Abstract
A dramatic SARS-Cov-2 outbreak is hitting Italy hard. To face the new scenario all the hospitals have been re-organised in order to reduce all the outpatient services and to devote almost all their personnel and resources to the management of Covid-19 patients. As a matter of fact, all the services have undergone a deep re-organization guided by: the necessity to reduce exams, to create an environment that helps reduce the virus spread, and to preserve the medical personnel from infection. In these days a re-organization of the endoscopic unit, sited in a high-incidence area, has been adopted, with changes to logistics, work organization and patients selection. With the present manuscript, we want to support gastroenterologists and endoscopists in the organization of a “new” endoscopy unit that responds to the “new” scenario, while remaining fully aware that resources, availability and local circumstances may extremely vary from unit to unit.
Key words: Endoscopy, Covid-19, Coronavirus, SARS-CoV-2
1. Introduction
Italy reported its first native case of severe acute respiratory syndrome from SARS-CoV-2 virus infection on February the 21st, after a major outbreak in China. As of March 26th the cases of Covid-19 have been rising exponentially up to 74,386 people infected and 7,505 deaths. This enormous number of patients has put severe stress on the healthcare systems of the most stricken areas of northern Italy, especially Lombardy.
The current national guidelines have reserved nasopharyngeal swabs for the PCR assay of SARS-CoV-2 mostly to symptomatic patients, who require hospitalization, in the attempt of maximizing cost-effectiveness. However, preliminary data not yet published on the experience of Vò Euganeo, a small town in Veneto where an extensive surveillance protocol by nasal swab testing has been adopted, prove that up to 50-75% of subjects carrying SARS-CoV-2 are actually asymptomatic and are an insidious source of infection. This report is consistent with some recent epidemiological Chinese studies that estimate up to 50-70% of asymptomatic carriers [1], [2], [3]. Moreover, the transmission of this pathogen through subjects with no symptoms has already been described [4], [5]. These reports lead to the obvious concern that in the Lombard epidemiological scenario there is a general underestimation of prevalent cases.
From the abovementioned reasons we must deduce that:
-
-
in high SARS-CoV-2 incidence areas where PCR assays are not extensively performed, Covid-19 cannot be ruled out by simple clinical examination or epidemiological link;
-
-
the greatest amount of efforts and precautions are required to minimize the spread of the disease and to preserve medical staff from infection.
Endoscopy is generally looked at as a high-risk activity given the obvious exposure of doctors and nurses to a patient's aerosol during the procedures [6], [7]. Furthermore, recent reports have confirmed the presence of SARS-CoV-2 RNA in stool samples, thus raising the question of a possible faecal-oral transmission of the disease [8], [9], [10].
The aim of this paper is to offer endoscopists a quick reference guide to adapt their endoscopic activity during an outbreak of Covid-19. Specifically, we will discuss the stratification of patients in need of endoscopic assessment and the operational management of the healthcare personnel as to personal protection, endoscopic spaces and endoscopic tools.
“Staff, Space, Stuff and Systems” preservation and optimization are core concepts taken into account in this report.
This paper is based on the first-hand experience of a tertiary Endoscopy unit at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milano, Lombardy (Italy).
2. SARS-CoV-2 and Covid-19
2.1. Biology
The 2019 novel coronavirus (SARS-CoV-2) has been identified in late December 2019 as the causative pathogen of the viral pneumonia outbreak started in Wuhan, in the Hubei province of China.
Coronaviruses belong to the Coronaviridae family, within the order Nidovirales. They are characterized by a non-segmented single-strand positive-sense RNA genome. Coronaviruses have been first identified in the 1960s and they are known to infect many different animal species (e.g., bats, snakes, mice) in addition to humans. An epidemiological study held coronaviruses responsible for about 15% of adult common colds [11]. Coronaviruses have been associated with respiratory illnesses with relatively low virulence, usually in the upper respiratory tract, but occasionally involving the lungs.
