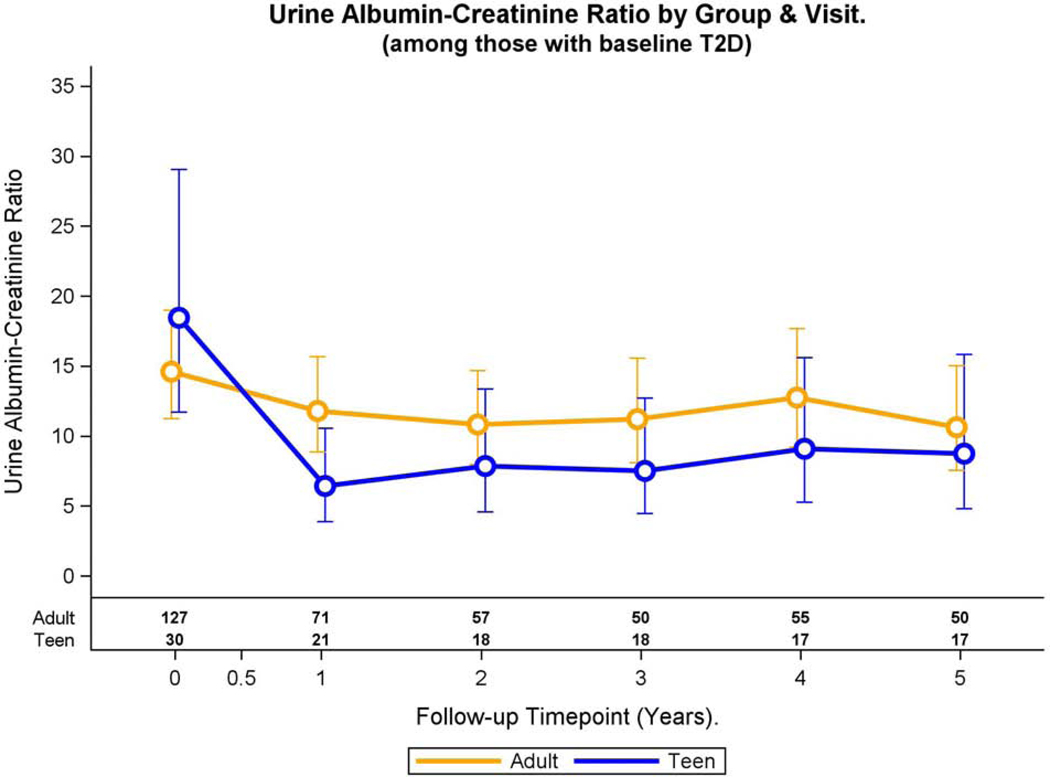
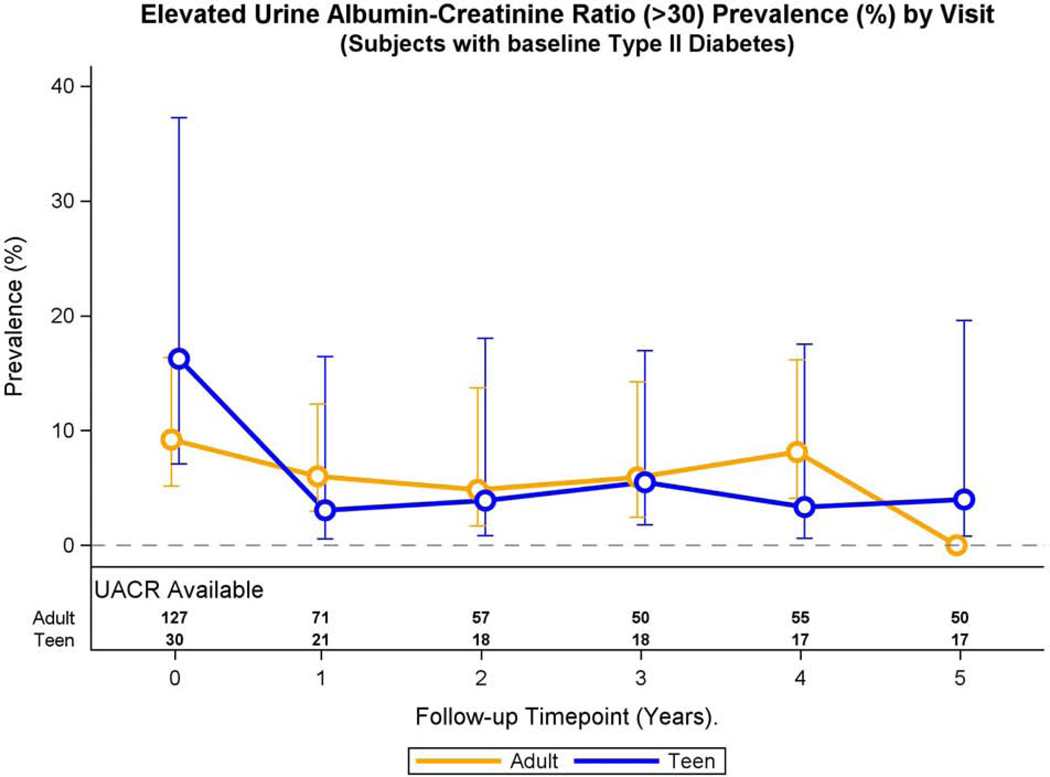
Adolescents with pre-operative type 2 diabetes demonstrated an increased statistically significant prevalence of elevated UACR at baseline compared to their adult counterparts (22.5 vs. 9.0%, p=0.03, Table 2) in multivariable models. Change in adjusted prevalence of elevated UACR differed over time in adolescents and adults with pre-operative type 2 diabetes (p<0.001, Figure 1A). In adolescents, the adjusted prevalence of elevated UACR declined from baseline to year 1 (p=0.06) after which the prevalence remained stable. In adults, adjusted prevalence of elevated UACR was stable from baseline to year 4 and then significantly declined at year 5 (p<0.001).
Table 2.
