Abstract
Background
Cerebrospinal fluid tau and neurofilament light (NfL) are two biomarkers of neurodegeneration in Alzheimer disease. Previous reports have shown that the influence of tau on cognitive decline depends on levels of amyloid burden whereas NfL predicts decline independently of amyloid. Most studies use a global cognitive composite as the primary outcome, and it is unknown if critical cognitive domain scores are similarly sensitive to rates of decline due to neurodegeneration.
Objective
To examine the unique contribution of amyloid, tau and NfL to rates of cognitive decline in multiple cognitive composites in a cognitively healthy, middle-aged to older adult cohort.
Methods
A total of 255 participants (55% female; mean age = 66.2 years, range = 42.5 – 86.7 years) completed CSF studies and serial cognitive assessments to measure global cognition, episodic memory and attentional control. Linear mixed effects models were used to examine rates of change on each composite score as a function of baseline biomarker levels.
Results
Total tau predicted decline in attention regardless of amyloid status, but the relationship to global cognition and episodic memory was dependent on amyloid, replicating prior literature. NfL predicted decline in attention and global cognition, but not memory, and this effect was independent of amyloid status.
Conclusions
These findings suggest that NfL can be used to monitor cognitive decline in aging and Alzheimer disease and that an attentional control composite may be a better outcome for tracking general neurodegenerative effects on cognition.
Keywords: Neurofilament light, attention, memory, CSF, Alzheimer’s disease
The amyloid plaques and neurofibrillary tangles characteristic of Alzheimer disease (AD) accumulate for decades before clinical symptoms become apparent [1,2]. This pathology results in neuronal injury and neurodegeneration and ultimately culminates in substantial cognitive decline and eventual loss of function. The ability to measure biomarkers of the AD pathological cascade in vivo has allowed for assessment of risk of future cognitive decline even among individuals who are currently cognitively healthy [3,4]. Individuals at highest risk of progression can then be targeted for recruitment into clinical trials or selected for treatment once a drug or other intervention becomes available. Thus, it is of critical importance to identify the biomarkers that are most predictive of longitudinal cognitive change.
Current theories place individuals along a disease continuum based on the number of abnormal biomarkers (amyloid, tau and neurodegeneration) they exhibit [5]. The relationship between amyloid (measured via the CSF or with PET imaging) and tau (CSF phosphorylated tau or tau PET) with cognition has been thoroughly examined [6–10]. However, while markers of amyloid and tau are well-established, there is substantial variation in how neurodegeneration is defined and measured, with a number of markers available both via neuroimaging [11] and biofluid measurements [12]. Although neurodegenerative markers are not specific to AD, their inclusion in predictive models tends to improve the diagnostic separation of AD from healthy controls. The most commonly studied biofluid neurodegenerative marker is CSF levels of total tau, which has been shown to relate to cognitive performance in a number of studies [13–16]. Although total tau levels are considered a neurodegenerative biomarker [5], in AD cohorts levels of phosphorylated and total tau are highly correlated [17,18] which limits the ability to use these biomarkers to truly separate neurodegenerative and tau pathologies.
Neurofilament light chain (NfL) is another protein that can measure neurodegeneration. Neurofilaments are structural proteins that support axonal integrity, and when axons are damaged, NfL is released. NfL has been shown to be elevated across a number of neurodegenerative disorders including amyotrophic lateral sclerosis, fronto-temporal dementia, Huntington’s disease and AD [19]. NfL has been shown to increase in AD and correlate with cognitive decline, at least in mixed samples of healthy and mildly impaired participants [14,20–22]. Interestingly, although both tau and NfL are markers of neurodegeneration, they appear to capture different aspects of AD pathological progression. This is evidenced by the fact that the correlation between total tau and cognition depends critically on levels of amyloid burden in populations at risk for AD [13,16,23], whereas the association of NfL with cognition [14] appears to be amyloid independent. These findings suggest that NfL-related cognitive decline is not uniquely tied to AD processes per se and that NfL-related decline may be detected earlier compared to total-tau as NfL is sensitive to cognitive changes even in the absence of increased amyloid accumulation.
The majority of studies of NfL have focused on older adult cohorts with mixed levels of cognitive impairment and it is currently unknown whether NfL has an influence on cognition earlier in the lifespan of individuals who are free from dementia symptoms. Furthermore, previous studies [14,20–22] have used a global measure of cognition as the primary outcome and whether the rate of decline due to NfL varies by domain is unknown. A notable exception from Mielke et al. showed that baseline levels of plasma NfL correlated with change on a global composite score but not on individual sub-domains including memory, executive function and language [20]. Therefore, the goals of this study were two-fold: First, we aimed to evaluate the influence of NfL on baseline cognitive performance and longitudinal change in a global composite score and determine whether the influence of NfL is functionally independent of amyloid burden. We extend prior findings to a middle-aged and older adult cohort that is entirely cognitively healthy at the initial assessment and directly compare effects across CSF tau and NfL. Our second goal was to examine whether the relationship of NfL to cognition varied by domain by analyzing two cognitive composite sub-scores, episodic memory and attentional control.
Episodic memory loss is the hallmark of AD. Assessments of memory feature heavily in the primary cognitive endpoints of ongoing clinical trials [24,25]. Memory is relatively consistently correlated with amyloid burden in otherwise healthy individuals [7,26], and is often targeted as an outcome measure due to findings that brain regions related to memory are particularly vulnerable to pathology [27]. Attentional control, the ability to direct attention towards relevant information and ignore distracting information, also evinces correlations with AD biomarkers [13,28–30], and is subserved primarily by prefrontal and parietal regions [31,32], which tend to preferentially atrophy during normal aging [33]. There is evidence to suggest that measures of attentional control may be particularly sensitive to AD processes, and in some studies, more so than memory [34,35]. Given that there is a well-established association between memory and attention processes [36], our second goal of this project was to directly compare rates of decline in these two cognitive domains.