Three strains differ from the usual pathogenicity in humans, causing serious respiratory syndromes and leading to higher mortality rates. They are: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [12]. Coronaviruses have the potential to transmit across species. In fact, phylogenetic analysis has considered bats as the reservoir of coronaviruses in general, and for SARS-CoV-2 in particular. However, according to current studies, an intermediate host between bats and humans is to be identified amongst the wild animals sold at the Huanan wet market of Wuhan, where the first infection has apparently taken place [13].
2.2. Transmission
The animal-to-human spread had been identified in the earliest patients infected with SARS-CoV-2 in Wuhan. Soon, a person-to-person spread developed. The emission of respiratory droplets which are deposited on the mucous membranes of mouth, nose and eyes of nearby people and by close personal contact seem to be the main routes of inter-human diffusion. However, a faecal-oral route of transmission has been recognized as well [9], [10], [12]. Furthermore, transmission of SARS-CoV-2 by fomites is plausible, since it has been shown that the virus can remain viable and infectious on surfaces even for days [14].
ACE2 protein is the cell receptor for SARS-CoV-2 entering host cells. Such a protein is widely expressed in AT2 lung cells but also in the gastrointestinal epithelial cells cytoplasm [9].
2.3. Diagnosis
According to the WHO guidelines, it is recommended to collect samples from both the upper (naso- and oropharyngeal samples) and lower respiratory tract, such as expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage. The diagnosis of Covid-19 is confirmed by the reverse transcription polymerase chain reaction (RT-PCR). In patients with a confirmed Covid-19 diagnosis, the RT-PCR test should be repeated in order to assess viral clearance before their being considered noninfectious [15].
However, some studies suggest a low sensitivity of this method, reporting a positive rate of nasopharyngeal swabs between 30% and 60% at initial presentation. This might worsen virus spreading because of underestimation of infected patients [16].
Some studies show that almost all affected patients show CT abnormalities (e.g., ground-glass opacities, bilateral patchy shadowing) [17], [18]. A Chinese report on 1,014 cases highlights the benefit provided by chest CT for the diagnosis of Covid-19 with 97% sensitivity, 25% specificity and 68% accuracy [17]. Interestingly, 30% of patients with symptoms had suggestive CT findings despite negative RT-PCR assays.
Therefore, in case of negative RT-PCR assays, it is important to evaluate chest CT, clinical manifestations and exposure history in order to diagnose Covid-19 [17]. However, in high-incidence clinical settings with limited resources, only patients with severe signs and symptoms would probably undergo CT scan, thus limiting its potential in diagnosis.
3. Endoscopic patients’ selection and assessment
3.1. Selection of patients and subdivision of the endoscopic procedures
The aim is that priority should be given to those interventions that can substantially improve a patient's survival and postpone those which are not strictly necessary [19].
Reducing or cancelling scheduled routine activities are among the first attempts to be implemented in order to ease the pressure of a rapidly spreading infectious agent such as SARS-CoV-2 on such a healthcare facility as a hospital. Also, good triage allows to perform essential procedures, thus reducing further viral spread from asymptomatic infected patients to medical staff and vice versa.
Another important objective is preserving healthcare professionals. As a matter of fact, they are valuable yet limited resources during an epidemic outbreak and can encounter shortage. The current guidelines on medical emergencies recommend providing safety and security to medical personnel and their families [19].
As regards patients undergoing endoscopy, we have identified three main categories:
-
-
endoscopic emergencies (life-threatening gastrointestinal bleedings and foreign body ingestion);
-
-
oncological patients with a proven impact of endoscopy on their prognosis;
-
-
non-oncological patients.