Main Outcomes by baseline type 2 diabetes status and study group.
| Baseline Type 2 Diabetes | Without Baseline Type 2 Diabetes | |||||||
|---|---|---|---|---|---|---|---|---|
| Teen-LABS (N=30) | LABS-2 (N=140) | Teen-LABS (N=131) | LABS-2 (N=248) | |||||
| Baseline | 5 years | Baseline | 5 years | Baseline | 5 years | Baseline | 5 years | |
| UACR (μg/mg) | ||||||||
| No. with data | 30 | 17 | 127 | 50 | 127 | 106 | 233 | 137 |
| Modeled mean (95% CI) | 19.9 (12.5, 31.5) | 8.9 (4.9, 16.2) | 14.1 (10.9, 18.3) | 9.8 (6.9, 14.0) | 7.8 (6.4, 9.6) | 6.8 (5.6, 8.3) | 6.1 (5.2, 7.2) | 6.5 (5.4, 7.8) |
| Elevated UACR | ||||||||
| No. with data | 30 | 17 | 127 | 50 | 127 | 106 | 233 | 137 |
| Modeled Prevalence % (95% CI) | 22.5 (10.1, 50.4) | 5.0 (0.9, 27.6) | 9.0 (5.1, 15.8) | 0.0 (<0.1, <0.1) | 9.4 (5.6, 15.9) | 2.8 (1.3, 6.4) | 4.5 (2.7, 7.7) | 1.7 (0.6, 4.9) |
| eGFR (FAS) | ||||||||
| No. with data | 30 | 17 | 135 | 54 | 130 | 106 | 241 | 138 |
| Modeled mean (95% CI) | 116.1 (107.9, 124.3) | 118.1 (107.7, 128.5) | 106.8 (102.1, 111.5) | 111.9 (105.9, 118.0) | 112.5 (108.2, 116.9) | 115.0 (111.0, 119.0) | 108.8 (105.2, 112.4) | 112.0 (108.4, 115.6) |
| Hyperfiltration | ||||||||
| No. with data | 30 | 17 | 135 | 54 | 130 | 106 | 241 | 138 |
| Modeled Prevalence % (95% CI) | 8.6 (3.3, 22.8) | 10.4 (3.8, 28.7) | 4.3 (2.0, 9.4) | 4.3 (1.6, 11.4) | 6.0 (2.9, 12.7) | 4.2 (2.2, 7.8) | 3.0 (1.4, 6.1) | 5.7 (3.3, 9.8) |
The modeled prevalence was adjusted for: study cohort, study visit, cohort by visit interaction, race, hypertension, BMI, sex, and the following baseline traits: education, insulin, HbA1c, hs-CRP, HDL cholesterol, LDL cholesterol, RAAS inhibitor use, and eGFR.
Fig. 1A: elevated UACR and continuous UACR over time in adolescents and adults with pre-operative type 2 diabetes.
Modeled change in prevalence of elevated UACR over time differed by group (group * time interaction, p<0.001).
In an adjusted model, change in UACR over time differed in adolescents and adults with pre-operative type 2 diabetes (group by time interaction, p=0.008, Figure 1B). For both adolescents (p<0.001) and adults (p=0.05), UACR significantly declined from baseline to 1 year, with values remaining similar to year 1 values at all time points thereafter.