Materials and Methods
A total of 257 participants were recruited from two ongoing, longitudinal studies on aging and AD, the Healthy Aging and Senile Dementia (HASD) study and the Adult Children Study (ACS), conducted at the Knight Alzheimer Disease Research Center at Washington University in St. Louis. Individuals who are less than 65 years of age are given clinical and cognitive assessments every 3 years and all other participants are assessed annually. If an individual reaches their 65th birthday during the course of the study, they are assessed annually thereafter. Participants in the present analyses also underwent at least one CSF collection with measurement of NfL and were required to have had a clinical / cognitive evaluation within 3 years of the first CSF collection1. Any individual who did not complete a cognitive assessment within the 3-year window was not considered further. As we are interested in rates of cognitive decline, we measure “time” as the number of years since the first CSF measurement up until the most recent cognitive evaluation. We removed one individual who had abnormally high values of CSF tau (> 4 standard deviations from the group mean) and one individual who had abnormally high values of NfL (> 4 standard deviations from the group mean). Although we included a wide window between CSF and the psychometric evaluation, it is important to note that the average interval was approximately 3 months2. Full demographic information on the final cohort is provided in Table 1. All participants provided consent to participate according to the Declaration of Helsinki and all study procedures were approved by the Human Research Protection Office at Washington University in St. Louis.
Table 1:
Baseline characteristics of the sample
| Amyloid Negative | Amyloid Positive | |||
|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD |
| Total N | 161 | NA | 94 | NA |
| Age (yrs) | 65.60 | 8.47 | 67.31 | 8.99 |
| % Female | 62% | NA | 43% | NA |
| % APOE ε4 positive | 24% | NA | 49% | NA |
| Education (yrs) | 16.05 | 2.42 | 16.27 | 2.53 |
| Clinical Dementia Rating | 0 | 0 | 0 | 0 |
| MMSE | 29.25 | 1.00 | 29.03 | 1.38 |
| NfL | 1356.29 | 574.42 | 1505.72 | 703.91 |
| Aβ42 (pg/ml) | 1780.47 | 561.21 | 773.98 | 204.09 |
| Total Tau (pg/ml) | 218.59 | 73.16 | 219.46 | 110.19 |
| P-tau (pg/ml) | 19.22 | 6.94 | 20.80 | 11.87 |
| CSF-Cognition Interval (Days) | 87.71 | 142.59 | 83.11 | 110.39 |
| Attention-Cognition Interval (Days) | 36.9 | 49.0 | 23.9 | 34.8 |
| Global Cognition | 0.10 | 0.53 | −0.14 | 0.58 |
| Attentional Control | 0.07 | 0.62 | −0.11 | 0.74 |
| Episodic Memory | 0.14 | 0.74 | −0.14 | 0.78 |
| Time in Study (yrs) | 6.13 | 3.05 | 5.31 | 2.68 |
| Number of Assessments | 5.46 | 3.00 | 5.02 | 2.67 |
| Number of Attention Assessments | 3.26 | 1.62 | 3.12 | 1.45 |
Note: MMSE = Mini Mental State Examination; CSF-Cognition interval refers to the number of days between the baseline CSF assessment and the baseline cognitive assessment; Attention-Cognition interval refers to the number of days between the baseline cognitive battery and the baseline attention battery
Clinical and Cognitive Evaluation
All participants were rated for the presence and severity of dementia using the Clinical Dementia Rating (CDR) Scale [37]. A CDR score of 0 indicates absence of dementia and ratings of 0.5, 1, 2 or 3 indicate very mild, mild, moderate and severe dementia, respectively. All participants in the current study were rated as CDR 0 at the time of their CSF collection. Each participant at the Knight ADRC completes a comprehensive cognitive test battery, however, the specific test versions used in each cohort varies slightly. Tests given in common across the batteries include the Free and Cued Selective Reminding Test [38], Trail Making Part A and B [39], Category Fluency for Animals [40], the Stroop color naming task [41,42], Simon task [43,44] consonant-vowel / odd-even task switching [45]. Participants in the HASD cohort also receive the WMS-R Logical Memory Story A Immediate and Delayed Recall and associate learning test [46] whereas the ACS cohort completes the WMS-III Logical Memory Stories A and B, Immediate and Delayed Recall as well as the Verbal Pairs Associates task [47].
Cognitive Composite Scores
Each test was z-scored to the mean and standard deviation of the entire cohort. The global cognitive composite was formed as a z-score of all the available measures that were given in common across the batteries. We then formed two cognitive subscores: The attentional control composite was formed from the accuracy score from the more difficult condition of each task (e.g., incongruent items in Stroop and Simon or switch trials in task switching) which were averaged together [28]. The episodic memory score consisted of the free recall score from the Free and Cued Selective Reminding test, Logical Memory Delayed Recall and the associate learning tests. Both of these composites were selected due to their demonstrated sensitivity to CSF markers in our prior studies [28].
CSF Collection
Cerebrospinal fluid (20 – 30 mL) was collected following an overnight fast. Aβ42, total tau and ptau181 were measured with Elecsys immunoassays (Roche Diagnostics, Basel, Switzerland) on an automated platform using two lots of assays for each protein. This assay has demonstrated excellent lot-to-lot comparability [57]. NfL was measured using a commercial enzyme-linked immunosorbent assay (ELISA) from Uman Diagnostics (Umeå, Sweden). In order to facilitate comparisons to other cohorts that may use different markers of amyloid pathology (e.g., PiB), we classified participants as amyloid negative (>= 1,098 pg/ ml) or positive (< 1,098 pg/ml) using published Aβ42 cut-offs [48]. Only the CSF measurements from the first CSF collection were used in the present analyses.
