Abstract
Purpose
Proton pump inhibitors (PPIs) are used in cancer patients to manage treatment-related gastrointestinal symptoms and to prevent damage to the gastric mucosal lining during treatment. However, PPI use may contribute to cognitive problems. To compare PPI-users and non-users, breast cancer survivors reported cognitive problems in three studies.
Methods
In Study 1, breast cancer survivors (N=209; n=173 non-users, n=36 PPI-users; stages 0-IIIC) rated their cognitive function on the Kohli scale prior to cancer treatment, as well as one and two years later. In Study 2, women (N=200; n=169 non-users, n=31 PPI-users, stages 0-IIIa, M=11 months post-treatment) rated their cognitive function on the Kohli scale and BCPT checklist at three visits over a six-month period. In Study 3, participants (N=142; n=121 non-users, n=21 PPI-users; stages I-IIIa, M=4 years post-treatment) rated their cognitive function on the Kohli scale, BCPT checklist, and Functional Assessment of Cancer Therapy cognitive scale (FACT-cog).
Results
In Study 1, PPI-users reported more severe concentration problems (p=0.039) but not memory problems (p=0.17) than non-users. In Study 2, PPI-users reported more severe concentration problems (p=0.022) than non-users, but not memory problems or symptoms on the BCPT (ps=0.11). Study 3’s PPI-users reported more severe memory problems (p=0.002), poorer overall cognitive function (p=0.006), lower quality of life related to cognitive problems (p=0.005), greater perceived cognitive impairment (p=0.013), and poorer cognitive abilities (p=0.046), but not more severe concentration problems (p=0.16), compared to non-users.
Conclusions/Implications
PPI use may impair breast cancer survivors’ memory, concentration, and quality of life.
Keywords: Proton pump inhibitors, breast cancer survivors, cognitive symptoms, concentration, memory
Introduction
Proton pump inhibitors (PPIs; esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole) are considered safe and are available over the counter in the U.S. PPIs treat conditions involving hyper-acidity, such as erosive esophagitis and gastroesophageal reflux disease. However, up to 70% of PPI usage may be inappropriate, e.g., for ongoing non-specific heartburn and indigestion symptoms [1]. Additionally, stopping PPI use can cause rebound acid hypersecretion, facilitating chronic use [2]. The potential adverse effects arising from prolonged PPI use are largely unknown, as long-term clinical trials have had a limited scope [3].
Two novel, off-label uses for PPIs have been discovered in cancer patients: gastro-protection, or shielding the intestinal mucosal lining from erosion caused by chemotherapy [4,5], and chemo-sensitization, or increasing the tumor’s responsivity to chemotherapy [6]. To reap these benefits, cancer patients may need to use PPIs throughout, and even prior to, chemotherapy, which may last several months or more. To avoid rebound acid hypersecretion, cancer patients may use PPIs long into survivorship. However, it is difficult to estimate rates or duration of PPI-usage in cancer survivors because they are available over-the-counter. In some cases, PPIs may not be listed on medical records, and physicians may be unaware of their patients’ intermittent or chronic usage.
PPIs may trigger cognitive decline or even dementia. Two epidemiological studies of dementia-free elderly individuals (≥ 75 years old) found PPI-users were about 40% more likely to develop dementia, compared to non-users after adjusting for age, sex, education, the Apolipoprotein E4 allele status, polypharmacy, and comorbidities [7,8]. This increased risk would translate into 10,000 new cases of dementia per year among those aged 75 to 84 alone [9]. A recent systematic review of 11 relevant studies (1 randomized, controlled trial, 4 cohort studies, 1 case control study, 1 cross-sectional study, and 6 case reports), concluded that, despite mixed findings, the majority of studies found an increased risk of dementia and cognitive impairment among PPI users [10].
Overview of Present Studies
The present studies are the first to examine potential cognitive effects of PPI use among breast cancer survivors - a group inherently at risk for cognitive decline [11]. To assess the functional implications of PPI use in breast cancer survivorship, we utilized data from breast cancer survivors at various points of survivorship in three distinct parent studies. Study 1 was a two-year observational study of fatigue in newly-diagnosed breast cancer patients. Study 2 was a 6-month randomized controlled trial investigating yoga’s effect on inflammation and fatigue among breast cancer survivors (M=11 months post-adjuvant treatment). Study 3 was a randomized controlled trial examining breast cancer survivors (M=4 years post-adjuvant treatment) inflammatory and mood-related vaccine responses. The current study used data from Study 3’s screening and placebo visit only. These secondary analyses utilized self-reported cognitive function data from all three studies.
Breast cancer survivors’ reports of cognitive problems provide important and meaningful data [12]. Cognitive difficulties predict distress, fatigue, and poorer quality of life, and may reflect brain structure abnormalities (e.g., white matter lesions) [13–15]. Indeed, self-report measures may detect subtle lapses in memory and concentration that neurocognitive tests are not sensitive enough to detect [11,14].