Fig. 1B:
Modeled change in geometric mean UACR and 95% CI over time was differed by group (group * time interaction, p=0.008).
Participants without pre-operative type 2 diabetes
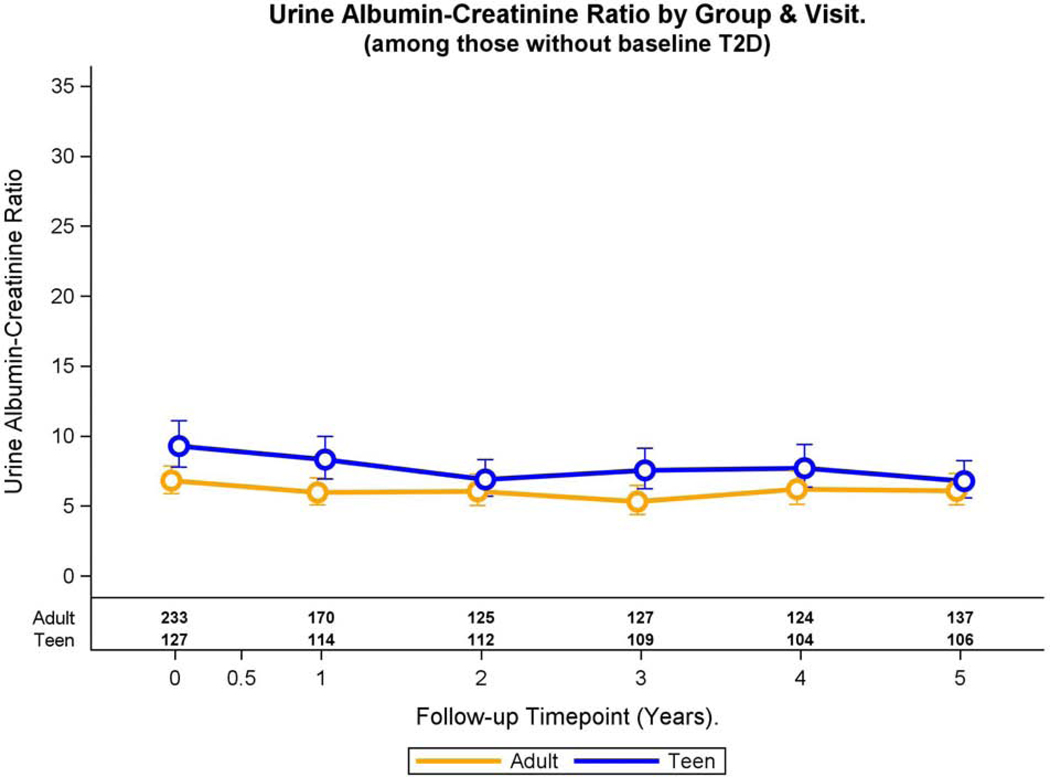
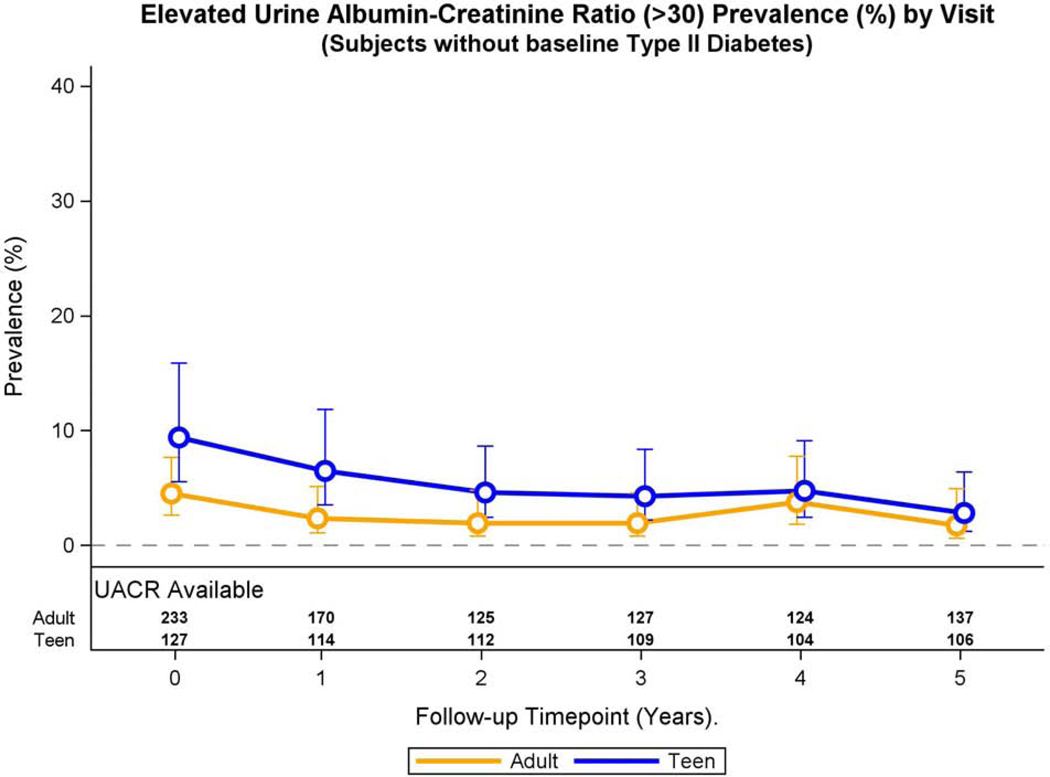
Adolescents without pre-operative type 2 diabetes demonstrated higher adjusted prevalence of elevated UACR at baseline compared to their adult counterparts (9.4 vs. 4.5%, Figure 2A), and the difference remained significant throughout the study period (prevalence ratio: 2.04, 95% CI 1.26, 3.28, p=0.004) in multivariable models. The adjusted prevalence of elevated UACR did not significantly change across study visits for Teen-LABS and LABS participants (p=0.89), nor was the interaction term significant (p =0.89)
Fig. 2A: Elevated UACR and continuous UACR over time in adolescents and adults without pre-operative type 2 diabetes.
Modeled change in prevalence of elevated UACR over time was similar by group (group * time interaction, p=0.89).
In adjusted models, Teen-LABS participants without pre-operative type 2 diabetes demonstrated a higher baseline geometric mean UACR compared to their adult counterparts (Table 2), and this difference remained throughout the study period (p=0.0009, Figure 2B). The geometric mean UACR did not significantly change over time for Teen-LABS and LABS participants (p=0.48), and the group by time interaction term was not significant. (p=0.45).
Fig. 2B:
Modeled change in UACR over time was similar by group (group * time interaction, p=0.45).
Effect of gastric bypass on eGFR and hyperfiltration in adolescents and adults
Participants with pre-operative type 2 diabetes
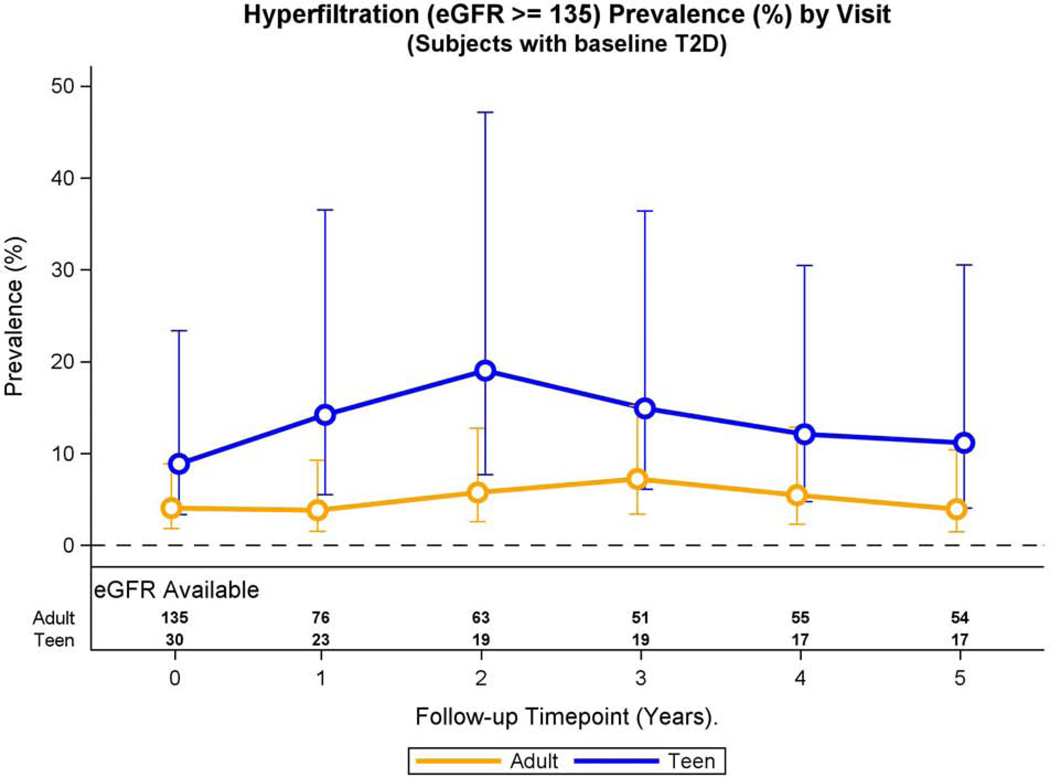
Over the 5-year period, adolescents were more likely than adults to have hyperfiltration (prevalence ratio: 2.36, 95% CI 1.03, 5.33, p=0.04) after multivariable adjustments. The adjusted prevalence of hyperfiltration did not change across the 5-year study period (p=0.15), and interaction term to test for a differential effect of bariatric surgery also did not reach significance (p=0.86, Figure 3A).
