Abstract
Objectives:
Bile acid (BA) homeostasis is regulated by intestinal cellular signaling involving the farnesoid X receptor (FXR) and fibroblast growth factor 19 (FGF19) secretion. Using preterm and term pigs as a model, we examined postnatal changes in expression of the FXR-FGF19 axis that is poorly characterized in human infants.
Methods:
Pigs delivered by cesarean-section at 10 d preterm and near full term (115 d gestation) were fitted with orogastric and umbilical arterial catheters. Pigs were fed combined parenteral nutrition and minimal enteral nutrition for 5 d, followed by milk formula until 26 d. Plasma and tissue samples were collected at d 0, 5, 11, and 26. Plasma FGF19 concentration and liver and distal intestinal gene expression of FGF19 and other FXR target genes were quantified.
Results:
Plasma FGF19 levels were lower in preterm vs. term newborn pigs (P < 0.05), increased markedly by 5 d, especially in preterm pigs, and decreased in both groups until d 26. Likewise, intestinal FXR and FGF19 expression was lower (P ≤ 0.05) in premature vs term newborn pigs and decreased (P ≤ 0.05) between d 5 and 26. Hepatic expression of cholesterol 7α-hydroxylase (CYP7A1) was inversely correlated with plasma FGF19 in both groups.
Conclusions:
We conclude that the activity of FXR-FGF19 axis is lower in preterm than in term newborn pigs but increases transiently and then declines by the first month of age. We also provide supportive evidence of negative feedback between plasma FGF19 and hepatic CYP7A1 expression.
Keywords: bile acids, premature pig, FXR-FGF19 axis
INTRODUCTION
Prematurity is a world-wide problem and leads to suboptimal postnatal growth, stunting and adverse neurodevelopmental outcomes (1, 2). Premature infants also have suboptimal digestive function and fat digestion that has been linked to poor development of digestive lipases and bile salt production (3, 4). Bile acids (BAs) are a key detergent like molecules synthesized by the liver and secreted into bile and function as essential cofactors in the digestion of lipids (5). The developmental regulation and expression of genes involved in BA homeostasis are poorly understood in premature infants.
A major nuclear receptor that regulates BA homeostasis is farnesoid X receptor (FXR) which is highly expressed in the liver and intestine (6, 7). Bile acids act as ligands that activate FXR and regulate the expression of target genes involved in synthesis and transport of BAs. Fibroblast growth factor 19 (FGF19) is a key FXR target gene whose secretion is triggered by luminal BAs and functions as an intestinal endocrine hormone (8). FGF19 secreted after a meal in response to gut BA flow functions mainly as a negative feedback signal to suppress hepatic BA production via inhibition of the rate limiting enzyme in bile synthesis, cholesterol 7α-hydroxylase (CYP7A1). However, FGF19 also has insulin-like functions on the liver and skeletal muscle metabolism and thus may function as a nutrient-responsive anabolic endocrine hormone (9, 10).
There are few reports describing the changes in FXR-FGF19 pathway in response to feeding and/or development during early development and the premature stage (11, 12). To our knowledge, there is only one report detailing FGF19 levels in preterm vs term neonates (13). Newborn preterm infants had high levels of FGF19 while their CYP7A1 activity, as measured by 7α-hydroxy-4-cholestene-3-one (C4) levels in plasma was undetectable in newborns less than 30 weeks’ gestation. Further understanding of FXR-FGF19 signaling may be considered important for premature infants and have implications for feeding protocols.
Given the importance of the FXR-FGF19 signaling pathway in BA homeostasis and metabolism, we aimed to investigate the impact of premature birth on the FXR-FGF19 axis in the context of postnatal development. We quantified the concentration of circulating FGF19 and expression of key genes involved in the FXR-FGF19 pathway in preterm (90% gestation) and term pigs. The preterm pig has been extensively used as a model to investigate the effects of prematurity on the gastrointestinal (GI) system (14, 15). Additionally, preterm pigs have been shown to suffer from the same morbidities and GI and liver physiological dysfunctions as premature human infants (16, 17). Importantly, FGF19 is expressed in the intestine and liver of both humans and pigs, unlike rodents that express the human ortholog FGF15 only in the intestine (18), thus making pigs a relevant model of human FGF19 biology. We hypothesize that the transcription levels of key genes in the FXR-FGF19 pathway, and FGF19 protein secretion from the distal ileum (DI) are suppressed due to prematurity and upregulated by feeding and postnatal age.