Clearly, endoscopic emergencies cannot be delayed and should be performed according to the standard emergency endoscopic guidelines. On the other side, patients scheduled for endoscopy for a non-oncologic illness should be evaluated according to the motivation and clinical urgency of the examination. Particular attention should be paid to more advanced endoscopic techniques. In specialized secondary and tertiary centers, enteroscopy (device-assisted endoscopy or video-capsule endoscopy) should be reserved and guaranteed for patients affected by midgut bleeding with moderate to severe anaemia as well as endoscopic ultrasound and operational endoscopic retrograde cholangio-pancreatography in the setting of biliary obstructions leading to jaundice and pancreatitis.
Certainly, other non-oncological patients, such as those with suspected functional or motility disorders, should be postponed after the resolution of the Covid-19 outbreak.
Otherwise, oncological endoscopy represents quite a challenge for both clinical and ethical issues.
When taking the clinical status of these patients (ongoing or previous chemotherapy) into consideration, it is general opinion that surveillance endoscopy can be safely re-scheduled. An algorithm considering both the underlying pathology and the required period of surveillance is potentially useful to decide about re-scheduling until routine procedures are restarted.
The correct allocation of resources and appropriate triage during a biological disaster must be taken into consideration at the moment of each decision.
The availability at the Center of prompt surgical and oncological treatments together with the patient's prognosis should guide physicians to perform diagnostic or therapeutic endoscopy in suspected oncological patients [20], [21].
We therefore recommend applying case-by-case judgement based on the expertise and endoscopic resources at the Center.
Furthermore, when assessing patients, tele-medicine is potentially a promising tool to help physicians in their evaluation. Some studies have acknowledged its potential role and usefulness in de-centralizing patients during such clinical disastrous epidemics [22], [23].
When it comes to operative and non-operative endoscopic procedures, when considering medical staff exposure, we have identified three main categories of endoscopy:
-
-
Oral route: any procedure through mouth or nose
-
-
Anal or stomal route: any procedure passing through the anus or an entero-cutaneous stoma
-
-
Capsule endoscopy.
3.2. Patients’ Risk Assessment
In our current situation, which is characterized by high incidence of Covid-19 and relative scarcity of surveillance assays in asymptomatic subjects, for the abovementioned reasons we recommend different modalities of individual protection based on a strict clinical and epidemiological stratification of patients with potential SARS-CoV-2 infection undergoing endoscopic examination.
Contrary to recent pre-published recommendations, we consider every patient a potential carrier of SARS-CoV-2 unless proven otherwise [24]. Establishing maximum prevention within the endoscopy unit is a necessary countermeasure to the large spectrum of signs and symptoms expressed by Covid-19 patients (fever, cough but also, in smaller percentages, nausea, vomiting, diarrhoea and even neurological symptoms) and the large quantity of asymptomatic carriers [1], [2], [3], [25].
As awareness of Covid-19 clinical manifestations grows further, the digestive tract seems to be more involved. A recent multi-center Chinese retrospective study has demonstrated that up to 48.5% of patients admitted to hospital with SARS-CoV-2 infection presented at Emergency with one or more digestive symptoms as their main complaint, and after a significantly longer time from onset to access to Emergency (9.0 days vs. 7.3 days).
Also, prolonged viral shedding from the respiratory and gastrointestinal tracts (up to 37 and 12 days respectively, to the best of our current knowledge) has been described in Covid-19 patients [4], [9], [26]. Given the paucity of symptoms in certain patients, there might be the risk that clinically healed patients could still shed viral particles [27]. It is also possible that patients may not declare suspicious symptoms in the days before the endoscopic examination ruling themselves out the possibility to be infected with SARS-CoV-2 for fear or because of mild symptomatology [27].