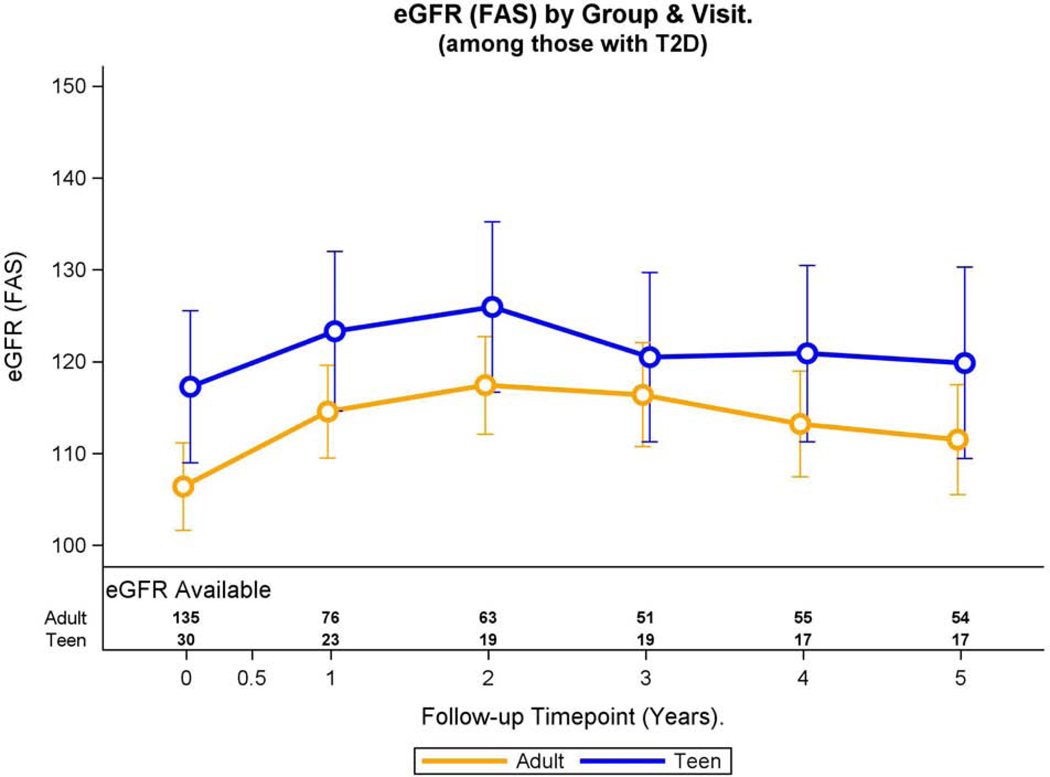
Fig. 3A: Hyperfiltration and eGFR over time in adolescents and adults with preoperative type 2 diabetes.
Modeled change in prevalence of hyperfiltration over time was similar by group (group * time interaction, p=0.86).
Over the 5-year period, adjusted mean eGFR in adolescents was greater than that for adults (mean difference: 7.4, 95% −0.3, 15.2 ml/min/1.73m2, p=0.06), although the difference did not reach statistical significance. The adjusted mean eGFR significantly increased from base to year 1 (p<0.001), then remained consistent thereafter. Furthermore, the interaction term to test for a difference in the change in mean eGFR over time between study groups was not significant (p=0.96, Figure 3B).
Fig. 3B:
Modeled change in eGFR over time was similar by group (group * time interaction, p=0.96).
Participants without pre-operative type 2 diabetes
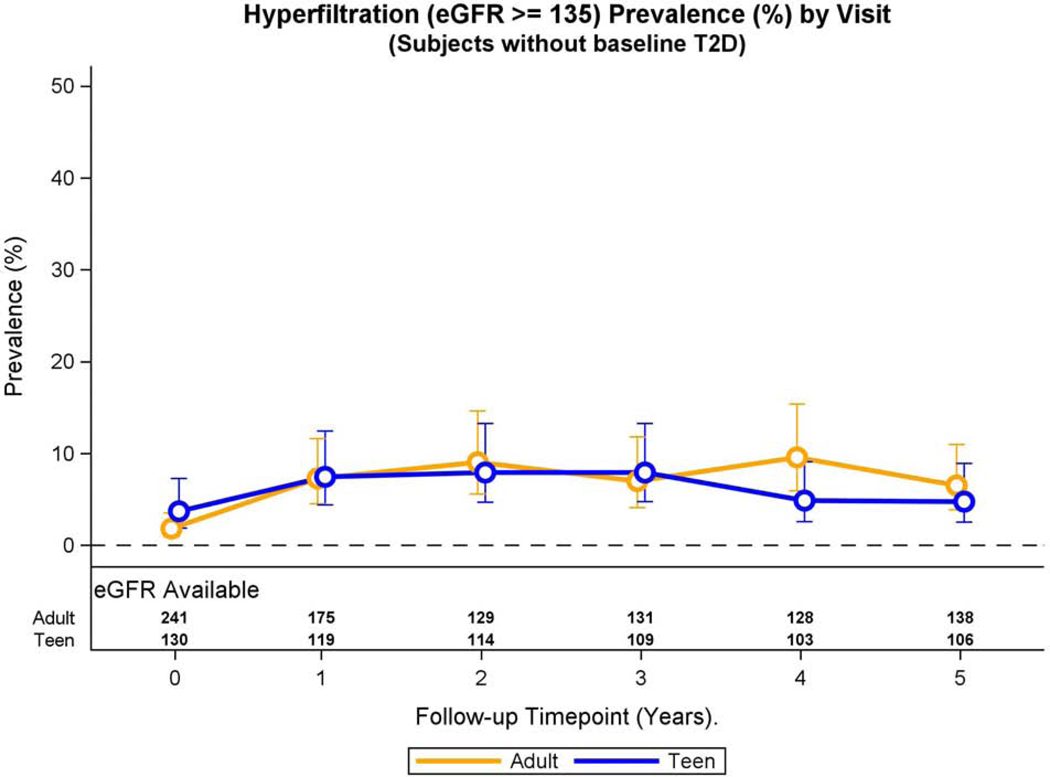
Over the 5-year period, the prevalence of hyperfiltration was similar in adolescents and adults without pre-operative type 2 diabetes (prevalence ratio: 0.98, 95% CI 0.61, 1.57, p=0.94) after multivariable adjustments. Furthermore, the adjusted prevalence of hyperfiltration did not change across study visits in either the Teen-LABS or LABS participants (Figure 4A), nor was the interaction term significant (p =0.11).