METHODS
Study Design and Animal Protocols
All experimental procedures were approved by the National Ethics Committee on Animal Experimentation at University of Copenhagen. The plasma and tissue samples used in this study were collected during two animal studies in which the pigs were treated almost identically and are described in detail in recent reports (19–21). A total of 134 pigs (Danish Landrace × Large White × Duroc) from 12 sows were delivered by cesarean section at 90 % gestation age (Preterm; 106 d, n = 58) or 100 % gestation age (Term; 118 d, n = 59) as previously described (19). Umbilical catheters (infant feeding tube 4F; Portex, Kent, UK) and orogastric feeding tubes (infant feeding tube 6F; Portex) were placed in each piglet within 3 h of delivery as described previously (19, 20). All piglets received a subcutaneous injection of iron dextran post-catheterization (200 mg Uniferon; Pharmacosmos, Holbaek, Denmark), and 3 boluses of maternal sow plasma co-infused with parenteral nutrition after the cesarean section to provide passive immunity. Pigs were euthanized on d 1, d 5, and d 26 for Study 1 (19, 20); and d 1 and d 11 for Study 2 (21) using the same anesthesia protocol described for the sows followed by intracardiac injection of pentobarbital (see Figure, Supplemental Digital Content 1) . Animals were euthanized on d 5 because this was when parenteral nutrition ended and exclusive enteral feeding began (see below). Additionally, in Study 2, d 11 was chosen as an end-of-study time point because 11-d preterm pigs would have a postnatal age of 11d, and thus would be the same gestational age as the term pigs on d 1 of the study (term-corrected age for preterm pigs). Blood was drawn via cardiac puncture while animals were sedated prior to euthanasia, and the plasma fraction was snap-frozen. Tissue from DI and liver was immediately collected and snap-frozen in liquid nitrogen.
Nutritional Support
Pigs were weighed daily and the volumes of different forms of nutrition were adjusted per body weight in both studies (see Figure, Supplemental Digital Content 1). All pigs received minimal enteral nutrition (MEN) with enteral administration of bovine colostrum (Biofiber Damino, Vejen, Denmark) from 16 ml·kg−1·day−1 on d 1, increasing to 64 ml·kg−1·day−1 on d 5 (see Table, Supplemental Digital Content 2). Also during d 1–5, pigs received parenteral nutrition (PN; 96 ml·kg−1·day−1 on d 1) and on d 5, parenteral nutrition was discontinued (19). From d 5 until d 9 (Study 1) or d 11 (Study 2) all piglets were enterally fed increasing amounts of raw bovine milk via feeding troughs. On d 9 of Study 1 piglets were transferred to whole milk powder (Arla Foods Ingredients, Viby, Denmark) until d 26 as described previously (19). All pigs had ad libitum access to water from d 5.
Plasma FGF-19 Analysis
Concentration of FGF19 in plasma (Preterm n = 58, Term n = 55) was measured using a commercial porcine ELISA Kit (RayBiotech, Norcross, GA). Plasma was diluted 5-fold (preterm) or 10-fold (term) and 100 μL of diluted plasma was assayed.
RT-qPCR Analysis
Total RNA was isolated from DI (Preterm n = 57, Term n = 52) and liver tissue (Preterm n = 28, Term n = 26) using TRIzol (Thermo Fisher Scientific, Waltham, MA) and expression of target genes was completed by reverse transcription polymerase chain reaction using standard protocols. Primer sequences for one reference gene, β-Actin, and 8 target genes: FXR, FGF19, small heterodimer partner (SHP), ileal lipid binding protein (ILBP), organic solute transporter (OST) α, apical sodium dependent BA transporter (ASBT), bile salt export pump (BSEP), and CYP7A1(see Table, Supplemental Digital Content 3); FXR, FGF19, SHP, ILBP, OSTα, and ASBT primers were previously designed (22). A relative standard curve was used to correct for differences on RT-qPCR reaction efficiency between plates (23). The 2−ΔΔCT method, as previously described (24) was used to compare gene expression levels between samples, which were analyzed to determine the fold change of mRNA expression.
Statistical Analysis
The results of Study 1 and 2 were combined, analyzed and presented as one study. We did this because the experimental protocols, gestational age at preterm and term birth, and postnatal nutrition support were the same in both studies. Plasma FGF19 concentrations and fold changes in gene expression were analyzed by a 2-way ANOVA using a linear mixed-model procedure (PROC MIXED) of SAS that included gestational age of birth (preterm vs term) and postnatal age (1, 5, 11, 26) as fixed effect. Normality of the residuals was tested using the Shapiro-Wilk test under the UNIVARIATE procedure (SAS Inst. Inc., Cary, NC). Correlations between circulating FGF19 levels gene expression of FGF19 in DI and CYP7A1 in liver were determined using the PROC CORR in SAS. Pearson correlation coefficient (R) was considered significant at P ≤ 0.05.