Statistical Analysis
Our modeling proceeded in several steps. First, we predicted cross-sectional performance and longitudinal decline in the global composite score from a linear mixed effects model with full information maximum likelihood using the lme4 package in R [49]. Each model included the following terms: a main effect of baseline age, neurodegeneration biomarker (tau or NfL, in separate models), years since the first NfL measurement (hereafter referred to as “time”) and baseline amyloid status (coded as −0.5, + 0.5 contrasts, hence lower order interactions and main effects are averaged across amyloid groups), as well as all the two and three-way interactions among the latter three terms. All variables were standardized prior to analysis and we included random effects of participant and time in all models. Furthermore, we provide an approximation of Bayes factors [50] for the three-way interaction (biomarker by amyloid by time) to quantify the degree of evidence for or against this interaction. Bayes factors can be interpreted using the following guidelines [51]: 1–3.2 = no evidence, 3.2 – 10 = substantial evidence, 10 – 100 = strong evidence and > 100 = decisive evidence.
Finally, to determine if total tau and NfL capture different aspects of AD progression, we compared a series of models using Akaike’s Information Criteria (AIC) in our sample of amyloid positive participants. Specifically, we compared models that included a term for NfL by time only, tau by time only and a model that included both terms. All models controlled for baseline age. We report the AIC for each model (smaller is better) and the probability that the lowest AIC model is the best approximating model (i.e., the AIC weight) as a measure of effect size. Our second set of analyses repeated the above models but separately for the attentional control and episodic memory composites. As the focus was on NfL and tau mediated decline, we only discuss model parameters relating to these terms in the text; however, full model output is provided in Tables 2 (for NfL models) and 3 (for Tau models).
Table 2:
Beta weights (Standard Errors) of the NfL models.
| Global | Attention | Episodic Memory | |
|---|---|---|---|
| Age | −0.217*** (0.040) | −0.145*** (0.044) | −0.016 (0.057) |
| NfL | −0.049 (0.043) | −0.056 (0.051) | −0.143** (0.063) |
| Time | −0.025*** (0.006) | −0.013 (0.009) | 0.005 (0.008) |
| Amyloid Status | −0.194*** (0.066) | −0.137 (0.079) | −0.240** (0.097) |
| NfL*Time | −0.019*** (0.006) | −0.036*** (0.009) | −0.009 (0.008) |
| NfL*Amyloid Status | 0.031 (0.072) | −0.043 (0.086) | 0.080 (0.106) |
| Amyloid Status*Time | −0.011 (0.011) | −0.004 (0.017) | −0.033** (0.015) |
| NfL*Amyloid*Time | 0.017 (0.012) | 0.016 (0.019) | 0.013 (0.016) |
| Constant | 0.006 (0.033) | −0.016 (0.040) | 0.050 (0.049) |
| Observations | 1,352 | 818 | 1,352 |
| Log Likelihood | −639.514 | −774.778 | −1,019.821 |
| Akaike Inf. Crit. | 1305.028 | 1575.555 | 2065.642 |
| Bayesian Inf. Crit. | 1372.750 | 1636.745 | 2133.363 |
Note:
p<0.05
p<0.01
Table 3:
Beta weights (Standard Errors) of the Tau models.
| Global | Attention | Episodic Memory | |
|---|---|---|---|
| Age | −0.227*** (0.035) | −0.156*** (0.038) | −0.080 (0.051) |
| Tau | −0.061 (0.036) | −0.090** (0.042) | −0.035 (0.053) |
| Time | −0.023*** (0.005) | −0.012 (0.009) | 0.007 (0.007) |
| Amyloid Status | −0.201*** (0.066) | −0.144 (0.077) | −0.258*** (0.098) |
| Tau*Time | −0.014** (0.006) | −0.022** (0.009) | −0.013 (0.008) |
| Tau*Amyloid Status | 0.002 (0.068) | −0.186** (0.080) | −0.023 (0.101) |
| Amyloid Status*Time | −0.015 (0.011) | −0.018 (0.017) | −0.033** (0.014) |
| Tau*Amyloid*Time | −0.037*** (0.011) | −0.015 (0.019) | −0.034** (0.015) |
| Constant | 0.006 (0.033) | −0.021 (0.039) | 0.053 (0.049) |
| Observations | 1,352 | 818 | 1,352 |
| Log Likelihood | −636.222 | −772.349 | −1019.494 |
| Akaike Inf. Crit. | 1298.444 | 1570.697 | 2064.988 |
| Bayesian Inf. Crit. | 1366.165 | 1631.886 | 2132.709 |
Note:
p<0.05
p<0.01
Data availability statement
Data used in this project are available by submitting a formal data request to the Knight Alzheimer Disease Research Center Administrative Core. Instructions and polices can be found at https://knightadrc.wustl.edu/Research/ResourceRequest.htm
Results
Participant Characteristics
All participants were cognitively healthy (CDR 0) at entry into the study. A total of 161 (62% female) participants were determined to be amyloid negative based on CSF and 94 (43% female) were amyloid positive. The groups did not differ in terms of age, years of education or MMSE scores (see Table 1).
Global Composite
NfL was not related to cognition at baseline, however, amyloid status was (β =−0.19, SE = 0.07, p-value = 0.004), indicating that only amyloid predicted cognition at baseline. NfL significantly predicted decline over time (β = −0.02, SE = 0.01, p-value = 0.002), but amyloid status did not, although the effect was in the expected direction. The three-way interaction among NfL, amyloid and time was not significant with strong evidence for the non-significant interaction (β =0.02, SE = 0.012, p-value = 0.17, Bayes factor = 14.38).