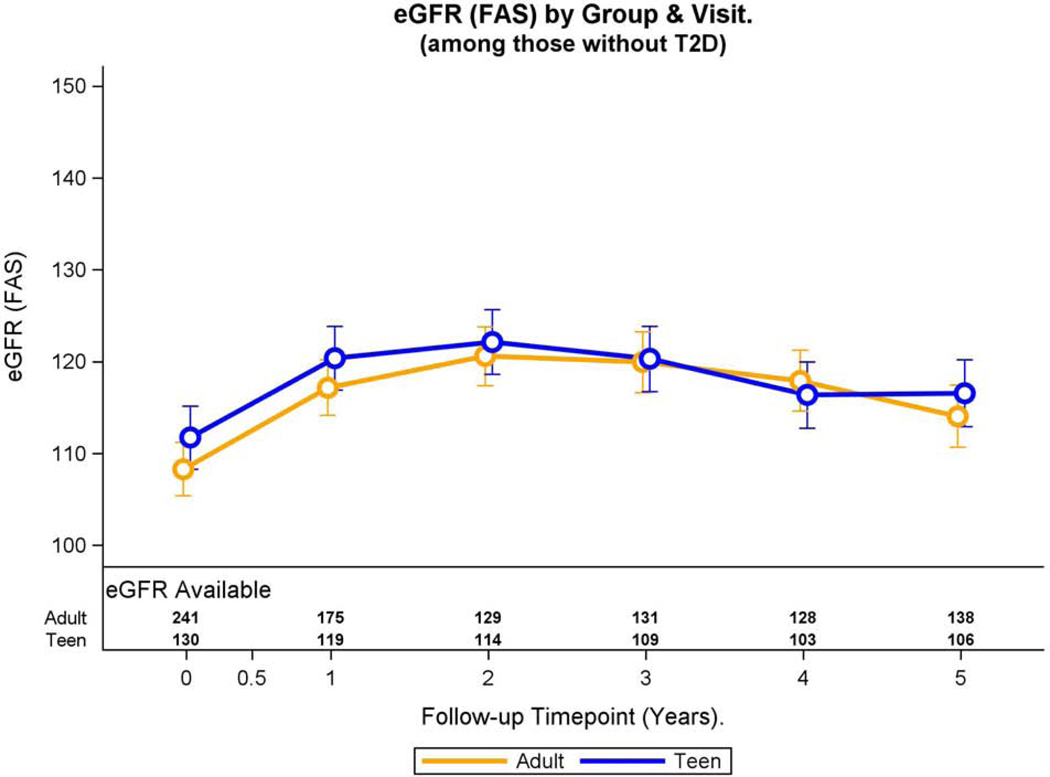
Fig. 4A: Hyperfiltration and eGFR over time in adolescents and adults without pre-operative type 2 diabetes.
Modeled change in prevalence of hyperfiltration over time was similar by group (group * time interaction, p=0.11).
Over 5-year period adjusted mean eGFR in adolescents was significantly greater than adults (mean difference: 3.1, 95% CI 0.05, 6.1 ml/min/1.73m2, p=0.047). Both groups experienced a significant change in eGFR over time (p<0.001). Specifically, the adjusted mean eGFR increased from baseline to year 1 (p<0.001), year 1 to year 2 (p<0.001) and then decreased from year 3 to year 4 (p=0.002), and year 4 to year 5 (p=0.02). Baseline and 5-year eGFR were similar (p=0.08). Furthermore, interaction term was not significant indicating that the adjusted eGFR trajectories of both groups during the study period were similar (p=0.07, Figure 4B).
Fig 4.B:
Modeled change in eGFR over time was similar by group (group * time interaction, p=0.07).
Effect of gastric bypass on impaired GFR in adolescents and adults
There were only 4 adults and 1 adolescent with eGFR <60 ml/min/1.73m2 pre-operatively, which limits our ability to evaluate the impact of bariatric surgery on impaired GFR.
Discussion
This study represents the first analysis to compare kidney outcomes in severely obese adolescents and adults undergoing gastric bypass. The impact of bariatric surgery on kidney outcomes may depend on age at surgery and on the type 2 diabetes status pre-operatively. Adolescents with pre-operative type 2 diabetes experienced a more precipitous resolution of elevated UACR following gastric bypass compared to their adult counterparts.