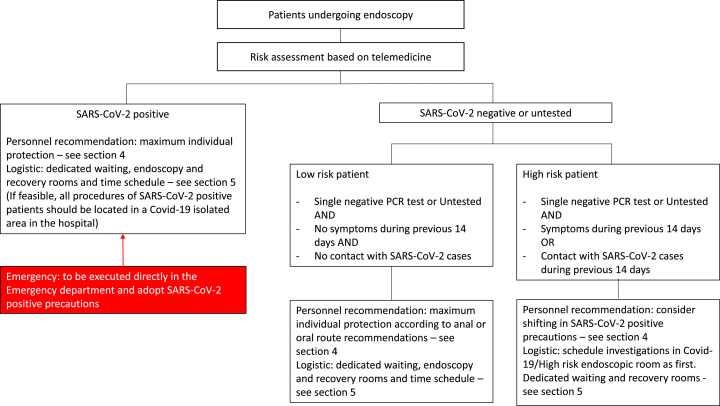
We have distinguished patients as follows: confirmed Covid-19 cases, low-risk subjects and high-risk subjects (Fig. 1 ).
Fig. 1.
Algorithm for patients’ stratification in high-incidence Covid-19 scenarios.
Before entering the endoscopy unit, a patient should be contacted for medical triage and appropriately profiled. Evaluation by tele-medicine when feasible is strongly recommended.
Low-risk subjects are defined as people without any symptoms during the previous 14 days and no contact with someone found to be SARS-CoV-2 positive. In clinical scenarios where shortage of RT-PCR assays is a fact, suspicious contacts should also be considered, for instance: a patient with a member of the family with possible or probable Covid-19 symptoms.
On the other side, high-risk patients are defined as those subjects that had a contact with someone SARS-CoV-2 positive and/or manifested one or more of the following symptoms and signs during the previous 14 days: fever, cough, dyspnoea, diarrhoea, rhinitis or conjunctivitis. From our experience we have detected also ageusia and anosmia in quite a few of pauci-symptomatic patients. However, further evidence and statistical analysis are needed.
We suggest that at point of care the patient's body temperature is checked, allocating the person to the high-risk category if the temperature results higher than 37,3°Celsius.
Whenever available, a single RT-PCR negative result should not hinder a patient's clinical evaluation since false negative results have been described in Covid-19 patients [28].
Generally, we consider a patient as free from SARS-CoV-2 when illness signs and symptoms are not detectable and a double-negative RT-PCR swab test is available.
Because of overall high specificity, patients with positive RT-PCR should always be considered as Covid-19 [5], [28].
Endoscopic emergencies must be considered as Covid-19 positive patients and treated accordingly.
4. Patients and Personnel preventions
4.1. Patients’ Management
Patients should be invited to wear surgical masks from entering the unit onwards. For low-risk patients wearing gloves can be optional, it depending on the Centre's supplies availability. Covid-19 positive and high-risk patients must wear a pair of gloves and a disposable light-fabric isolation gown.
Access to the endoscopy area may not be allowed to any other person. Access by family members or caregivers is allowed only for strictly necessary clinical or organizational issues, and they must be requested to wear masks and, when appropriate, gloves and a gown.
Patients should keep the surgical mask on until the very beginning of oral-route endoscopy and throughout their anal-route endoscopy examination.
One should consider early oxygen administration through a nasal cannula in order to prevent desaturation and to maximize the patient's comfort.
A specific colonoscopy short or at least a piece of cloth should be placed on the endoscope during anal route endoscopy to prevent any environmental dispersion of faecal material.
As soon as the endoscopic procedure is finished, before the patient is sent to a dedicated recovery room, a new surgical mask must be worn.
4.2. Healthcare Professionals Management
In our current clinical high-incidence scenario, trying to estimate the probability of Covid-19 infection in order to set adequate individual preventive measures during this outbreak can be misleading and ineffective.
As a consequence, when feasible, we recommend resorting to the highest-performance set of personnel protections.
However, considering the shortage of Personal Protection Equipment (PPE) there might be some exceptions to this general rule.
Oral-route endoscopy must always be considered a high-risk activity as well as tracheal intubation or bronchoscopy [29].