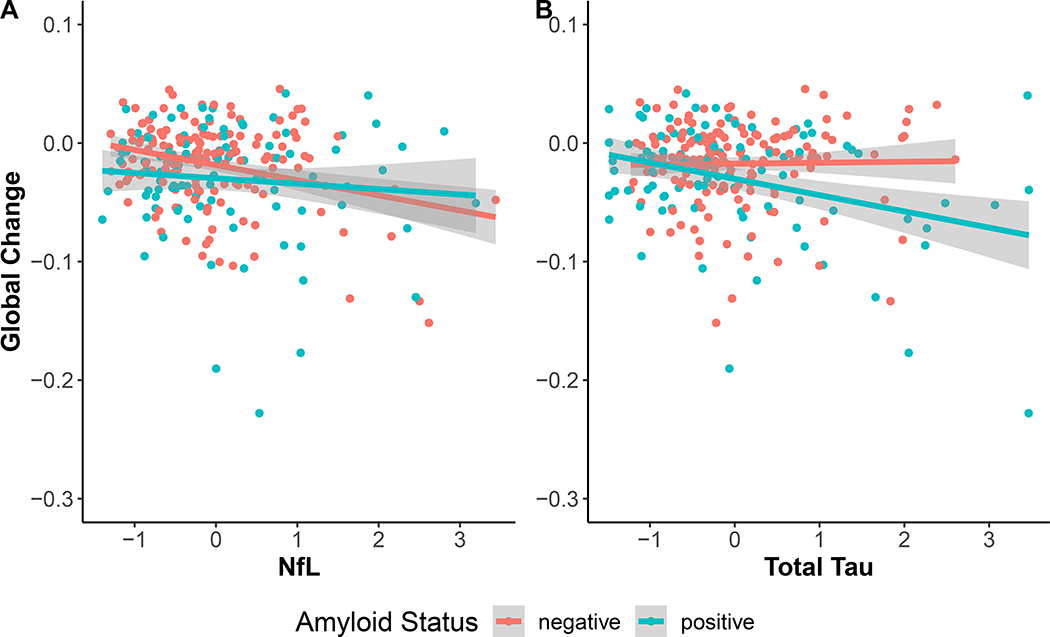
Turning now to the influence of total tau, tau was not related to the global composite at baseline whereas amyloid status was (β =−0.20, SE = 0.07, p-value = 0.003). Furthermore, there was a three-way interaction among tau, amyloid and time (β =−0.04, SE = 0.01, p-value = 0.001, Bayes factor = 4.68 in support of the interaction). This interaction indicated that tau predicted global cognitive decline when amyloid was positive (β =−0.032, SE = 0.008, p-value < 0.001) but not when amyloid was negative (β =0.005, SE = 0.008, p-value = 0.57), replicating the interactive effects of tau and amyloid that have been observed in the literature. This relationship is illustrated in Figure 1.
Figure 1:
Influence of NfL (Panel A) and total tau (Panel B) on rates of change in global cognition, split by amyloid status defined using CSF cutoffs (N amyloid negative = 161, amyloid positive = 94). NfL was significantly related to decline regardless of amyloid status. Tau correlated with decline only in amyloid positive participants.
Given that both tau and NfL predicted decline in the global composite score, it is of interest to know whether both markers equally and independently contribute to decline in preclinical AD (i.e., amyloid positive participants) or whether one marker outperforms the other. We compared models that included either NfL or tau as predictors of global decline against a model that included both. Results show that a tau only model was clearly preferred over the NfL model (AICs: NfL = 498.93, Tau = 488.16, Both = 491.70) with the tau model AIC weight estimated at 85%. This result indicates that total tau is clearly a better predictor of global decline than NfL in preclinical AD.
Attentional Control Composite
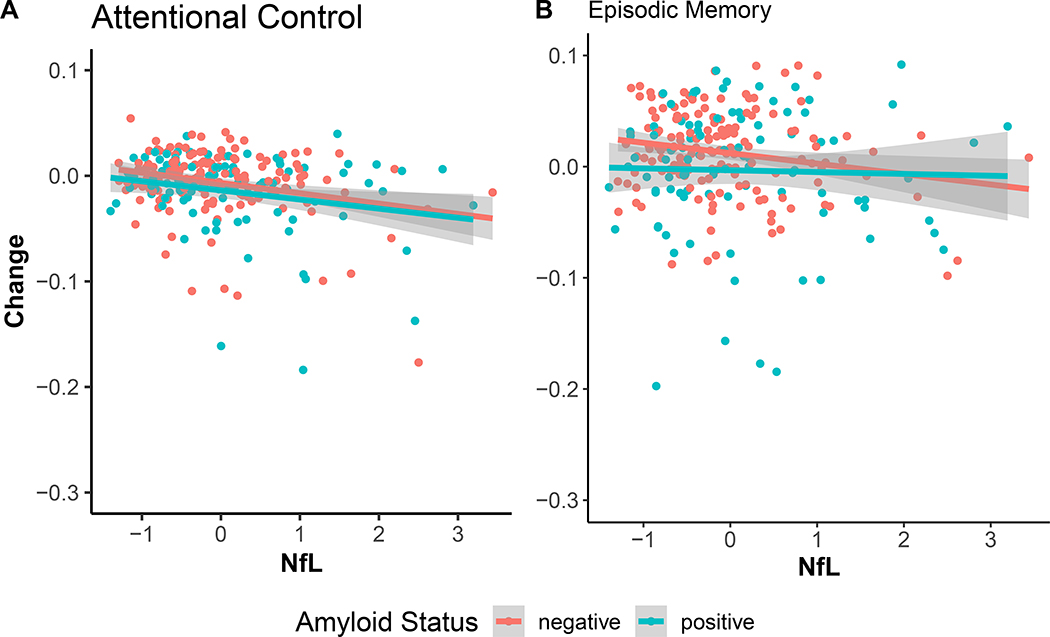
Neither NfL nor amyloid status were correlated with attention at baseline. Only NfL (β =−0.036, SE = 0.01, p-value < 0.001) predicted change over time, amyloid status did not. Once again, the three-way interaction among NfL, amyloid and time was not significant (p-value = 0.41, Bayes factor = 20.23 in support of the null, see Figure 2).
Figure 2:
Influence of NfL on annualized change in attentional control (panel A) and episodic memory (panel B), split by amyloid status. NfL was associated with decline in attentional control only.