Severe obesity now afflicts 4–6% of US youth and is associated with poor health outcomes, including increased risk of cardiovascular disease, type 2 diabetes mellitus, and premature death (12). We recently also described an increased prevalence of early kidney disease in this population (13), and these adolescents are at high risk for progression to end-stage kidney disease in adulthood (14). In the current analysis, we found that the adjusted prevalence of elevated UACR prior to surgery was almost 2-fold higher in adolescents in the Teen-LABS cohort compared to their adult counterparts in the LABS study irrespective of pre-operative type 2 diabetes status. This finding was unexpected considering that the adult comparison group had onset of obesity by 18 years of age, and were therefore subject to longstanding adverse renal effects of obesity. The discrepancy in elevated UACR indicates that adolescents with severe obesity who undergo bariatric surgery may represent a more severe phenotype of obesity-related nephropathy and DKD. Interestingly, Teen-LABS participants also demonstrated increased serum insulin concentrations prior gastric bypass compared to adults, both among adolescents with and without type 2 diabetes. Puberty is associated with a marked insulin resistance and may at least partially explain the difference observed in serum insulin concentrations between the adolescent and adult participants in this analysis (15). Hyperinsulinemia and insulin resistance are putative mechanisms of obesity-related nephropathy and diabetic kidney disease, especially among those with type 2 diabetes (16, 17). In fact, we previously reported that in adolescents with type 2 diabetes, insulin resistance was the strongest determinant of kidney disease, including incident hyperfiltration and elevated UACR (18, 19). The results of the current study suggest that high insulin concentrations may have contributed to the increased prevalence of preoperative kidney disease in adolescents undergoing bariatric surgery.
Bariatric surgery is now increasingly recognized as an accepted treatment option for severe obesity that has beneficial effects on long-term obesity-related comorbidities, including kidney disease (8, 20). In a recent and separate analysis by our group, we demonstrated that Teen-LABS participants with type 2 diabetes experienced regression of hyperfiltration, elevated UACR and hypertension during a 5 year follow-up period after bariatric surgery (gastric bypass and vertical sleeve gastrectomy)(21). In contrast, adolescents receiving medical treatment in Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) study experienced increased rates of hyperfiltration, elevated UACR and hypertension during the same time period (7). We also recently reported that adolescents more often experienced remission of both type 2 diabetes and hypertension in response to bariatric surgery, but abdominal reoperations and short-term nutritional deficiencies were more prevalent among adolescents than adults (11). In the current analysis we demonstrate that younger age at bariatric surgery is associated with higher rates of hyperfiltration and earlier remission of kidney disease in those with type 2 diabetes pre-operatively, despite higher prevalence of kidney disease before surgery. These data support the indication of bariatric surgery in adolescents with severe obesity and type 2 diabetes.
The earlier and more pronounced remission of elevated UACR in youth with type 2 diabetes may provide additional evidence that adolescents have greater plasticity for comorbidity reversal compared to adults. Longer duration of diabetic kidney disease is associated with fibrosis and nephron loss, which may explain differences in timing of remission of elevated UACR in adolescents compared to adults with pre-operative type 2 diabetes. Fibrosis and histological remodeling may have a more delayed response to surgical weight loss (22). Earlier improvements in kidney function in adolescents may reflect greater impact of surgery on reversible intrarenal hemodynamic and neurohumoral factors (23). Accordingly, a reasonable explanation of our findings may be that gastric bypass improves type 2 diabetes-related, reversible physiological-functional mechanisms underlying early kidney disease in youth, while less reversible anatomic/structural changes that have occurred with aging or disease duration contribute to relatively delayed remission in adults following surgical weight loss. The higher prevalence of hyperfiltration, an early marker of diabetic nephropathy(24), among adolescents with type 2 diabetes supports the concept that bariatric surgery in this population may treat existing DKD earlier in its natural history. Noteworthy, differences in onset of kidney disease remission between adolescents and adults were not observed in participants without type 2 diabetes before surgery. The discrepancy between participants with and without type 2 diabetes before surgery may relate to functional and structures differences between obesity-related nephropathy and DKD, and how these injuries respond to bariatric surgery.
There are strengths and weaknesses of this study worth mentioning. The major strengths of the present analysis include data from studies with prospective enrollment of consecutive cases of gastric bypass surgery across multiple institutions participating in two multi-center studies using synchronized data collection and standardized methods. Limitations include the observational study design and low prevalence of certain outcomes, and small sample size of adolescents with type 2 diabetes. The lack of non-surgical control groups also precludes any strictly causal inference between surgical intervention and improved outcomes, though our data contribute to a larger body of evidence indicating that bariatric surgery has a beneficial effect on long-term kidney outcomes.(25) Additionally, while the adult study sample selected for comparison had a longer duration of obesity than the participants in the adolescent study, there may be residual biases in the adult comparison group for which we cannot fully account. For example, although adult participants were obese at 18 years age as assessed by questionnaire at the time of surgery, their weight at this at 18 years of age was not objectively measured. Accordingly, we cannot exclude that the weight at age 18 was higher in Teen-LABS participants compared to their adult counterparts in LABS and influenced the decision to proceed with earlier surgical intervention. Other limitations of our methods include use of estimated GFR rather than directly measured GFR. However, repeated gold-standard assessments of GFR would have been difficult within such large, longitudinal studies. Moreover, our data were limited to random UACR collections rather than timed urine collections. Spot collections are prone to more random variation, possibly due to or changes in urine concentration.(26, 27) Finally, differential missing data between cohorts over time is also a limitation. Specifically, 52% of LABS participants did not have urine available for analysis at the 5-year follow up time point, compared to only 24% of LABS subjects. However, statistical techniques that addressed missing data were applied and sensitivity analyses indicated missing data pattern assumptions were reasonable.
It is important to note that although our findings suggest earlier remission of kidney disease in adolescents compared to adults with type 2 diabetes in response to gastric bypass, the potential risks associated with bariatric surgery should be recognized. Complications of bariatric surgery include the possibility of the need for repeat surgery, the requirement for lifelong nutrient supplementation to prevent or treat dietary deficiencies, deleterious implications on bone health, potential impacts on the offspring, as well as the concerning mental health burden. Clinical adverse events in Teen-LABS and LABS have previously been reported (28). It is also worth noting that abdominal reoperations and short-term nutritional deficiencies were more common among adolescents than adults following gastric bypass (17).