Across studies, we examined the effect of PPIs on self-reported cognitive function, predicting that PPI-users would report worse cognitive function than non-users. These studies were approved by the Ohio State Institutional Review Board, and all participants consented before participating.
Study 1: Method
Participants
For the longitudinal observational parent study addressing fatigue in breast cancer survivors, 209 cancer patients (n=173 non-users, n=36 PPI-users) were recruited from The Ohio State University cancer clinics shortly after their diagnosis. Women with stage IV cancer, a prior history of cancer (excluding basal or squamous cell skin carcinomas), or significant visual, auditory, or cognitive impairments were excluded. Data collection occurred between June 2008 and February 2014.
Procedure
Participants self-reported their cognitive symptoms at three visits: prior to treatment (Visit 1), and an average of 8.4 months (Visit 2) and 20.2 months (Visit 3) post-adjuvant treatment. Overall, 187 women (n=149 non-users, n=38 PPI-users) returned for Visit 2 and 171 (n=139 non-users, n=31 PPI-users, n=1 unknown) women returned for Visit 3. We hypothesized that PPI-users would report more severe cognitive problems than PPI non-users.
Measures
To assess cognitive symptoms, at each visit participants completed the two-item Kohli Scale [11], rating their worst memory and concentration problems over the past five days bounded by 0 (“not present”) to 10(“as bad as you can imagine”). The two items were designed to be analyzed separately [11]. A rating of seven or greater on either scale is considered severe. Due to ease and speed of administration, the Kohli is well-suited to a clinic setting and is less burdensome than longer measures for those experiencing cognitive impairment.
For the covariates, both medical chart data and women’s self-reported medical diagnoses at the screen visit were used to calculate the Charlson Comorbidity Index, which was developed in a breast cancer population [17]. Using the Center for Epidemiological Depression Scale (CES-D), women reported how often they experienced certain depressive symptoms during the past week, ranging from ‘rarely or none of the time’ to ‘most or all of the time’ [18]. The continuous CES-D score was included as a covariate due to depression’s links with acid reflux [19] and cognitive performance [20]. Women also recalled regular and recent medication usage at each visit, and a PPI dummy variable was coded as 0 for ‘no current use’ and 1 for ‘current use.’ Lastly, we obtained cancer status and treatment information from medical records and computed separate dummy variables for chemotherapy, radiation, and cancer-related hormone therapy, which can all impair cognition [13,21,22].
Statistical Methods
To assess between-group differences in relevant demographic information at baseline, we employed independent sample t-tests and χ2 tests. For the questions of interest, we used SAS 9.5 (Cary, NC) to construct hierarchical linear models with a compound symmetry covariance matrix to make use of and account for the within-subjects repeated measures design. This analytical technique provides a pooled estimate of PPI use on cognition (i.e., the average effect of PPI use throughout the study). PPI use was the independent variable, and the Kohli scales were the dependent variables, in separate models. These models adjusted for the following conceptually-relevant variables: comorbidities [23], depressive symptoms [20], cancer stage, chemotherapy treatment [11], radiation treatment [22], age [24], hormone therapy [21], and education [25]. No imputation was performed to address missing data as hierarchical linear modeling is equipped to handle missing values without excluding entire cases. In all analyses, alphas were set at 0.05 and two-tailed tests of significance were conducted.
Study 1: Results
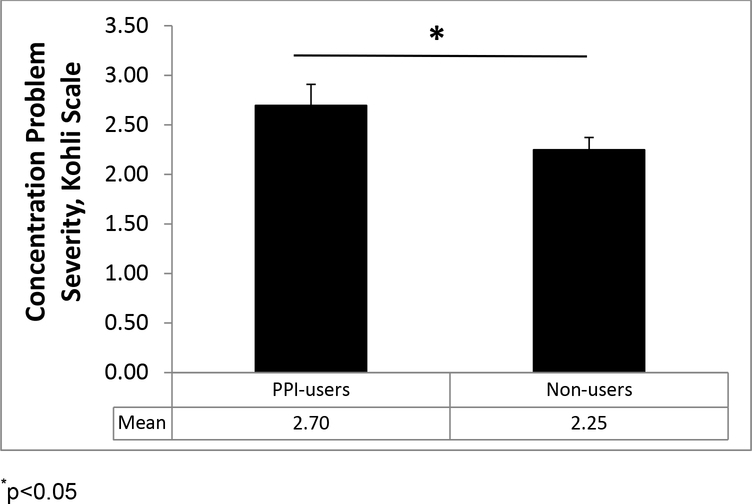
Due to fewer exclusionary criteria especially concerning comorbidities, this study’s sample was more heterogeneous than the samples described below. On average, participants were middle-aged (M=55.8 years old) with considerable range (26 to 88 years old). Overall, 78% of the women were White and 15% were Black. At baseline, 17% of women reported PPI use, and 66% of these women continued to report PPI usage at both of the annual follow-up visits. PPI-users and non-users did not differ on variables of interest, except that PPI-users were older (M=59.6 years old) than non-users (M=54.8 years old) (p=0.039). Throughout the study, PPI-users reported more severe concentration problems (estimated marginal mean; EMM=2.697, SE=0.212) than non-users (EMM=2.249, SE=0.124) on the Kohli scale (F(1, 386)=4.30, p=0.039), Figure 1. However, PPI-users did not report more severe memory problems on the Kohli scale, compared to non-users (p=0.17).
