Editor—The rapid and devastating progression of the coronavirus disease 2019 (COVID-19) pandemic is driving innovation, collaboration, and a need for solutions at an exceptional rate. The risk of transmission during aerosol-generating procedures such as bag mask ventilation, tracheal intubation/extubation, tracheostomy, cardiopulmonary resuscitation, manual noninvasive ventilation, and bronchoscopy is alarming.1 The widespread concern for a shortage and lack of appropriate personal protective equipment (PPE) compounds this frightening outcome and can lead to noncompliance with protective guidelines for healthcare providers up to the point that we are allowing extended use and reuse of N95 respirators.
The current gold standard to manage COVID-19 suspected or confirmed patients is enhanced PPE and airborne isolation in negative pressure rooms if available2 , 3; however, these are very limited resources in a pandemic situation. Strategies such as negative air flow in rooms and wards have been considered in the past to try to mitigate and reduce the risk of nosocomial transmission of viruses. Unfortunately, newer evidence suggests that the COVID-19 model of droplet/aerosol spread and transmission might be too simplistic as the virus can remain in aerosols for hours and on surfaces for days.4, 5, 6, 7 The Centers for Disease Control and Prevention now recommends wearing of masks by the general population.8
Humanity is in the midst of an unprecedented health crisis. It is probable that beyond the ongoing surge there will be a second wave of cases and eventually recurring viral outbreaks in the community. At the London Hospitals in London, ON, Canada, we recognise the need for viable solutions to protect healthcare providers, especially during aerosol-generating procedures. A reasonable approach to address this objective is the creation of a physical barrier that helps protect healthcare providers and the surrounding environment. Two methods recently explored and described in the literature and social media are generating much interest: (1) a simple covering of the patient with a plastic drape over the upper body9 and (2) a partially enclosed working space.10 Our proposal adopts concepts learned from both approaches; however, we added significant functional improvements while keeping the idea as simple and reproducible as possible for others to replicate.
We created a rigid cubic frame chamber that relies almost exclusively on materials available at hardware stores, which is then draped with a clear plastic bag. Our goals are that it is: (1) constructed with readily available and affordable materials, (2) acceptable to users, (3) portable to allow its use as an isolation device in different locations and scenarios of healthcare providing facilities, (4) effective as a physical barrier that allows creation of an enclosed continuous negative air-flow environment through suction/vacuum mechanisms, (5) allows oxygen delivery and nebulisation of infection control chemicals, and (6) shared with the wider medical community so that healthcare teams can customise and adapt the frame concept to their needs.
Using a collaborative feedback approach with researchers at the Robarts Research Institute and Western University, we shared ideas amongst a design team. Working in person, by e-mail, videoconferencing, and using CAD software and 3D printers, we conducted several design and testing cycles. Each step involved simulated activities to qualitatively assess the functionality of the initial idea and subsequent designs. Simulations were completed using videolaryngoscopy for intubation. Suction was applied to the isolation space using standard wall suction connectors. Clear plastic bags (400–800 L capacity) were used to enclose the space. Transparent dressing (Tegaderm™ film; 3M Health Care, St Paul, MN, USA) was used to reseal working ports. Ultraviolet light traceable aerosol (fluorescein) was created using an ultrasonic nebuliser (Trust Life Brand Quality™; Shoppers Drug Mart®, Toronto, ON, Canada) to assess gross leakage from the bag and surroundings contamination.
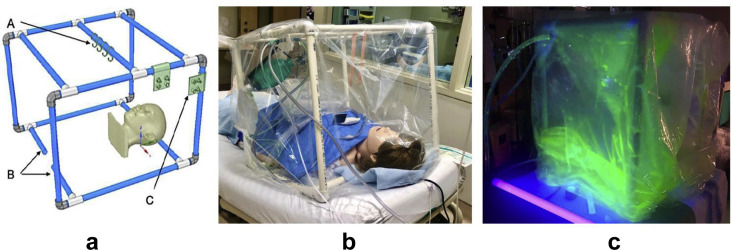
Our prototypical polyvinyl chloride (PVC) rigid frame chamber is shown in Fig. 1 . Clearance of the fluorescein tracer, contamination of the operator, bag, table, and support structures were qualitatively assessed. A simulated cough to assess protection of the operator's PPE was performed (available for visualisation at https://youtu.be/dVP3XinNIhg). We demonstrated containment of both droplets and aerosols, the ability to clear the bag of aerosolised particles with negative air flow, and the protection of the operator and environment. Airway devices are supported on hooks, which increases the working space available. During a simulated airway management training session of our COVID-19 intubation team, direct vision, communication, and manoeuverability were accomplished for 12 operators.
Fig 1.
Depiction of multipurpose portable negative air flow protection chamber. (a) CAD drawing of a PVC tube frame of 60×60×60 cm. (A) 3D-printed hooks on the central bar for hanging equipment. (B) Stabilising tube can be added or removed in different lengths depending on the clinical circumstances. (C) Multiple 3D-printed suction/oxygen/nebuliser ports can be snapped on any of the supporting tubes to accommodate the physical location of these sources according to individual needs. CAD, computer-aided design; PVC, polyvinyl chloride. (b) The mannequin, resuscitation bag (on a hook), videolaryngoscope, tracheal tube, tape, and collection bag for contaminated equipment are shown inside the enclosed environment of the PVC frame draped with a clear plastic bag. Suction and oxygen supply are connected to the snap ports. (c) Fluorescein solution aerosolisation was created within the proposed chamber using the ultrasound nebuliser. With the help of ultraviolet light, visualisation of aerosol/droplet effective containment, and continuous negative airflow through the suction tubing in the upper left corner, was successfully achieved. Plans and instructions for this frame are available under a Creative Commons License, Attribution-NonCommercial-ShareAlike 4.0 International.
Even though our results are preliminary and qualitative in nature, we demonstrate proof of concept for an additional physical barrier during aerosol-generating procedures. We believe that this easily constructed barrier, and others, have the potential to protect healthcare providers in caring for confirmed or suspected COVID-19 patients. These include patients undergoing monitored anaesthesia care or regional anaesthesia, treated with noninvasive ventilation, in overcrowded emergency department or wards, in endoscopy/bronchoscopy suites, in radiology suites, and during patient transportation.
We acknowledge that further research and testing are necessary to quantify the contamination level within the multipurpose portable negative air flow protection isolation chamber and whether it is possible to inactivate any virus before removal and doffing of the plastic drape and frame. We remain concerned that healthcare providers may develop a false sense of security if these barriers are used, so we strongly emphasise that local guidelines for PPE be maintained, and that new devices being developed during this healthcare crisis are used mainly as an additional complementary resource, not a replacement, to face the undeniable scarcity of PPE.
Declarations of interest
The authors declare that they have no conflicts of interest.
References
- 1.Peng P.W., Ho P.L., Hota S.S. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anesth. 2020 doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020 doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X., Liu Y., Gong Y. Perioperative management of patients infected with the novel coronavirus: recommendation from the joint task force of the Chinese society of anesthesiology and the Chinese association of anesthesiologists. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Z.-D., Wang Z.-Y., Zhang S.-F. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020 Jul doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Doremalen N., Bushmaker T., Morris D.H. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 7.Ong S.W.X., Tan Y.K., Chia P.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020 doi: 10.1001/jama.2020.3227. [PMID: 32129805] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.USA Today. Coronavirus might spread much farther than 6 feet in the air. CDC says wear a mask in public. Available from: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html (accessed 04 May 2020).
- 9.Matava C.T., Yu J., Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anesth. 2020 doi: 10.1007/s12630-020-01649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020 doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]