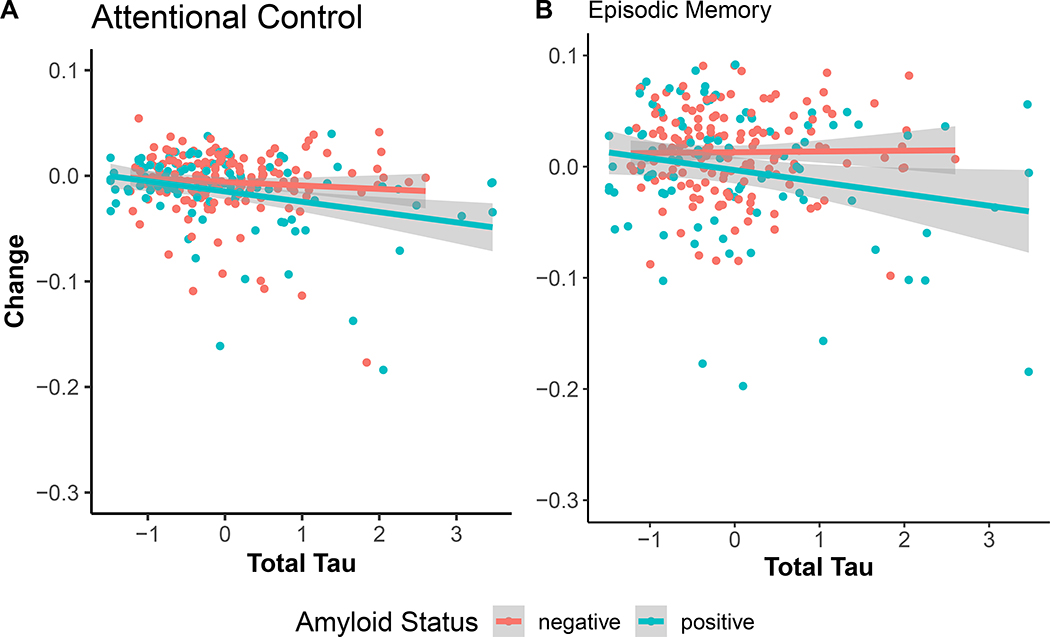
Using tau as the predicter revealed that tau was related to attention at baseline (β =−0.089, SE = 0.04, p-value = 0.03), however, amyloid status was not. Tau significantly predicted decline in attention over time (β =−0.02, SE = 0.01, p-value = 0.02) whereas amyloid status did not. This pattern indicates that increasing levels of tau accelerate attentional control decline and once this variance is accounted for, amyloid does not contribute to further declines in attention. The three-way interaction among tau, amyloid and time was not significant (β =−0.01, SE = 0.019, p-value = 0.45, Bayes factor = 21.33 in support of the null, see Figure 3).
Figure 3:
Influence of total tau on annualized change in attentional control (Panel A) and episodic memory (Panel B). Tau was associated with decline in attentional control regardless of amyloid status and was only associated with memory in amyloid-positive participants.
In the subset of amyloid positive participants, model comparisons clearly indicated that a tau only model outperformed models that included NfL (AICs: NfL = 568.10, Tau = 558.11, Both = 561.74). The estimated AIC weight for the tau only model was 86%.
Episodic Memory Composite
Both NfL (β =−0.14, SE = 0.06, p-value = 0.02) and amyloid (β =−0.24, SE = 0.10, p-value = 0.01) were correlated with memory performance at baseline. NfL did not predict change over time in memory whereas amyloid status did (β =−0.03, SE = 0.015, p-value = 0.03). The three-way interaction was not significant (β =0.01, SE = 0.016, p-value = 0.43, Bayes factor = 27.01 ). These results are shown in Figure 2.
In contrast, total tau was not related to memory at baseline, but amyloid status was (β =−0.26, SE = 0.10, p-value < 0.001). Once again, the three-way interaction among amyloid, tau and time was significant (β =−0.03, SE = 0.02, p-value = 0.02). However the Bayes factor in favor of this interaction was relatively uninformative (Bayes factor = 0.35). Nevertheless, follow-up tests indicate that tau accelerates memory decline in amyloid positive participants (β =−0.03, SE = 0.01, p-value < 0.005), but not in amyloid negative participants (β =0.004, SE = 0.01, p-value = 0.69), as expected (Figure 3).
As with the other composites, model comparisons indicated that a tau only model outperformed models that included NfL in amyloid positive individuals (AICs: NfL = 754.82, Tau = 749.88, Both = 751.99). The estimated AIC weight for the tau only model was 70%.
Discussion
The overarching goal of this project was to systematically evaluate the contribution of NfL and total tau to cognitive performance in a global cognitive composite as well as in two important cognitive domains, episodic memory and attentional control. The major findings of the study can be summarized as follows. First, in a global cognitive composite, both NfL and total tau were related to decline. However, NfL-related decline did not depend on levels of amyloid burden, whereas the influence of tau was only apparent when amyloid was elevated. Second, in an entirely preclinical (amyloid positive) cohort, tau was a better predictor of cognitive change than NfL. Third, NfL predicted decline in attentional control scores but did not predict decline in episodic memory. Finally, CSF tau predicted decline in attention that was not related to amyloid burden, however, tau was only related to memory decline in the presence of amyloid. We discuss the implications of each of these results in turn.
It has been repeatedly shown that individuals with multiple abnormal biomarkers have the greatest risk of progressing to full dementia. Including an indicator of subtle cognitive change also increases predictive power of disease progression [4]. It is important to find the “best” biomarkers that correlate most strongly with baseline performance and also best predict future risk. It has already been established that inclusion of both NfL and tau biomarkers substantially increases diagnostic separation of clinical groups relative to either marker alone [14]. In the current study, we have extended this finding to show that NfL can predict cognition even among cognitively normal participants. The majority of studies to date have relied on a global cognitive composite as the outcome. Specifically, Mielke et al. (2019) directly showed that plasma NfL at baseline is related to a global score only, and not to individual domain scores [20]. It is unclear whether the sensitivity of global scores are due to the increased reliability of combining multiple cognitive tests or whether NfL produces subtle declines in multiple domains that only become fully apparent when the domains are combined into a single score. Interestingly, in our study, the predictive power of NfL on longitudinal cognitive change depended somewhat on the domain of cognition that was evaluated.