RESULTS
Plasma FGF19 concentrations.
Compared to d 1, plasma FGF19 concentrations increased on d 5 in both preterm and term pigs (P ≤ 0.05 for preterm and term), and on d 11 in preterm group (P ≤ 0.001), and were higher in term compared to preterm on d 1 (P ≤ 0.001; Fig. 1.). There was no difference in plasma FGF19 concentration between preterm and term pigs on d 5, 11 and 26. In both preterm and term pigs, plasma FGF19 was lower (P ≤ 0.01) at d 26 than at d 5.
Fig. 1.
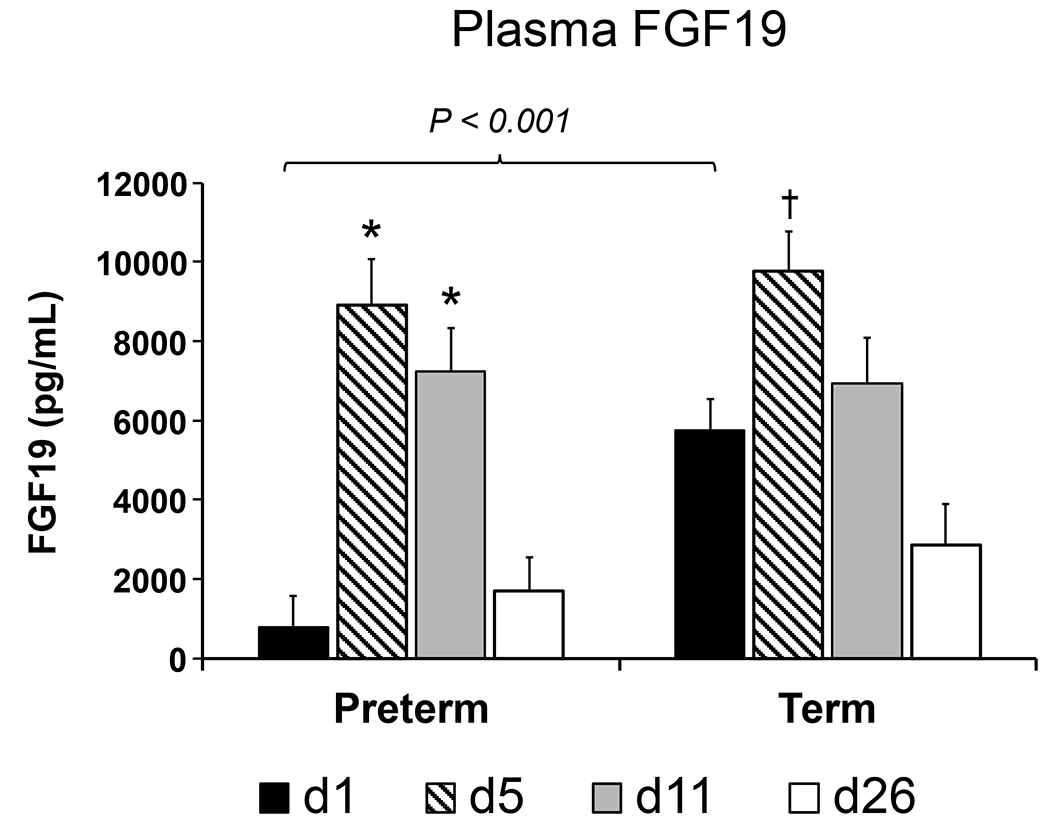
FGF19 concentration in pg/mL in peripheral plasma of preterm and term pigs on d-of-age 1, 5, 11, and 26. Values are least square means ± SE. Means differ from respective preterm or term D1 value *P ≤ 0.01, †P ≤ 0.05. Bracket denotes significant difference in values between preterm and term groups on the same day (d1 preterm n = 11, term n = 11; d5 preterm n = 8, term n = 10; d11 preterm n = 10, term n = 12; d26 preterm n = 10, term n = 10).
Intestinal gene expression.