In conclusion, we report an increased prevalence of early kidney disease in severely obese adolescents with type 2 diabetes compared to adults prior to bariatric surgery. This might be due to a higher prevalence of hyperinsulinemia in Teen-LABS participants with type 2 diabetes. Earlier remission of elevated UACR following gastric bypass was apparent in adolescents with type 2 diabetes compared to their adult counterparts. Kidney disease is associated with increased risk for cardiovascular events and early death in type 2 diabetes. However, it remains unclear whether earlier remission of DKD will translate to better long term cardiovascular and kidney health. Therefore, further research with extended follow up is needed to refine the risks and benefits of bariatric surgery on long-term health outcomes in severely obese adolescents with and without type 2 diabetes.
Material and Methods:
Study Design and Participants
The Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS; NCT00474318) and Longitudinal Assessment of Bariatric Surgery (LABS; NCT00465829) studies were designed similarly as prospective multicenter observational studies of consecutive cases of bariatric surgery (29–32) (Supplemental Figure 1). The adolescent study incorporated the design features and data collection forms of the adult study in order to facilitate valid comparisons of the two cohorts. Both studies recruited participants undergoing any first time bariatric surgical procedure. The adolescent study enrolled teens (age ≤19 years) at five participating centers from 2006–2012, while the adult study enrolled patients age ≥18 years at 10 centers, from 2006–2009. Adult study participants completed a weight history questionnaire (33) characterizing body weight by age 18, permitting the selection of adults with BMI ≥ 30 kg/m2 by age 18 for the present analysis. The current analysis included adolescents who had Roux-en-Y gastric bypass, as this was the dominant operation in adolescents in Teen-LABS. Comparisons were similarly limited to adult participants ages 25 to 50 at time of surgery who underwent gastric bypass surgery. The protocol and data and safety monitoring plans were approved by the institutional review boards at all participating institutions and by the data and safety monitoring boards for each study.
Research methods and data collection were described previously (29–31). Research visits for both studies occurred at baseline within 30 days prior to surgery, at 6 months after surgery, and annually up to five years after surgery. Data collected by each consortium were maintained in a central database by their respective data coordinating centers. Through the five-year study time point, 96% (154 of 161) of the adolescent cohort and 96% (379 of 396) of adults remained as active participants, completing 89% (698 of 784 [adolescent]) and 83% (1,623 of 1,957 [adult]) of all postoperative research visits. At the five-year visit, some data (at least body weight) were collected for 81% of all participants.
All laboratory assays were performed by the Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, Washington, as previously described (28). Concentrations of creatinine in serum and urine were determined annually by using the Creatinine Plus enzymatic Roche reagent on a Modular P analyzer (Roche Diagnostics, Inc., Indianapolis, IN). The results of this procedure are traceable to the IDMS reference method and allow for accurate estimated GFR (eGFR) ascertainment. The reportable range of creatinine in serum/plasma samples is: 0.03–60.0 mg/dL, and 0.03–1200.0 mg/dL in urine samples. Concentration of cystatin C in serum was determined at baseline and annually by immunochemically by using Siemens reagents (Siemens Healthcare Diagnostics. Inc, Newark, DE) on a Siemens nephelometer autoanalyzer (BNII). This method is standardized against the IFCC/ERM DA-471 Reference Material (RT Corp, Laramin WY). Due to the expected normal to elevated glomerular filtration rates for age, we calculated eGFR by Full Age Spectrum (FAS) combined serum creatinine and cystatin C equation, which has been validated in both pediatrics and adults and lends itself well to studies examining the transition from pediatric to early adulthood:
| (34). |
The FAS equation is based on normalized serum creatinine (SCr/Qcrea), where Qcrea is the median serum creatinine (SCr) from healthy populations to account for age and sex, and QcysC is defined as 0.82 mg/L for ages <70 years. The coefficient ‘α’ in the denominator is a weighting factor for the normalized renal biomarkers. We used α = 0.5, which means the denominator is equal to the average of both normalized biomarkers. We defined hyperfiltration as eGFR ≥ 135ml/min/1.73m2 (35). Urine albumin-to-creatinine ratio (UACR) was measured at baseline and annually thereafter unless a result was abnormal. Spot urine samples were obtained after a 10–14hr overnight fast. Elevated UACR was defined as an UACR of ≥30 μg/mg (19).
Statistical Analysis:
Due to the significant difference in prevalence of pre-operative type 2 diabetes between adolescents and adults, and also the greater risk of kidney disease in severely obese people with diabetes compared to those without diabetes, analyses were stratified by pre-operative type 2 diabetes status. Categorical variables are presented as frequencies and percentages, with group differences evaluated using chi-square tests. Continuous variables are reported as means with standard deviations or medians with first and third quartiles, with group comparisons evaluated using t-tests or Wilcoxon rank-sum tests. Repeated measures analyses were performed using mixed models with a subject-level random intercept term. Linear mixed models were used to compare eGFR and log-transformed UACR by study cohort. Poisson mixed modeling with robust error variance was used to compare prevalence of elevated UACR and hyperfiltration by study cohort. In addition to study cohort, study visit, and cohort by visit interaction, the following variables were considered for inclusion in the final models: race, hypertension, BMI, and the following baseline traits: insulin, HbA1c, hs-CRP, HDL cholesterol, LDL cholesterol, RAAS inhibitor use, and eGFR. Sex and baseline education were forced into each model, as these characteristics were associated with missing follow-up visits (11). Values from female participants in their second or third trimester of pregnancy and up to six months postpartum were omitted from analyses. Modeled estimates (geometric means for UACR) and 95% confidence intervals were calculated by cohort and study visit. These models addressed missing data values by maximum likelihood, under the data missing at random assumption. Sensitivity analyses were performed and were found to support the validity of this assumption (11). All statistical analyses were conducted using SAS v9.4; all reported p-values were two-sided and considered statistically significant when less than 0.05. No adjustments were made for multiple comparisons.