NfL did not predict baseline global performance (although the effect was clearly in the predicted direction), whereas amyloid status did. In contrast, only NfL predicted decline in global cognition whereas amyloid status did not. This pattern is consistent with the proposed temporal order of biomarker abnormalities (i.e., amyloid accumulates first followed by downstream effects on tau and neurodegeneration), and accords with previous findings that tau and neurodegeneration markers correlate more strongly with cognitive decline than amyloid [28,52,53]. The present study showed similar results using CSF tau as the neurodegeneration biomarker, however, the relationship with cognition was dependent on amyloid status. The finding that NfL does not interact with amyloid in our statistical models may indicate that NfL is capturing both AD-related pathology as well as unrelated neurodegeneration. Such a pattern possibly reflects a general consequence of aging as NfL tends to correlate with advancing age [19,54]. It is important to account for these extraneous neurodegenerative processes when building a model of cognitive function in AD.
Nevertheless, in direct comparisons using information criteria in a sub-sample of amyloid positive individuals, there was clear evidence for a model that included tau to outperform a model that included NfL in explaining decline in both a global composite. Therefore, if the interest is on tracking disease progression via cognitive decline in preclinical (i.e., amyloid positive) AD, total tau may be a better marker to target. Of course, NfL may still be a useful neurodegenerative marker in the context of preclinical AD for a number of reasons. First, CSF assays are cheaper to conduct compared to PET or MRI and do not expose participants to radiation. The fact that NfL can also be measured in plasma makes this marker even less invasive than a measurement via CSF. Furthermore, CSF and serum measures of NfL are tightly correlated [55]. Second, NfL should not be engaged by anti-tau therapies and thus can still be used as a marker of neurodegeneration in clinical trials that target tau.
The second primary goal of this study was to examine whether specific cognitive domains would be more or less sensitive to neurodegenerative processes than a global composite. To this end, we evaluated the relationship between both neurodegeneration markers and two well-established cognitive domain scores, attentional control and memory. Our results show that both tau and NfL were related to decline in attentional control. Interestingly, the magnitude of NfL-related decline in attentional control was almost double the magnitude of tau-related decline. In contrast, NfL did not predict change in episodic memory, and tau was only predictive among individuals positive for amyloid. Given that response time variability on attentional control tasks correlates with white matter integrity [56], it is possible that white matter connections are more important for attentional control than for memory. Thus, if the clinical goal is to track general neurodegenerative changes in cognition, an attention composite may be a better cognitive target than episodic memory. However, an episodic memory composite may be more specific to AD specific neurodegeneration (i.e., only among individuals who are positive for amyloid). Clearly, a study that compares the diagnostic performance of each composite directly is warranted.
Although there are many strengths of this study including a large, well-characterized cohort with comprehensive biomarker and longitudinal cognitive evaluations, there are some limitations that are worth noting. First, we only examined CSF markers in the current analysis, and it is not clear how the results may change when neuroimaging markers of either amyloid (via PET) or neurodegeneration (e.g., global atrophy) are considered. Furthermore, we modeled rates of decline using a linear function. It is possible that decline in different composites have a differential trajectory based on differing underlying pathologies. Future studies with longer duration of follow-up are needed to test this hypothesis. In addition, the attention measures were administered in a separate cognitive battery from the other tests and thus fewer observations are available for this composite. Finally, as with many cohort studies of AD, our participants may not be fully representative of the general population due to important differences in race or education, among others.
Conclusions
In summary, our results indicate that both NfL and total tau from the CSF, markers of neurodegeneration, exhibit a relationship to cognitive decline in a lifespan sample of clinically normal adults. The contribution of NfL was independent of amyloid load, whereas the influence of tau was synergistic with amyloid. Finally, NfL-related changes were more apparent in attentional control than episodic memory. Given that NfL and tau may reflect different processes that are more or less apparent in specific participants (i.e., amyloid positive vs. not), these results suggest that both NfL and total tau should be used to track decline in preclinical AD. Furthermore, an attentional control composite may be a better cognitive outcome for tracking general neurodegeneration (i.e., not dependent on amyloid) that is reflected by NfL, which ultimately may contribute to decline in memory performance.
Acknowledgements
We would like to express our gratitude to the research volunteers who participated in the studies from which these data were obtained and their supportive families. We thank the Clinical, Biomarker and Imaging Cores at the Knight Alzheimer Disease Research Center for sample and data collection. We acknowledge Ms. Rachel Henson for performing the assays for CSF NfL. We acknowledge Drs. Carlos Cruchaga, Tammie L.S. Benzinger, and Laura Piccio for providing partial funding of the NfL assays.
Funding
This study was supported by National Institute on Aging grants R03AG050921 and K23AG053426 (SE Schindler), RF1AG058501 (C Cruchaga and L Piccio), R01AG054567 (TLS Benzinger and Y Wang), the Foundation for Barnes-Jewish Hospital (JC Morris), P50AG005681 (JC Morris), P01AG003991 (JC Morris), and P01AG026276 (JC Morris).
Competing Interests
Drs. Aschenbrenner, Gordon, Schindler, Balota and Hassenstab report no competing interests. Dr. Fagan is supported by NIH grants including P01AG026276, P01AG03991 and P50AG0568 and has received past research funding from Biogen, Roche Diagnostics and Fujirebio. She is on the scientific advisory boards of AbbVie, Genentech and Roche Diagnostics and consults for Araclon/Grifols, Biogen and Diadem SLR. There are no conflicts. Dr. Morris is funded by NIH grants P50AG005681, P01AG003991, P01AG026276 and UF1AG032438. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
Footnotes
123 of the participants in the current sample were included in an earlier analysis of CSF markers and changes in attentional control [28].