Figure 1.
Estimated marginal means (± SEs) of self-reported cognitive problems for PPI-users and non-users in Study 1. PPI-users reported more severe concentration problems on the Kohli than non-users; *p<0.05.
Study 2: Methods
Participants
For the parent randomized, controlled trial investigating the effects of a yoga intervention on inflammation and fatigue, 200 breast cancer survivors were recruited via breast cancer support groups, oncologists’ referrals, and community announcements [26]. The exclusionary criteria included a prior history of other cancers as well as other chronic diseases known to alter metabolism and inflammation (i.e., diabetes, anemia, autoimmune disease or other inflammatory disease), alcohol or drug abuse, and more than five hours of vigorous physical activity per week. Participants had completed treatment (except hormonal treatment) 11 months earlier, on average. Data collection occurred between October 2007 and December 2012.
Procedure
At baseline, participants (N=200; n=169 non-users, n=31 PPI-users) were randomly assigned to the experimental group, a 12-week biweekly yoga intervention, or the waitlist control for the parent randomized controlled trial. Participants (N=184; n=154 non-users; n=30 PPI-users) returned for their first follow-up visit an average of 153 (SD=51) days after the baseline visit, and participants (N=181; n=152 non-users, n=29 PPI-users) returned for their second follow-up visit an average of 248 (SD=53) days after the baseline visit, respectively. At all three visits, women completed self-report cognitive assessments. We hypothesized that PPI-users would report more severe cognitive problems and more cognitive symptoms than non-users.
Measures
At each visit, women completed the Kohli scale and the Breast Cancer Prevention Trial (BCPT) cognitive symptoms checklist, which were designed to assess cognitive complaints among cancer survivors [11,16]. The BCPT cognitive subscale is the average of three five-point Likert scales that measure the extent to which an individual has been bothered by specific symptoms (i.e., forgetfulness, distractibility, difficulty concentrating) over the past four weeks. Thus, it measures more chronic cognitive complaints than the Kohli. From women’s self-reported medication usage, a PPI dummy variable was calculated at each visit. Covariate data were obtained as described in Study 1.
Statistical Methods
To assess between-group differences in relevant demographic information at baseline, we employed independent sample t-tests and χ2 tests. For the questions of interest, hierarchical linear models with a compound symmetry covariance matrix were constructed with SAS 9.5 software (Cary, NC). In separate models, PPI use was the independent variable and the self-reported cognitive measures were the dependent variables. Models adjusted for the same covariates used in Study 1 (age, depressive symptoms, education, comorbidities, cancer stage, chemotherapy treatment, radiation treatment, and hormone treatment). To address potential yoga group cohort effects, random, group-specific intercepts in the yoga condition were included in accordance with the study’s partially nested design. In all analyses, alphas were set at 0.05 and two-tailed tests of significance were conducted.
Study 2: Results
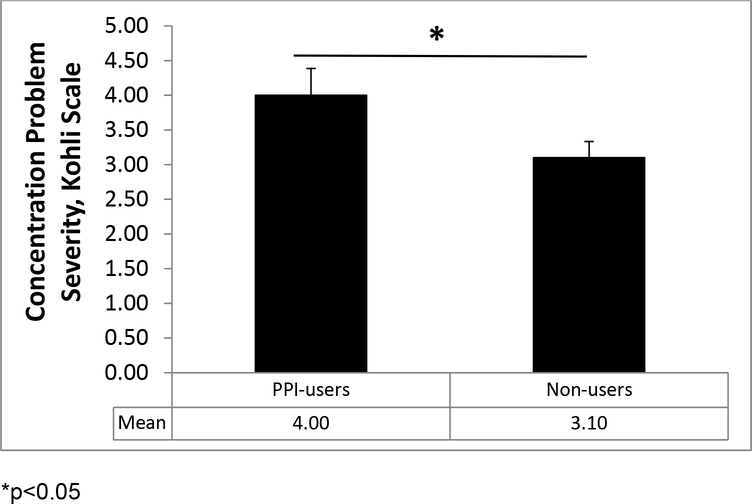
The majority of women were highly educated (college graduate or graduate training) (69.5%), stage 0 or I breast cancer survivors (53.5%), and Caucasian (88%). Compared to non-users, PPI-users had longer post-adjuvant recovery (p=0.029), but did not differ on other relevant variables (ps>0.09). See Table 2 for participant characteristics. Throughout the study, PPI-users reported more severe concentration problems (EMM=4.000, SE=0.386) on the Kohli than non-users (EMM=3.101, SE=0.232) (F(1, 207)=5.36, p=0.022. PPI-users did not endorse more memory problems on the Kohli (p=0.11) or cognitive symptoms on the BCPT symptoms checklist (p=0.11) than nonusers. See Figure 2.