Gene expression of FXR in DI decreased in preterm and term animals on d 11 (P ≤ 0.001 and ≤ 0.001 for preterm and term, respectively; Fig. 2A.) and d 26 (P ≤ 0.001 and ≤ 0.001) compared to d 1, whereas DI expression of FGF19 increased on d 5 (P ≤ 0.001; Fig. 2B.), 11 (P ≤ 0.001), and 26 (P ≤ 0.001) in preterm pigs, and was higher on d 5 (P ≤ 0.001) and lower on d 11 (P = 0.008) in preterm compared to term. Compared to d 1, SHP levels in DI increased on d 11 in preterm pigs (P = 0.005; Fig. 2C.), and on d 26 in both preterm (P ≤ 0.001) and term (P ≤ 0.001) groups. Similarly, compared to d 1, both preterm and term pigs had increased DI gene expression of OSTα on d 5 (P ≤ 0.001 and ≤ 0.001 in preterm and term, respectively; Fig. 2D.), d 11 (P ≤ 0.001 and ≤ 0.001), and d 26 (P ≤ 0.001 and ≤ 0.001), increased ASBT on d 5 (P = 0.006 and 0.039; Fig. 2E.) and d 11 (P = 0.007 and ≤ 0.001), and increased ILBP on d 5 (P = 0.026 and 0.017; Fig. 2F.), d 11 (P ≤ 0.001), and in term pigs, on d 26 (P = 0.036).
Fig. 2.
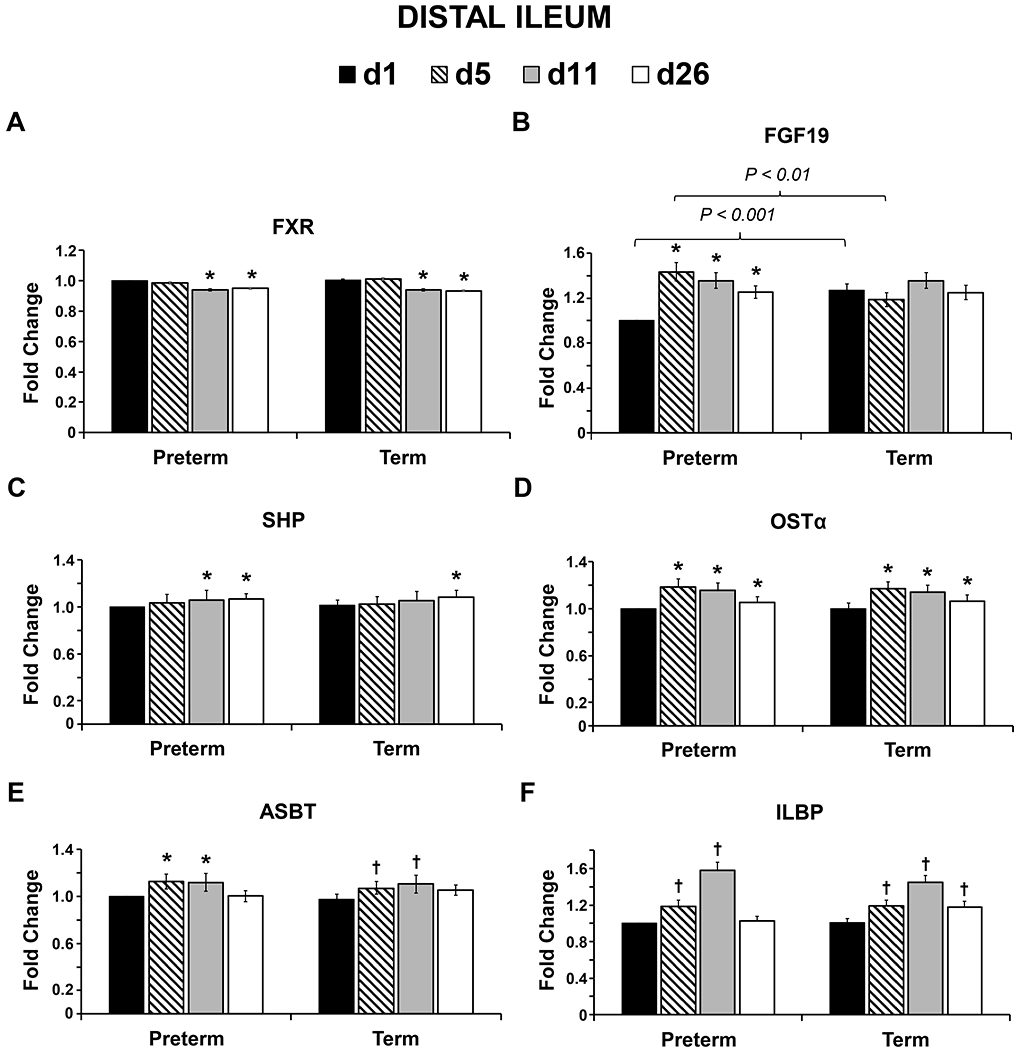
Relative mRNA abundance of farnesoid x receptor (FXR), fibroblast growth factor (FGF19), small heterodimer partner (SHP), organic solute transporter alpha (OSTα), apical sodium dependent bile acid transporter (ABST), and ileal lipid binding protein (ILBP) in the distal ileum of preterm and term pigs on d-of-age 1, 5, 11, and 26. Values are least square means ± SE. Means differ from respective preterm or term D1 value *P ≤ 0.01, †P ≤ 0.05. Brackets denote significant differences between preterm and term groups on the same day (d1 preterm n = 7, term n = 7; d5 preterm n = 7, term n = 7; d11 preterm n = 7, term n = 7; d26 preterm n = 7, term n = 5).