Furthermore, we analyzed our data with only a 1-year interval (N = 246) to ensure the wide interval was not biasing our results. In both cases, the results were qualitatively identical to what is reported. We retain the 3-year window for purposes of having the largest possible sample size.
References
- [1].Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, others (2012) Clinical and biomarker changes in Dominantly Inherited Alzheimer’s disease. New England Journal of Medicine 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, others (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiology of aging 30, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, Sutphen CL, Benzinger TLS, Mintun MA, Holtzman DM, Morris JC (2013) Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 80, 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. The Lancet Neurology 12, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B (2016) A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Villemagne VL, Maruff P, For the AIBL Research Group (2012) Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology 79, 1645–1652. [DOI] [PubMed] [Google Scholar]
- [7].Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, Maruff P (2017) Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: A meta-analysis. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 6, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ (2018) Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 91, e859–e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, Hanseeuw BJ, Buckley R, Chhatwal J, Hedden T, Marshall GA, Quiroz YT, Donovan NJ, Jackson J, Gatchel JR, Rabin JS, Jacobs H, Yang H, Properzi M, Kirn DR, Rentz DM, Johnson KA (2018) The impact of Aβ and tau on prospective cognitive decline in older individuals. Ann Neurol ana.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lowe VJ, Bruinsma TJ, Wiste HJ, Min H-K, Weigand SD, Fang P, Senjem ML, Therneau TM, Boeve BF, Josephs KA, Pandey MK, Murray ME, Kantarci K, Jones DT, Vemuri P, Graff-Radford J, Schwarz CG, Machulda MM, Mielke MM, Roberts RO, Knopman DS, Petersen RC, Jack CR (2019) Cross-sectional associations of tau-PET signal with cognition in cognitively unimpaired adults. Neurology 93, e29–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Allison SL, Koscik RL, Cary RP, Jonaitis EM, Rowley HA, Chin NA, Zetterberg H, Blennow K, Carlsson CM, Asthana S, Bendlin BB, Johnson SC (2019) Comparison of different MRI-based morphometric estimates for defining neurodegeneration across the Alzheimer’s disease continuum. NeuroImage: Clinical 23, 101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. The Lancet Neurology 15, 673–684. [DOI] [PubMed] [Google Scholar]
- [13].Aschenbrenner AJ, Balota DA, Tse C-S, Fagan AM, Holtzman DM, Benzinger TL, Morris JC (2015) Alzheimer disease biomarkers, attentional control, and semantic memory retrieval: Synergistic and mediational effects of biomarkers on a sensitive cognitive measure in non-demented older adults. Neuropsychology 29, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, Blennow K, Hansson O, the Alzheimer’s Disease Neuroimaging Initiative (2016) Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Molecular Medicine 8, 1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schindler SE, Jasielec MS, Weng H, Hassenstab JJ, Grober E, McCue LM, Morris JC, Holtzman DM, Xiong C, Fagan AM (2017) Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiology of Aging 56, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Timmers M, Tesseur I, Bogert J, Zetterberg H, Blennow K, Börjesson-Hanson A, Baquero M, Boada M, Randolph C, Tritsmans L, Van Nueten L, Engelborghs S, Streffer JR (2019) Relevance of the interplay between amyloid and tau for cognitive impairment in early Alzheimer’s disease. Neurobiology of Aging 79, 131–141. [DOI] [PubMed] [Google Scholar]
- [17].Blennow K, Wallin A, Ågren H, Spenger C, Siegfried J, Vanmechelen E (1995) tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Molecular and Chemical Neuropathology 26, 231–245. [DOI] [PubMed] [Google Scholar]
- [18].Storandt M, Head D, Fagan AM, Holtzman DM, Morris JC (2012) Toward a multifactorial model of Alzheimer disease. Neurobiology of Aging 33, 2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFL Group, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, Bjerke M, Blennow K, Boxer A, Brundin L, Burman J, Christensen T, Fialová L, Forsgren L, Frederiksen JL, Gisslén M, Gray E, Gunnarsson M, Hall S, Hansson O, Herbert MK, Jakobsson J, Jessen-Krut J, Janelidze S, Johannsson G, Jonsson M, Kappos L, Khademi M, Khalil M, Kuhle J, Landén M, Leinonen V, Logroscino G, Lu C-H, Lycke J, Magdalinou NK, Malaspina A, Mattsson N, Meeter LH, Mehta SR, Modvig S, Olsson T, Paterson RW, Pérez-Santiago J, Piehl F, Pijnenburg YAL, Pyykkö OT, Ragnarsson O, Rojas JC, Romme Christensen J, Sandberg L, Scherling CS, Schott JM, Sellebjerg FT, Simone IL, Skillbäck T, Stilund M, Sundström P, Svenningsson A, Tortelli R, Tortorella C, Trentini A, Troiano M, Turner MR, van Swieten JC, Vågberg M, Verbeek MM, Villar LM, Visser PJ, Wallin A, Weiss A, Wikkelsø C, Wild EJ (2019) Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol 76, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, Machulda MM, Kremers WK, Knopman DS, Jack C, Petersen RC, Kern S (2019) Plasma and CSF neurofilament light: Relation to longitudinal neuroimaging and cognitive measures. Neurology 93, e252–e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, Klimova J, Mead S, Blennow K, Rossor MN, Schott JM, Zetterberg H, Fox NC (2017) Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 89, 2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K, for the Alzheimer’s Disease Neuroimaging Initiative (2016) Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurology 73, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale AM, Alzheimer’s Disease Neuroimaging Initiative for the (2012) Amyloid-β–Associated Clinical Decline Occurs Only in the Presence of Elevated P-tau. Arch Neurol 69,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, Fanning K, Farlow MR, Hassenstab J, McDade EM, others (2017) The DIAN-TU Next Generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimer’s & dementia: the journal of the Alzheimer’s Association 13, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, Weiner M, Aisen PS (2014) The Preclinical Alzheimer Cognitive Composite: Measuring Amyloid-Related Decline. JAMA Neurology 71, 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hedden