Table 2:
Study 2 sample demographics at baseline
| PPI Users (n = 31) | PPI Non-users (n = 169) | ||||
|---|---|---|---|---|---|
| Measure | N (%) | M (SD) | N (%) | M (SD) | |
| Age | 54.4(9.9) | 51.1(9.0) | |||
| Comorbidities | 0.3(0.7) | 0.1(0.4) | |||
| Months since cancer tx | 14.0(8.6)* | 10.3(7.7)* | |||
| Race (% White) | 28(90%) | 148(88%) | |||
| Education | High School | 2(6%) | 10(6%) | ||
| Some College | 7(23%) | 42(25%) | |||
| College Grad | 9(29%) | 53(31%) | |||
| Grad Training | 13(42%) | 64(38%) | |||
| Chemo tx (% Yes) | 15(48%) | 107(54%) | |||
| Yoga group (% Yes) | 17(55%) | 83(49%) | |||
| Hormone tx (% Yes) | 9(29%) | 67(40%) | |||
| Radiation tx (% Yes) | 23(74%) | 105(62%) | |||
| CES-D score | 9.0(6.3) | 11.0(8.5) | |||
| Cancer Stage | |||||
| Stage 0 | 5(16%) | 13(11%) | |||
| Stage I | 13(42%) | 76(45%) | |||
| Stage II | 10(32%) | 65(38%) | |||
| Stage III | 3(10%) | 15(9%) | |||
p<0.05
Figure 2.
Estimated marginal means (± SEs) of self-reported cognitive problems for PPI-users and non-users in Study 2. PPI-users reported more severe concentration problems on the Kohli compared to non-users; *p<0.05.
Study 3: Methods
Participants
Breast cancer survivors (N = 142; n=121 non-users; n=21 PPI-users) were recruited via breast cancer support groups, oncologists’ referrals, and community announcements for the ongoing parent study ( NCT02415387). Participants were post-menopausal, between 40 and 80 years old, and had completed primary cancer treatment an average of 4 years earlier for stage I-IIIA breast cancer. The exclusionary criteria for the parent study included a prior history of other cancers as well as other chronic diseases known to alter metabolism and inflammation (i.e., diabetes, anemia, autoimmune disease or other inflammatory disease), alcohol or drug abuse, statin usage, and current smoking. Data collection is ongoing and began in January 2014.
Procedure
Following a screening visit and confirmation of eligibility, participants signed a release for their medical records. At two visits an average of 39 days apart (SD=19), they received a typhoid vaccine and saline injection (placebo) – one per visit – in a randomized order. Only data from the screening and placebo visits were used in this study. Participants reported PPI use and cognitive symptoms at each visit, and covariate data (i.e., chemotherapy and hormone treatment, comorbidities) were collected as described in Study 1. We hypothesized that PPI-users would report more severe cognitive problems, more cognitive symptoms, and more impaired cognitive function than PPI non-users.
Measures
Women completed the Kohli scale to assess recent cognitive functioning at both visits and the BCPT checklist to assess longer-term cognitive functioning at Visit 2. One additional self-report cognitive measure was included at the screening visit: the Functional Assessment of Cancer Therapy cognitive scale (FACT-cog), a 37-item measure that yields a total score and four subscale scores (i.e., perceived cognitive impairments subscale, 0 – 80; impact on quality of life (QoL) subscale, 0 −16; comments from others subscale, 0 – 16; and perceived cognitive abilities subscale, 0 – 36) [27]. Among a sample of breast cancer survivors, the total FACT-cog score and three of the subscale were internally consistent (Cronbach alphas=0.77–0.86) and predicted objective cognitive test performance: the total FACT-cog score was related to immediate and delayed verbal memory; the perceived cognitive impairment and perceived cognitive abilities subscales both tracked with immediate and delayed verbal memory and executive function; and comments from others predicted immediate verbal memory [28]. On the FACT-cog, women rated their perceived cognitive function on five-point Likert scales measuring the frequency of cognitive complaints over the past seven days - bounded by “never” and “several times a day.” Higher scores indicate better cognitive function in the last week.
At each visit, women reported current PPI usage, as PPIs are available without a prescription and are not always listed on medical records. Participants also listed other medication usage at each visit, and this information was used to tabulate number of prescription medications used. Medical records provided data on chemotherapy and cancer-related hormonal treatment status. Lastly, participants reported medical diagnoses at the screen visit, which was corroborated with medical records.