Hepatic gene expression.
Hepatic gene expression of FXR and SHP did not differ between term and preterm groups at any time point (Fig. 3A. and 3B.). Gene expression of BSEP in liver decreased on d 5 compared to d 1 in term animals (P = 0.006; Fig. 3C.) and was lower on d 5 (P ≤ 0.001) and higher on d 26 (P = 0.038) in preterm compared to term. There was no significant difference in hepatic gene expression of BSEP in preterm animals (Fig. 3C.). Finally, compared to d 1, term pigs had decreased CYP7A1 expression in liver on d 5 (P = 0.035; Fig. 3D.).
Fig. 3.
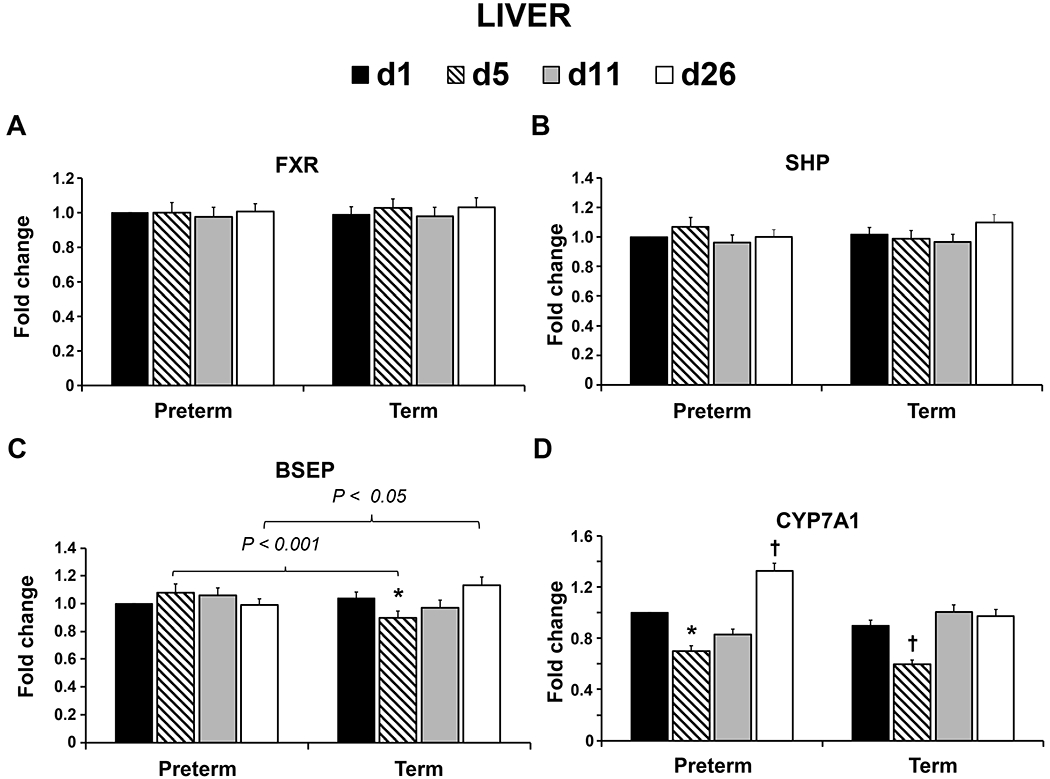
Relative mRNA abundance of farnesoid X receptor (FXR), small heterodimer partner (SHP), bile salt export pump (BSEP), and cytochrome P450 family 7 subfamily A member 1 (CYP7A1) in the liver of preterm and term pigs on d-of-age 1, 5, 11, and 26. Values are least square means ± SE. Means differ from respective preterm or term D1 value *P ≤ 0.01, †P ≤ 0.05. Brackets denote significant differences between preterm and term groups on the same day (d1 preterm n = 7, term n = 7; d5 preterm n = 7, term n = 7; d11 preterm n = 7, term n = 7; d26 preterm n = 7, term n = 5).