In this setting, regardless of the classification of patients (high/low-risk, Covid-19), in order to prevent the medical staff from becoming infected, we suggest high-performance personal protection equipment, i.e. a N95 or FFP2/FFP3 respirator, a hairnet, a double pair of gloves, a disposable waterproof surgical gown, a face shield (which we prefer because it allows to protect, and then spare, respirators) or goggles, and work safety clogs (Table 1 ).
Table 1.
PPE recommended for low-risk and high-risk procedures in PPE-shortage scenarios. In case of availability, consider always wearing high-risk procedure PPE.
|
Low risk procedures Low risk patient - anal route Low risk patient - VCE |
High risk procedure Oral route endoscopy |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
On the contrary, in order to spare PPE in shortage circumstances, lighter equipment may be suggested with low-risk patients undergoing anal-route endoscopy. For instance, in this setting we suggest to wear a surgical mask, a hairnet, a double pair of gloves, a disposable light-fabric isolation gown, goggles or a face shield, clogs.
We also recommend that male operators trim their beard to improve their respirator adherence to their face skin. Before starting the endoscopic activity, healthcare personnel should remove any personal jewelry items, such as bracelets, rings, earrings and watches. Also, such personnel must frequently perform hand hygiene (before and after each procedure, after touching any possibly contaminated item) that is having a thorough hands wash with soap rub or application of a suitable alcohol-based solution. When hands are visible dirty, water-and-soap rub washing is always recommended. Spaces for getting dressed and undressed are discussed in the “Endoscopic Time and Space Management” section.
How to don PPE
-
1.
Wear a first pair of gloves (inner pair). These should be considered as the operator's second skin and should be dismissed as last items. Long-tailed above-the-wrist gloves should be used not to expose the wrist skin during procedures.
-
2.
Wear the gown. When possible, get assistance from a colleague to fasten the rear strap or strings.
-
3.
Wear hairnet. If long hair is present, fit all of it inside the product. Cover your ears.
-
4.
Wear a respirator/surgical mask. Try to avoid as much as possible to manipulate the item, especially the front nose and mouth part. Rubbers or strings must be placed above the hairnet.
-
5.
Wear goggles or a face shield.
-
6.
Wear clogs or overshoes (2 pairs are suggested).
-
7.
Wear a second pair of gloves (outer pair).
How to Remove PPE
-
1.
Take off the gown. While folding it, keep the external contaminated part on the inside. Do not press the item to prevent aerosol spreading. Break and do not untie its rear closure to prevent manipulating.
-
2.
Take off the second pair of gloves (outer pair).
-
3.
Remove the face shield or goggles from the back and place them in proper container for “items to highly disinfect”.
-
4.
Take off the mask/respirator. Do not manipulate its front respiratory part. Take off firstly the lower string/rubber band and secondly the upper string/rubber band.
-
5.
Take off the hairnet. Start from the back.
-
6.
Take off the clogs or overshoes.
-
7.
Take off the first pair of gloves (inner pair).
When feasible, the filter area should be a room connected with the Covid-19 area. When this cannot be arranged, the personnel should consider initial undressing (point 1 and 2) in the Covid-19 endoscopy area and then complete the following steps in the filter area. Instead, personnel of the low-risk rooms should initially undress (point 1 and 2) in the endoscopic rooms and then carry out the following operations out of it.
Considering any PPE shortage in high-incidence scenarios, we suggest changing the outer clothing layer and disinfect face shield/goggles after each Covid-19 negative patient endoscopy and to keep PPE on in the dedicated Covid-19 positive room until all endoscopies are completed. This recommendation does not count when there are more than one high-risk patients. In this case, outer-layer clothing should be changed after each procedure but can be kept on if there is only one high-risk patient and changed later after Covid-19 positive patients. Of course, the outer pair of gloves should be dismissed after each procedure. While treating Covid-19 patients, the inner pair of gloves must not be changed between procedures but rather cleaned with hand wash or alcoholic solutions.
For dressing and undressing see the “Endocopic Time and Space Management” section.