Statistical Methods
Twelve participants (n=1 PPI-user, n=11 non-users) were excluded from analyses due to prior participation in Study 2. Compared to those who were included in analyses, those who were excluded had longer recovery since cancer treatment and were more likely to be White (ps<0.020) but were not different in terms of age, education level, comorbidities, chemotherapy or hormonal treatment, number of prescription medications, or PPI use (ps>0.19). Analyses using screening data included 121 non-users and 21 PPI-users; analyses using the placebo visit included 96 non-users and 14 PPI-users. Data from the vaccine visit were not used. As the BCPT was only measured at Visit 2, only those who had their placebo injection at this visit were included in the BCPT analysis. To assess between-group differences in relevant demographic information at the screening visit, we employed independent sample t-tests and χ2 tests. For the questions of interest, we used analysis of covariance. The same covariates were used (i.e., comorbidities, depressive symptoms, cancer stage, chemotherapy treatment, radiation treatment, age, cancer-related hormone therapy, and education). However, CES-D scores were not included as a covariate in FACT-cog models, as the CES-D was not measured at the screening visit. To address cases with missing covariate data (n=8 for screen analyses; n=2 for placebo visit analyses), imputation was performed five times. The overall pattern of results was the same with or without the imputed data. Below we report the results with the imputed data. In all analyses, alphas were set at 0.05 and two-tailed tests of significance were conducted.
Study 3: Results
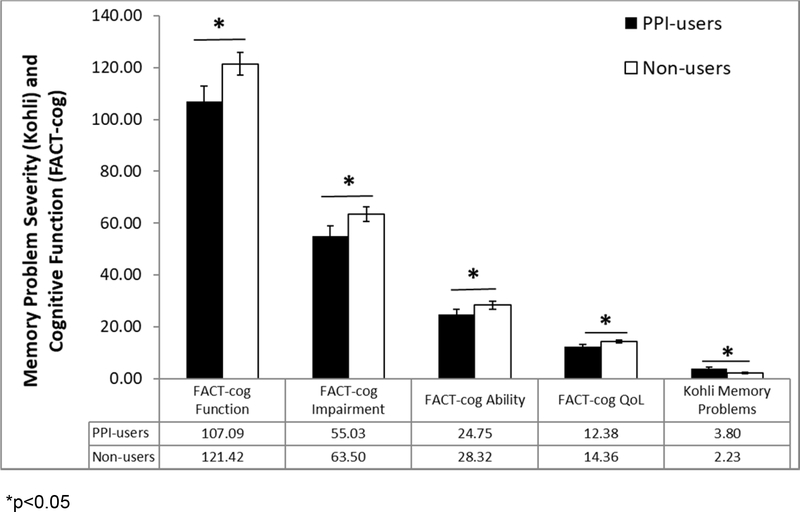
PPI-users (n=21) were not different from non-users (n=121) in terms of age, comorbidities, race, education, months since cancer treatment, and chemotherapy or hormonal treatment rates (ps>0.09). See Table 3 for participant characteristics. PPI-users reported more severe memory problems (EMM=3.800, SE=0.548) than non-users (EMM=2.233, SE=0.342) (F(1, 109) = 9.736, p=0.002).However, PPI-users did not report more severe concentration problems (p=0.16) or more symptoms on the BCPT (p=0.56) than non-users. At the screening visit, PPI-users had poorer total FACT-cog scores (EMM = 107.088, SE=5.923) than non-users (EMM = 121.423, SE=4.356), (F(1,141) = 7.838, p=0.006). PPI users’ lower total FACT-cog scores were driven by their reports of poorer quality of life (p=0.005), greater cognitive impairment (p=0.013), and poorer cognitive abilities (p=0.046), but not their reports of others’ comments about their cognitive function (p=0.34) (Figure 3).
Table 3.
Study 3 sample demographics at screen
| PPI Users (n=21) | PPI Non-users (n=121) | ||||
|---|---|---|---|---|---|
| Measure | N (%) | M (SD) | N (%) | M (SD) | |
| Age | 55.7(9.1) | 55.6(7.7) | |||
| Comorbidities | 0.3(0.6) | 0.2(0.5) | |||
| Months since cancer tx | 53.1(19.9) | 43.0(27.7) | |||
| Race (% White) | 20(95%) | 106(88%) | |||
| Education | High School | 6(29%) | 16(13%) | ||
| Some College | 2(10%) | 25(21%) | |||
| College Grad | 6(29%) | 37(31%) | |||
| Grad Training | 7(33%) | 43(34%) | |||
| Chemo tx (% Yes) | 16(80%) | 88(73%) | |||
| Hormone tx (% Yes) | 14(65%) | 95(79%) | |||
| Radiation tx (% Yes) | 9(43%) | 68(56%) | |||
| CES-D score | 9.1(8.0) | 7.1(7.3) | |||
| Cancer Stage | Stage I | 6(29%) | 50(41%) | ||
| Stage II | 11(52%) | 63(52%) | |||
| Stage III | 1(5%) | 8(7%) | |||
p<0.05
Figure 3.