There was a significant negative correlation between hepatic CYP7A1 expression and plasma FGF19 levels in both preterm (R=0.685, P<0.001) and term pigs (R=0.677, P<0.001)(Fig. 4, top panels). There was also a significant positive correlation between plasma FGF19 and intestinal FGF19 gene expression in preterm (R=0.486, P<0.003) and term (R=0.336, P<0.028) pigs (Fig. 4, bottom panels).
Fig. 4.
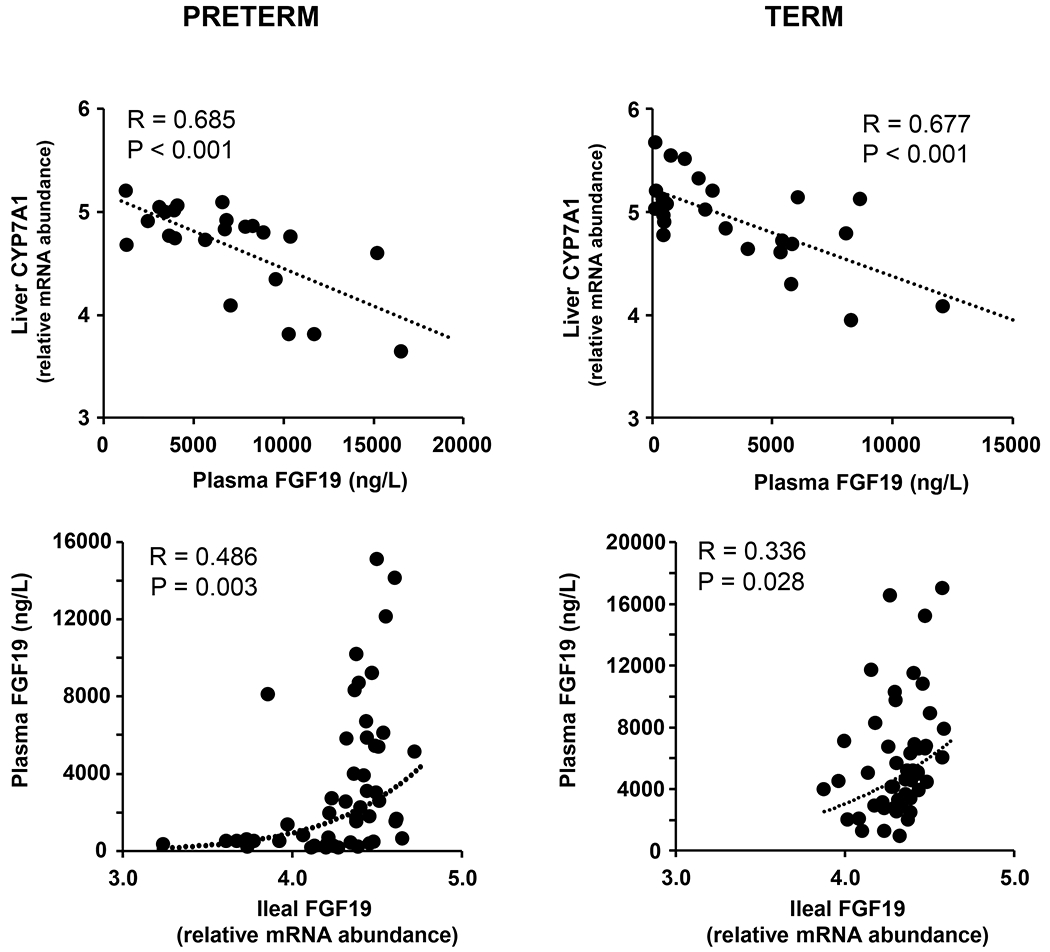
Correlations between circulating FGF19 (ng/L) and relative mRNA abundance of liver CYP7A1 (top panels) or relative mRNA abundance of ileal FGF19 (bottom panels) in preterm and term pigs.