References to the proper use and protocols regarding PPE are available at the American Center for Disease Control webpage: https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf [23]. Video example about wearing PPE are reported in supplementary file 2.
5. Endoscopic Time and Space Management
Previous scientific evidence matured during the Ebola epidemic shows that during an outbreak of an infectious disease there is an obvious reduction in non-urgent day surgery [30].
The low numbers of patients should encourage a Center to re-organize its endoscopy rooms in order to avoid further spreading of SARS-CoV-2.
Also, appropriate time scheduling of procedures should be implemented thus optimizing the room cleaning and virus dissemination controls.
Patients should enter the endoscopy unit based on their risk for Covid-19 infection. Ideally, low-risk patients should be the first procedures to carry out. On the contrary, any Covid-19 positive patients’ endoscopy should be the last, with high-risk patients undergoing endoscopy just before them.
In order to contain spread of SARS-CoV-2 infection within the hospital, every effort should be made to place one set of endoscopic equipment in a Covid-19 area, in order to perform all endoscopic procedures in Covid-19 inpatients in an isolated environment.
According to recent findings, the half-life of SARS-CoV-2 in aerosol is estimated at approximately 1.1 hour (95% CI 0.64–2.64) [14]. To prevent possible patient-to-patient transmission, the current guidelines suggest performing endoscopy in negative-pressure rooms [31]. Therefore, when possible, high-risk and Covid-19 patients should undergo the procedure in such rooms. However, such a type of facilities is not always available in all the Centers. As an alternative well-ventilated rooms may be sufficient especially if an adequate time interval between examinations is set [7], [32], [33]. One hour may be a fair amount of time to reduce the possible airborne viral load.
When it comes to the management of endoscopic space, we suggest to set three different endoscopic rooms with stable specifically tasked teams of doctors and nurses organized with a weekly rotation: one team for low-risk patients – oral route, one for low-risk patients – anal route, and one for high-risk and Covid-19 patients. Performing both upper and lower endoscopy in the same session is discouraged; however, if necessary, they should be placed in the oral-route room. Whenever there is not enough space to set different rooms for the anal and oral procedures, the careful management of endoscopy times can overcome the risk of spreading the infection. As it is, anal-route procedures in low-risk subjects should be carried out before any oral-route endoscopy.
After each procedure, the rooms shall undergo thorough cleaning with appropriate disinfectants. Special attention should be paid to the surfaces where any biofilm might hold viral particles.
There shall be no distinction in the disinfection protocols between low-risk / high-risk / Covid-19 rooms since in high-incidence scenarios, as mentioned before, maximum precautions are required.
A filter area should be arranged for healthcare professionals to perform their adequate dressing and undressing as described before. Different “clean to dirty” and “dirty to clean” routes should be organized according to local space availability. To avoid mistakes and to get acquainted with dressing and undressing procedures, visual warning by green, yellow and red signs can be put in place.
Furthermore, separate recovery rooms are required with the usual distinction of low-risk patients, high-risk patients and Covid-19 patients.
When it comes to the management of patients in the waiting room, adequate social distancing must be ensured (at least 2-3 meters).
When feasible, adequate natural ventilation and open windows can help reducing the environmental viral load build-up, [23], [32], [34].