Supplementary Material
Supplemental Figure 1. Cohort Flow Diagram
Acknowledgements:
The research protocols of Teen-LABS and LABS were approved by the relevant institutional review boards and all human participants gave written informed consent.
Support: The Teen-LABS consortium is funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grants: UM1DK072493 (PI, Dr. Thomas Inge, University of Colorado, Denver), and UM1DK095710 (PI, Dr. Changchun Xie, University of Cincinnati). Dr. Petter Bjornstad is funded by NIDDK (K23-DK116720), in addition to research support from JDRF (2-SRA-2018–627-M-B, 2-SRA-2019–845-S-B), Thrasher Research Fund, NIH/NIDDK DiaComp, ISPAD, Diabetes Guild, Center for Women’s Health Research at University of Colorado, Children’s Hospital Colorado Research Institute and Colorado Clinical and Translational Sciences Institute.
Footnotes
Potential Conflicts of Interest: PB has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk and Horizon Pharma. PB serves on the advisory board of XORTX. All support was outside the submitted work. All other authors have indicated they have no relationships relevant to this article to disclose.
Supplementary Material:
Supplementary information is available on Kidney International’s website
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011;57(1 Suppl 1):A8, e1–526. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R, Jr., Dolan L, Imperatore G, et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA. 2017;317(8):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes care. 2013;36(6):1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Saeed AH, Constantino MI, Molyneaux L, D’Souza M, Limacher-Gisler F, Luo C, et al. An Inverse Relationship Between Age of Type 2 Diabetes Onset and Complication Risk and Mortality: The Impact of Youth-Onset Type 2 Diabetes. Diabetes care. 2016;39(5):823–9. [DOI] [PubMed] [Google Scholar]
- 5.Bjornstad P, Cherney DZ, Maahs DM, Nadeau KJ. Diabetic Kidney Disease in Adolescents With Type 2 Diabetes: New Insights and Potential Therapies. Current diabetes reports. 2016;16(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71(3S1):A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornstad P, Hughan K, Kelsey MM, Shah AS, Lynch J, Nehus E, et al. Effect of Surgical versus Medical Therapy on Diabetic Kidney Disease over 5 Years in Severely Obese Adolescents with Type 2 Diabetes. Diabetes Care (under review). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nehus EJ, Khoury JC, Inge TH, Xiao N, Jenkins TM, Moxey-Mims MM, et al. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int. 2017;91(2):451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjornstad P, Hughan K, Kelsey MM, Shah A, Lynch J, Nehus E, et al. Effect of Surgical versus Medical Therapy on Diabetic Kidney Disease over 5 Years in Severely Obese Adolescents with Type 2 Diabetes. Diabetes Care. 2019;(under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inge TH, King WC, Jenkins TM, Courcoulas AP, Mitsnefes M, Flum DR, et al. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132(6):1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA, et al. Five-Year Outcomes of Gastric Bypass in Adolescents as Compared with Adults. N Engl J Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. 2013;128(15):1689–712. [DOI] [PubMed] [Google Scholar]
- 13.Xiao N, Jenkins TM, Nehus E, Inge TH, Michalsky MP, Harmon CM, et al. Kidney function in severely obese adolescents undergoing bariatric surgery. Obesity (Silver Spring). 2014;22(11):2319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelsey MM, Zeitler PS. Insulin Resistance of Puberty. Curr Diab Rep. 2016;16(7):64. [DOI] [PubMed] [Google Scholar]
- 16.De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013;28(1):29–36. [DOI] [PubMed] [Google Scholar]
- 17.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(3):550–62. [DOI] [PubMed] [Google Scholar]
- 18.Bjornstad P, Maahs DM, Cherney DZ, Cree-Green M, West A, Pyle L, et al. Insulin sensitivity is an important determinant of renal health in adolescents with type 2 diabetes. Diabetes care. 2014;37(11):3033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornstad P, Nehus E, El Ghormli L, Bacha F, Libman IM, McKay S, et al. Insulin Sensitivity and Diabetic Kidney Disease in Children and Adolescents With Type 2 Diabetes: An Observational Analysis of Data From the TODAY Clinical Trial. Am J Kidney Dis. 2018;71(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjornstad P, Hughan K, Kelsey M, Shah AS, Lynch J, Nehus E, et al. Effect of Surgical versus Medical Therapy on Diabetic Kidney Disease over 5 Years in Severely Obese Adolescents with Type 2 Diabetes. Diabetes Care (under review). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43(8):1388–95. [DOI] [PubMed] [Google Scholar]
- 23.Streeten DH, Anderson GH, Jr., Wagner S. Effect of age on response of secondary hypertension to specific treatment. Am J Hypertens. 1990;3(5 Pt 1):360–5. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636–42. [DOI] [PubMed] [Google Scholar]
- 25.Chang AR, Grams ME, Navaneethan SD. Bariatric Surgery and Kidney-Related Outcomes. Kidney Int Rep. 2017;2(2):261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naresh CN, Hayen A, Craig JC, Chadban SJ. Day-to-day variability in spot urine protein-creatinine ratio measurements. Am J Kidney Dis. 2012;60(4):561–6. [DOI] [PubMed] [Google Scholar]
- 27.Yang CY, Chen FA, Chen CF, Liu WS, Shih CJ, Ou SM, et al. Diagnostic Accuracy of Urine Protein/Creatinine Ratio Is Influenced by Urine Concentration. PLoS One. 2015;10(9):e0137460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML, et al. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA Pediatr. 2018;172(5):452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. 2014;168(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surg Obes Relat Dis. 2013;9(6):926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins TM, Buncher CR, Akers R, Daniels SR, Lawson ML, Khoury PR, et al. Validation of a weight history questionnaire to identify adolescent obesity. Obes Surg. 2013;23(9):1404–12. [DOI] [PubMed] [Google Scholar]
- 34.Pottel H, Delanaye P, Schaeffner E, Dubourg L, Eriksen BO, Melsom T, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant. 2017;32(3):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol. 2017;28(4):1023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cohort Flow Diagram