DISCUSSION
Bile acid homeostasis is critically important for lipid digestion as well as nutrient availability for rapidly growing preterm infants. Using preterm pigs as a model for preterm infants, we show that prematurity significantly reduces circulating FGF19 levels at birth. Additionally, the intestinal expression of FGF19 correlated with the level of FGF19 in circulation suggesting that FGF19 is partially transcriptionally regulated. The expression of FXR, the nuclear BA receptor that targets FGF19 was not different between preterm and term pigs but expression decreased in the first 26 d. The intestinal expression of several other FXR targets genes involved in BA homeostasis, namely OST, ASBT, and ILBP, were lower in both preterm and term newborns and increased with postnatal age. The developmental increase observed in plasma FGF19 and intestinal FXR target genes are similar to that reported previously in term infants and pigs (12). However, our results in preterm vs term pigs are in contrast to the recent report in preterm infants showing that plasma FGF19 is high at birth and declines with advancing gestational age (13). This difference between neonatal pigs and infant could be due to species differences in developmental ontogeny, but also potential confounding issues of heterogeneity of feeding practices in infants. The reduced secretion of FGF19 in preterm vs term pigs could be simply a function of lower intestinal mass relative to body weight observed previously in the pigs used from our study (19, 20). Further studies are warranted to test the FGF19 secretion patterns in response to feeding and different diets in preterm pigs.
After birth, the plasma FGF19 concentration surged in both preterm and term pigs likely triggered by the gradual increase in enteral feeding and resultant secretion of bile acids into the intestine. This finding demonstrates that despite having much lower plasma FGF19 at birth, the preterm pig has a remarkable adaptive capacity to upregulated FGF19 secretion within 5 d to levels comparable to term pigs. The intestinal FGF19 expression also increased on d 5 in preterm and term pigs, after the onset of feeding, followed by a gradual decrease over time. The decline in plasma FGF19 between d 11 and 26 was consistent in both preterm and term pigs. The explanation for the decline in plasma FGF19 with advancing age is not clear, but may be related to changes in BA profiles marked by increases in hyocholic and hyodeoxycholic acids relative to chenodeoxycholic acid since the former BAs have been shown to be poor FXR agonists (12).
We also observed changes in hepatic FXR target genes involved in bile homeostasis, specifically CYP7A1 and BSEP. The expression of both genes tended to decrease between birth and 5 d of age and then rebounded by d- 26. CYP7A1 is the rate-limiting enzyme in BA synthesis, while BSEP is critical for hepatic BA efflux into bile canaliculi. Interestingly, hepatic CYP7A1 expression was negatively correlated with circulating levels of FGF19 in both preterm and term pigs between birth and d 26. This finding is consistent with the prevailing literature and suggests a functional negative feedback loop in neonatal pigs between circulating FGF19 and suppression of hepatic CYP7A1 and BA synthesis. We previously observed that plasma FGF19 concentrations are reduced during total parenteral nutrition, which may lead to unsuppressed CYP7A1 and hepatic synthesis and accumulation of BAs leading to parenteral nutrition-associated cholestasis (25).
In summary, the current study shows that plasma FGF19 concentrations are lower in preterm compared to term newborn pigs. We also show in both preterm and term pigs a surge in plasma FGF19 with the onset of enteral nutrition in the first two weeks followed by a decline after four weeks. The developmental patterns in expression of some FXR target genes correlate with plasma FGF19 and suggest a functional negative feedback with hepatic CYP7A1 expression. The metabolic significance of the postnatal surge in FGF19 in both pigs and human infants (11) and differences associated with prematurity and birth weight is unknown. Studies in mice show that FGF19 functions as an anabolic hormone with insulin-like actions on liver and skeletal muscle protein metabolism that are mediated via the mTOR pathway (9, 10). Very low birth weight, preterm infants suffer from growth failure in the early postnatal period and neurodevelopmental delays long-term. Importantly, our recent study in neonatal pigs shows that prematurity blunts the feeding-induced stimulation of translation initiation signaling and protein synthesis in muscle (26). Further studies are warranted to investigate the metabolic significance of FGF19 in infants and whether it functions as a nutrient-mediated growth factor.
Supplementary Material
What is Known
Premature birth is a worldwide problem and leads to growth failure and neurodevelopmental delay.
Reduced BA production and circulation may limit lipid digestion in premature infants
Fibroblast growth factor 19 (FGF19) is an important gut enterokine involved in BA homeostasis and metabolism.
What is New
Plasma FGF19 concentrations are lower in preterm vs. term newborn pigs, but levels increase rapidly with enteral feeding after birth.
We confirm evidence of a functional negative feedback relationship between plasma FGF19 and hepatic cholesterol 7α-hydroxylase (CYP7A1) in neonatal pigs.
Acknowledgments
FINANCIAL SUPPORT:
This work was supported in part by federal funds from the USDA, Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, and grants from the University of Copenhagen Innovation Fund Denmark (NEOMUNE)(P.S.), National Institutes of Health Grant DK-094616 (D.B.), and California Agriculture Research Institute #58873 (R.M.).