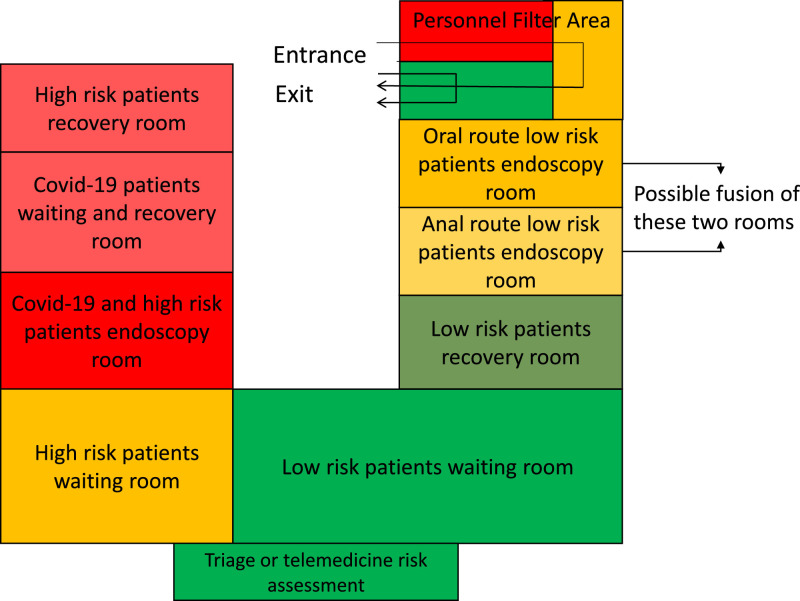
In Fig. 2 a possible subdivision of the endoscopic spaces is reported.
Fig. 2.
Example of space management for the Endoscopy Unit. Viral load and theoretical risk are depicted with a colour scale where red areas are those with a sure high viral load and yellow/green the ones with an estimated intermediate/lower viral load. Yellow areas are intermediate-risk areas. A Covid-19 waiting room is not planned because we assume that they are inpatients or on quarantine. However, a Covid-19 recovery room can be considered as a waiting room, when needed.
6. Reprocessing of flexible endoscopes and endoscopic accessories
No particular precautions should be additionally considered when disinfecting flexible endoscopes. Standard techniques as described in the American and European guidelines are sufficient to deal with minute viral particles. Although no data is still available for SARS-CoV-2, to the best of our knowledge there are no cases in the literature mentioning SARS contamination through infected endoscopic devices. Virucidal standard products are considered capable to handle the SARS-CoV-2 viral load.
The healthcare professionals in charge of disinfection should wear PPE as if treating Covid-19 patients [31], [35].
7. Video-capsule endoscopy
Video-capsule endoscopy may be (erroneously) considered a low-risk procedure, since aerosol are not generally produced. However, it is well known that patients during capsule swallowing can cough. Thus, we recommend the use of PPE: minimal PPE (light-fabric gown, goggles, surgical mask, double pair of gloves) while assisting low-risk patients and full PPE when managing high-risk/Covid-19 patients.
Adequate spaces for VCE administration should be arranged (in a well-ventilated area). Patients should be asked to wear PPE as already described before and, whenever possible, should swallow the capsule alone in a separate area.
When using VCE with a recorder (e.g. PillCam-3), it is suggested to wrap the recorder and its belt in plastic and secure the lot with tape.
Accurate disinfection of the instruments with appropriate products is then recommended.
8. Conclusions
With the present manuscript we aim to support endoscopy units in their daily practice during the current Covid-19 outbreak. We are conscious about the peculiarity of the moment and that many of the above described “ideal” instructions can be modified according to the local availability and quality of resources. Also, we acknowledge that only part of these indications is characterized by strong levels of evidence, and many rather are the result of discussion among experts, who have been facing these dramatic changes in everyday's activities. Nevertheless, we believe that this paper, as derived from the experience gained during these last weeks, can help colleagues dealing with SARS-CoV-2, while keeping in mind that, never like today, “tomorrow is another day”.
Declaration of Competing Interest
All the authors declare no conflict of interest.
Acknowledgments
The authors wish to thank Fondazione IRCCS Ca’ Granda for the permission to publish the dressing/undressing videos and Mr Mark Hinxmann for English revision.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dld.2020.04.018.