T, Oh H, Younger AP, Patel TA (2013) Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 80, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buckner RL (2005) Molecular, Structural, and Functional Characterization of Alzheimer’s Disease: Evidence for a Relationship between Default Activity, Amyloid, and Memory. Journal of Neuroscience 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aschenbrenner AJ, Balota DA, Fagan AM, Duchek JM, Benzinger TL, Morris JC (2015) Alzheimer disease cerebrospinal fluid biomarkers moderate baseline differences and predict longitudinal change in attentional control and episodic memory composites in the adult children study. Journal of the International Neuropsychological Society 21, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Duchek JM, Balota DA, Thomas JB, Snyder AZ, Rich P, Benzinger TL, Fagan AM, Holtzman DM, Morris JC, Ances BM (2013) Relationship between Stroop performance and resting state functional connectivity in cognitively normal older adults. Neuropsychology 27, 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gordon BA, Zacks JM, Blazey T, Benzinger TLS, Morris JC, Fagan AM, Holtzman DM, Balota DA (2015) Task-evoked fMRI changes in attention networks are associated with preclinical Alzheimer’s disease biomarkers. Neurobiology of Aging 36, 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Wright A, Shenker J, Magin R (2000) fMRI Studies of Stroop Tasks Reveal Unique Roles of Anterior and Posterior Brain Systems in Attentional Selection. Journal of Cognitive Neuroscience 12, 988–1000. [DOI] [PubMed] [Google Scholar]
- [32].Kane MJ, Engle RW (2002) The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review 9, 637–671. [DOI] [PubMed] [Google Scholar]
- [33].West RL (1996) An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin 120, 272–292. [DOI] [PubMed] [Google Scholar]
- [34].Twamley EW, Ropacki SAL, Bondi MW (2006) Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society 12,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Balota DA, Duchek JM (2015) Attention, variability and biomarkers in Alzheimer’s disease In Remembering: Attributions, Processes and Control in Humany Memory. Psychology Press, New York, NY, pp. 285–303. [Google Scholar]
- [36].McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ (2010) The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct. Neuropsychology 24, 222–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. [DOI] [PubMed] [Google Scholar]
- [38].Grober E, Buschke H, Crystal H, Bang S, Dresner R (1988) Screening for dementia by memory testing. Neurology 38, 900–903. [DOI] [PubMed] [Google Scholar]
- [39].Armitage SG (1945) An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs 60, 1–48. [Google Scholar]
- [40].Goodglass H, Kaplan E (1983) Boston Diagnostic Aphasia Examination Booklet, III, ORAL EXPRESSION, J. Animal Naming (Fluency in Controlled Association). In An Introduction to Model-Based Cognitive Neuroscience Lea & Febiger, Philadelphia. [Google Scholar]
- [41].Spieler DH, Balota DA, Faust ME (1996) Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance 22, 461–479. [DOI] [PubMed] [Google Scholar]
- [42].Stroop JR (1935) Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18, 643–662. [Google Scholar]
- [43].Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ (2007) Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer’s disease: Evidence for disproportionate selection impairments in the simon task. Neuropsychology 21, 170–182. [DOI] [PubMed] [Google Scholar]
- [44].Simon JR (1969) Reactions toward the source of stimulation. Journal of Experimental Psychology 81, 174–176. [DOI] [PubMed] [Google Scholar]
- [45].Tse C-S, Balota DA, Yap MJ, Duchek JM, McCabe DP (2010) Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attention tasks. Neuropsychology 24, 300–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wechsler D (1987) Manual: Wechsler Memory Scale- Revised, Psychological Corporation, San Antonio, TX. [Google Scholar]
- [47].Wechsler D (1997) Wechsler Memory Scale (3rd ed.): Administration and scoring manual, Psychological Corporation, San Antonio, TX. [Google Scholar]
- [48].Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, Wahl S, Benzinger TLS, Holtzman DM, Morris JC, Fagan AM (2018) Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer’s & Dementia 14, 1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67,. [Google Scholar]
- [50].Wagenmakers E-J (2007) A practical solution to the pervasive problems ofp values. Psychonomic Bulletin & Review 14, 779–804. [DOI] [PubMed] [Google Scholar]
- [51].Kass RE, Raftery AE (1995) Bayes Factors. Journal of the American Statistical Association 90, 773–795. [Google Scholar]
- [52].Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM (2016) Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science Translational Medicine 8, 338ra66–338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Soldan A, Pettigrew C, Cai Q, Wang M-C, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS, for the BIOCARD Research Team (2016) Hypothetical Preclinical Alzheimer Disease Groups and Longitudinal Cognitive Change. JAMA Neurology 73, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, Simonsen AH, Verbeek MM, Rosa-Neto P, Slot RER, Tainta M, Izaguirre A, Reijs BLR, Farotti L, Tsolaki M, Vandenbergue R, Freund-Levi Y, Verhey FRJ, Clarimón J, Fortea J, Frolich L, Santana I, Molinuevo JL, Lehmann S, Visser PJ, Teunissen CE, Zetterberg H, Blennow K (2019) Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s & Dementia 15, 742–753. [DOI] [PubMed] [Google Scholar]
- [55].Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, Vöglein J, Levin J, Masters CL, Martins R, Schofield PR, Rossor MN, Graff-Radford NR, Salloway S, Ghetti B, Ringman JM, Noble JM, Chhatwal J, Goate AM, Benzinger TLS, Morris JC, Bateman RJ, Wang G, Fagan AM, McDade EM, Gordon BA, Jucker M (2019) Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 25, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jackson JD, Balota DA, Duchek JM, Head D (2012) White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia 50, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12:517–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this project are available by submitting a formal data request to the Knight Alzheimer Disease Research Center Administrative Core. Instructions and polices can be found at https://knightadrc.wustl.edu/Research/ResourceRequest.htm