Estimated marginal means (± SEs) of self-reported cognitive problems for PPI-users and non-users in Study 3. PPI-users reported more severe memory problems on the Kohli at the placebo visit, and poorer cognitive functioning on the FACT-cog at the screening visit, than non-users; *p<0.05.
Discussion
The present studies highlight potential cognitive problems associated with PPI usage in three samples of women at different points in breast cancer survivorship. These are the first studies to demonstrate clinically relevant PPI-associated cognitive outcomes in a population already at risk for cognitive decline. PPI-users in Study 3 reported more severe memory problems than non-users, while PPI-users in Studies 1 and 2 reported more severe concentration problems. This poorer cognitive functioning may impede quality of life, and indeed, PPI-users in Study 3 reported lower quality of life due to cognitive symptoms.
Across studies, PPI-users’ cognitive problem severity was comparable to that of cancer patients currently undergoing chemotherapy treatment, as reported in a seminal study of 595 cancer patients [11]. In that study, cancer patients’ mean memory and concentration problem ratings on the Kohli scale prior to chemotherapy were 1.48 and 1.44, respectively, compared to 3.59 and 3.91 during chemotherapy and 2.93 and 2.30 six months post-treatment, respectively. A question for further research is whether PPI use – particularly chronic use – before, during, or after cancer treatment impedes post-treatment cognitive recovery.
Our results are underscored by a randomized trial that revealed significant cognitive deterioration among healthy, young participants following just one week of exposure to PPIs [29] as well as two large-sample studies that have found higher rates of dementia among PPI-users [7,8]. Although recent findings challenge the notion that PPI use increases the risk of developing dementia [30–32], cancer survivors may be particularly vulnerable to the impact of PPIs. Even non-intestinal cancers, such as breast cancer, are linked to taxonomic shifts in the gut microbiota [33] and elevated intestinal permeability [34], which could alter gut-brain communication, thereby amplifying the cognitive impact of PPIs. This subtle but significant cognitive decline among breast cancer survivors may be wrongly attributed to normal aging or “chemobrain.”
The growing concerns about PPI use have led family physicians in Canada to publish new clinical guidelines for de-prescribing PPIs [35]. The U.S. Food and Drug Administration has approved PPIs for short-term use (<8 weeks) to treat common gastric acid conditions (e.g., erosive esophagitis, gastroesophageal reflux disease), and for longer-term use in exceptional cases (e.g., pathological hypersecretory conditions, gastric ulcers). In cancer patients, off-label maintenance or prophylactic PPI therapy may last months or years, despite unknown side effects. Although long-term PPI use is generally regarded as safe in the U.S., in a recent survey of 94 healthcare providers at an academic medical center, 63% of gastrointestinal (GI) specialists and 47% of non-GI specialists reported changing their practice of prescribing PPIs (e.g., lowering dosage, replacing with histamine 2 blockers) due to recent reports of adverse side effects [36]. When asked about specific PPI-related concerns, 33% and 21% of GI specialists cited patient concerns or their own concerns about dementia, respectively.
Discontinuing PPI use is difficult. In a double-blind, placebo-controlled trial in which 97 patients who had been on PPIs for an average of 4 years were randomized to either three weeks of tapered dosage or three weeks of constant dosage prior to stopping PPI usage, only 27% of participants were able to successfully discontinue treatment [37]. Surprisingly, tapering was ineffective, as rates of discontinuation were similarly low for both groups [37]. The current studies showed that long-term PPI use is common among breast cancer survivors. In Study 1, 66% of breast cancer survivors taking PPIs at baseline continued to report usage one and two years later, and in Study 2, 84% of those taking PPIs at baseline continued usage throughout the six-month study. This long-term usage may be problematic, especially among a population that is already at risk for cognitive decline. Development and implementation of methods to facilitate successful PPI discontinuation is much needed.
Biological mechanisms likely underlie the association between PPI use and cognitive function. By reducing the acidity of the stomach (increasing the pH), PPIs alter the digestive process as well as the gastrointestinal environment such that notable shifts in gut microbiota populations occur [38] – potentially impacting brain function [39]. In fact, PPIs can even compromise the gut barrier [40] and enter into circulation. Once in the bloodstream, PPIs can cross the blood-brain barrier [41], triggering the build-up of amyloid beta proteins – a biological signature of dementia [42]. They may also reduce the acidity of microglial lysosomes [43]. Microglial cells are the “janitorial,” phagocytic immune-like brain cells, and decreased lysosomal acidity diminishes their ability to clean up debris, allowing amyloid to accumulate. Additionally, PPIs interact with – and even bind to – tau [41]. Thus, there are multiple pathways by which PPIs can lead to cognitive decline.