Appendix. Supplementary materials
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun K., Chen J., Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit Heal. 2020:7500. doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan. China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med2020:2019–20. 10.1056/nejmc2001468. [DOI] [PMC free article] [PubMed]
- 5.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020:19–20. doi: 10.1056/nejmoa2001316. Online pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston E.R., Habib-Bein N., Dueker J.M., Quiroz B., Corsaro E., Ambrogio M. Risk of bacterial exposure to the endoscopist's face during endoscopy. Gastrointest Endosc. 2019;89:818–824. doi: 10.1016/j.gie.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. E-pub ahea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J., Han B., Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.054. E-pub ahea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg S.B. Update on Human Rhinovirus and Coronavirus Infections. Semin Respir Crit Care Med. 2016;37:555–571. doi: 10.1055/s-0036-1584797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Peng F, Wang R, Guan K, Jiang T, Xu G, et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun2020:102434. 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed]
- 13.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 16.Yang Y, Yang M, Shen C, Wang F, Yuan J, Li J, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv2020:2020.02.11.20021493. 10.1101/2020.02.11.20021493. [DOI]
- 17.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;2019 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med2020:1–13. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 19.Seda G., Parrish J.S. Augmenting Critical Care Capacity in a Disaster. Crit Care Clin. 2019;35:563–573. doi: 10.1016/j.ccc.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Satkoske V.B., Kappel D.A., DeVita M.A. Disaster Ethics: Shifting Priorities in an Unstable and Dangerous Environment. Crit Care Clin. 2019;35:717–725. doi: 10.1016/j.ccc.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum L. Facing Covid-19 in Italy - Ethics, Logistics and Therapeutics on the Epidemic's Front Line. N Engl J Med2020:1–3. [DOI] [PubMed]
- 22.Rolston D.M., Meltzer J.S. Telemedicine in the Intensive Care Unit: Its Role in Emergencies and Disaster Management. Crit Care Clin. 2015;31:239–255. doi: 10.1016/j.ccc.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization Rational use of personal protective equipment for coronavirus disease (COVID-19) Interim guidance. 27 February 2020:2020. [Google Scholar]
- 24.Repici A., Maselli R., Colombo M., Gabbiadini R., Spadaccini M., Anderloni A. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020 doi: 10.1016/j.gie.2020.03.019. Journal Pr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol2020:24–7. 10.1002/jmv.25728. [DOI]
- 26.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Articles Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan. China : a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L., Mu M., Ren H.G., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China : a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000620. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo M.-Z., Huang Y.-G., Ma W.-H., Xue Z.-G., Zhang J.-Q., Gong Y.-H. Expert Recommendations for Tracheal Intubation in Critically ill Patients with Noval Coronavirus Disease 2019. Chinese Med Sci J = Chung-Kuo i Hsueh k'o Hsueh Tsa Chih. 2020:1–9. doi: 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolkan H.A., Van Duinen A., Samai M., Bash-Taqi D.A., Gassama I., Waalewijn B. Admissions and surgery as indicators of hospital functions in Sierra Leone during the west-African Ebola outbreak. BMC Health Serv Res. 2018;18:1–9. doi: 10.1186/s12913-018-3666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderwood A.H., Day L.W., Muthusamy V.R., Collins J., Hambrick R.D., Brock A.S. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Escombe A.R., Oeser C.C., Gilman R.H., Navincopa M., Ticona E., Pan W. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:0309–0317. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escombe A.R., Ticona E., Chávez-Pérez V., Espinoza M., Moore D.A.J. Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infect Dis. 2019;19:1–7. doi: 10.1186/s12879-019-3717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J.Y., Wong E.W., Wayne L. Practical Aspects of Otolaryngologic Clinical Services During the 2019 Novel Coronavirus Epidemic An Experience in Hong Kong. JAMA Otolaryngol Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0488. Published. [DOI] [PubMed] [Google Scholar]
- 35.Beilenhoff U., Biering H., Blum R., Brljak J., Cimbro M., Dumonceau J.M. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) - Up. Endoscopy. 2018;50:1205–1234. doi: 10.1055/a-0759-1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.