References
- 1.Kozuki N, Katz J, Christian P, et al. Comparison of US Birth Weight References and the International Fetal and Newborn Growth Consortium for the 21st Century Standard. JAMA Pediatr 2015;169(7):e151438. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JG, Baer RJ, Partridge JC, et al. Survival and Major Morbidity of Extremely Preterm Infants: A Population-Based Study. Pediatrics 2016;138(1). [DOI] [PubMed] [Google Scholar]
- 3.Hamosh M, Scanlon JW, Ganot D, et al. Fat digestion in the newborn. Characterization of lipase in gastric aspirates of premature and term infants. J Clin Invest 1981;67(3):838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamosh M, Sivasubramanian KN, Salzman-Mann C, et al. Fat digestion in the stomach of premature infants. I. Characteristics of lipase activity. J Pediatr 1978;93(4):674–9. [DOI] [PubMed] [Google Scholar]
- 5.Hegyi P, Maleth J, Walters JR, et al. Guts and Gall: Bile Acids in Regulation of Intestinal Epithelial Function in Health and Disease. Physiol Rev 2018;98(4):1983–2023. [DOI] [PubMed] [Google Scholar]
- 6.Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology 2017;65(1):350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman AG, Sokol RJ Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol 2019;16(6):346–60. [DOI] [PubMed] [Google Scholar]
- 8.Degirolamo C, Sabba C, Moschetta A Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016;15(1):51–69. [DOI] [PubMed] [Google Scholar]
- 9.Kir S, Beddow S, Samuel VT, FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benoit B, Meugnier E, Castelli M, et al. Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat Med 2017. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Infantes D, Gallego-Escuredo JM, Diaz M, et al. Circulating FGF19 and FGF21 surge in early infancy from infra- to supra-adult concentrations. Int J Obes (Lond) 2015. [DOI] [PubMed] [Google Scholar]
- 12.Gavalda-Navarro A, Pastor JJ, Mereu A, et al. Developmental regulation of the intestinal FGF19 system in domestic pigs. Am J Physiol Gastrointest Liver Physiol 2018;314(6):G647–G54. [DOI] [PubMed] [Google Scholar]
- 13.Memon N, Griffin IJ, Lee CW, et al. Developmental regulation of the gut-liver (FGF19-CYP7A1) axis in neonates. J Matern Fetal Neonatal Med 2018:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oosterloo BC, Premkumar M, Stoll B, et al. Dual purpose use of preterm piglets as a model of pediatric GI disease. Vet Immunol Immunopathol 2014;159(3–4):156–65. [DOI] [PubMed] [Google Scholar]
- 15.Sangild PT, Thymann T, Schmidt M, et al. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci 2013;91(10):4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 2006;130(6):1776–92. [DOI] [PubMed] [Google Scholar]
- 17.Burrin DG, Ng K, Stoll B, et al. Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition-associated liver disease. Adv Nutr 2014;5(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright TJ, Ladher R, McWhirter J, et al. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol 2004;269(1):264–75. [DOI] [PubMed] [Google Scholar]
- 19.Andersen AD, Sangild PT, Munch SL, et al. Delayed growth, motor function and learning in preterm pigs during early postnatal life. Am J Physiol Regul Integr Comp Physiol 2016;310(6):R481–92. [DOI] [PubMed] [Google Scholar]
- 20.Hansen CF, Thymann T, Andersen AD, et al. Rapid gut growth but persistent delay in digestive function in the postnatal period of preterm pigs. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016;310(8):G550–G60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren S, Hui Y, Obelitz-Ryom K, et al. Neonatal gut and immune maturation is determined more by postnatal age than by postconceptional age in moderately preterm pigs. Am J Physiol Gastrointest Liver Physiol 2018;315(5):G855–G67. [DOI] [PubMed] [Google Scholar]
- 22.Ng K, Stoll B, Chacko S, et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J Parenter Enteral Nutr 2016;40(5):656–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larionov A, Krause A, Miller WA standard curve based method for relative real time PCR data processing. BMC Bioinformatics 2005;6(62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 25.Jain AK, Stoll B, Burrin DG, et al. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am.J.Physiol Gastrointest.Liver Physiol 2012;302(2):G218–G24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naberhuis JK, Suryawan A, Nguyen HV, et al. Prematurity blunts the feeding-induced stimulation of translation initiation signaling and protein synthesis in muscle of neonatal piglets. Am J Physiol Endocrinol Metab 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