The strengths of this study include three samples of breast cancer survivors at different points in survivorship as well as the stringent exclusionary criteria eliminating potential confounds that are known to impact cognitive function. The repeated measurement of cognitive symptoms in Studies 1 and 2 provide a more robust estimate of the effect of PPI use on cognition – an additional strength. However, PPI use was not manipulated, limiting causal interpretations of findings. PPI use prior to the study and continuity of use between visits were not measured and could not be reliably ascertained from medical records, as over-the-counter, unsupervised use is common. Additionally, unequal PPI group sizes reduce the reliability of the findings. Importantly, only self-reported cognitive outcomes were assessed in this study, and the question remains whether PPI use relates to objective measures of cognitive function. Another question for further investigation is whether the observed PPI-related cognitive differences are clinically significant. PPI-induced biological changes (i.e., microbiota changes) make causal pathways plausible, and signal the need for a prospective trial with breast cancer survivors measuring both self-reported and objective cognitive function, mindful that self-report could capture subtle decline that objective measures may miss [11,14].
Conclusions
The current studies provide initial evidence that PPI use may impair cognitive function – including both memory and concentration – in breast cancer survivors. Due to their cancer history, this population may be particularly susceptible to PPI-related cognitive problems. The severity of PPI-users’ memory and concentration problems were on par with those reported by patients currently undergoing chemotherapy in a large observational study, whereas PPI non-users’ reported severity aligned more closely with those of cancer survivors several months post-treatment. PPI-related cognitive symptoms may impair breast cancer survivors’ quality of life, and therefore deserve further investigation.
Supplementary Material
Table 1:
Study 1 sample demographics at baseline
| PPI Users (n = 36) | Non-users (n = 173) | ||||
|---|---|---|---|---|---|
| Measure | N (%) | M (SD) | N (%) | M (SD) | |
| Age | 59.6(12.8)* | 54.8(11.1)* | |||
| Comorbidities | 1.1(1.5) | 0.7(1.3) | |||
| Months before cancer tx | 0.5(0.5) | 0.7(0.6) | |||
| Race (% White) | 25(69%) | 138(80%) | |||
| Education | High School | 16(44%) | 44(26%) | ||
| Some College | 6(17%) | 37(22%) | |||
| College Grad | 6(17%) | 44(26%) | |||
| Grad Training | 8(22%) | 47(27%) | |||
| Chemo tx (% Yes) | 13(36%) | 77(45%) | |||
| Radiation tx (% Yes) | 20(56%) | 92(53%) | |||
| Hormone tx (% Yes) | 22(60%) | 122(71%) | |||
| CES-D score | 17.3(10.1) | 16.4(10.8) | |||
| Cancer Stage | Stage 0 | 5(14%) | 34(20%) | ||
| Stage I | 20(56%) | 72(42%) | |||
| Stage II | 9(25%) | 46(27%) | |||
| Stage III | 2(6%) | 19(11%) | |||
p<0.05
Acknowledgments
This study was supported in part by NIH grants: R01 CA186251, R01 CA126857, R01 CA131029, R01 CA186720, K05 CA172296, UL1RR025755, and T32 DE014320.
Glossary
- PPI
Proton Pump Inhibitor
- BCPT
Breast Cancer Prevention Trial
- FACT-cog
Functional Assessment of Cancer Therapy cognitive scale
Footnotes
Conflicts of Interest: The authors had no conflicts of interest.
References
- 1.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimer C, Søndergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137:80–7. [DOI] [PubMed] [Google Scholar]
- 3.Attwood S, Ell C, Galmiche J, Fiocca R, Hatlebakk J, Hasselgren B, et al. Long‐term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther. 2015;41:1162–74. [DOI] [PubMed] [Google Scholar]
- 4.Sartori S, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Misoprostol and omeprazole in the prevention of chemotherapy-induced acute gastroduodenal mucosal injury. Cancer. 1996;78:1447–82. [DOI] [PubMed] [Google Scholar]
- 5.Sartori S, Trevisani L, Nielsen I, Tassinari D, Panzini I, Abbasciano V. Randomized trial of omeprazole or ranitidine versus placebo in the prevention of chemotherapy-induced gastroduodenal injury. J Clin Oncol. 2000;18:463–463. [DOI] [PubMed] [Google Scholar]
- 6.Wang B-Y, Zhang J, Wang J-L, Sun S, Wang Z-H, Wang L-P, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res. 2015;34:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–6. [DOI] [PubMed] [Google Scholar]
- 8.Haenisch B, von Holt K, Wiese B, Prokein J, Lange C, Ernst A, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci. 2015;265:419–28. [DOI] [PubMed] [Google Scholar]
- 9.Kuller LH. Do proton pump inhibitors increase the risk of dementia? JAMA Neurol. 2016;73:379–81. [DOI] [PubMed] [Google Scholar]
- 10.Batchelor R, Gilmartin JF, Kemp W, Hopper I, Liew D. Dementia, cognitive impairment and proton pump inhibitor therapy: a systematic review. J Gastroenterol Hepatol. 2017;32:1426–35. [DOI] [PubMed] [Google Scholar]
- 11.Kohli S, Griggs JJ, Roscoe JA, Jean-Pierre P, Bole C, Mustian KM, et al. Self-Reported Cognitive Impairment in Patients With Cancer. J Oncol Pract. 2007;3:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savard J, Ganz PA. Subjective or Objective Measures of Cognitive Functioning—What’s More Important? JAMA Oncol. 2016;2:1263–4. [DOI] [PubMed] [Google Scholar]
- 13.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–69. [DOI] [PubMed] [Google Scholar]
- 14.Shilling V, Jenkins V. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs. 2007;11:6–15. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–56. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Mizyed I, Fass S, Fass R. gastro‐oesophageal reflux disease and psychological comorbidity. Aliment Pharmacol Ther. 2009;29:351–8. [DOI] [PubMed] [Google Scholar]
- 20.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho‐Oncology. 2004;13:61–6. [DOI] [PubMed] [Google Scholar]
- 22.Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment-from bench to bedside. Neuro-Oncol. 2012;14:iv37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. [DOI] [PubMed] [Google Scholar]
- 24.Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br Med Bull. 2009;92:135–52. [DOI] [PubMed] [Google Scholar]
- 25.Evans DA, Beckett LA, Albert MS, Hebert LE, Scherr PA, Funkenstein HH, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol. 1993;3:71–7. [DOI] [PubMed] [Google Scholar]
- 26.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32:1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. [DOI] [PubMed] [Google Scholar]
- 28.Von Ah D, Tallman EF. Perceived Cognitive Function in Breast Cancer Survivors: Evaluating Relationships With Objective Cognitive Performance and Other Symptoms Using the Functional Assessment of Cancer Therapy—Cognitive Function Instrument. J Pain Symptom Manage. 2015;49:697–706. [DOI] [PubMed] [Google Scholar]
- 29.Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short-term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimers Res Ther. 2015;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, Nam JH, Lee H, Chung H, Lee E, Shin J. Beyond uncertainty: negative findings for the association between the use of proton pump inhibitors and risk of dementia. J Gastroenterol Hepatol. 2019; [DOI] [PubMed] [Google Scholar]
- 31.Hussain S, Singh A, Zameer S, Jamali MC, Baxi H, Rahman SO, et al. No association between proton pump inhibitors use and risk of dementia: Evidence from a meta‐analysis. J Gastroenterol Hepatol. 2019; [DOI] [PubMed] [Google Scholar]
- 32.Gray SL, Walker RL, Dublin S, Yu O, Aiello Bowles EJ, Anderson ML, et al. Proton pump inhibitor use and dementia risk: Prospective population‐based study. J Am Geriatr Soc. 2018;66:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor–positive female breast cancer. JNCI J Natl Cancer Inst. 2016;108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parrilli G, Iaffaioli RV, Martorano M, Cuomo R, Tafuto S, Zampino MG, et al. Effects of anthracycline therapy on intestinal absorption in patients with advanced breast cancer. Cancer Res. 1989;49:3689–91. [PubMed] [Google Scholar]
- 35.Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors. Can Fam Physician. 2017;63:354–64. [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Qaisi M, Kahn A, Crowell M, Burdick G, Vela M, Ramirez F. Do recent reports about the adverse effects of proton pump inhibitors change providers’ prescription practice? Dis Esophagus. 2018; [DOI] [PubMed] [Google Scholar]
- 37.Björnsson E, Abrahamsson H, Simren M, Mattsson N, Jensen C, Agerforz P, et al. Discontinuation of proton pump inhibitors in patients on long‐term therapy: a double‐blind, placebo‐controlled trial. Aliment Pharmacol Ther. 2006;24:945–54. [DOI] [PubMed] [Google Scholar]
- 38.Jackson MA, Goodrich JK, Maxan M-E, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701. [DOI] [PubMed] [Google Scholar]
- 40.Marlicz W, Koulaouzidis A, Loniewski I, Koulaouzidis G. Letter by Marlicz et al Regarding Article,“Proton Pump Inhibitors Accelerate Endothelial Senescence.” Circ Res. 2016;119:e31–2. [DOI] [PubMed] [Google Scholar]
- 41.Rojo LE, Alzate-Morales J, Saavedra IN, Davies P, Maccioni RB. Selective interaction of lansoprazole and astemizole with tau polymers: potential new clinical use in diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2010;19:573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badiola N, Alcalde V, Pujol A, Münter L-M, Multhaup G, Lleó A, et al. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PloS One. 2013;8:e58837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fallahzadeh M, Borhani Haghighi A, Namazi M. Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther. 2010;35:125–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.