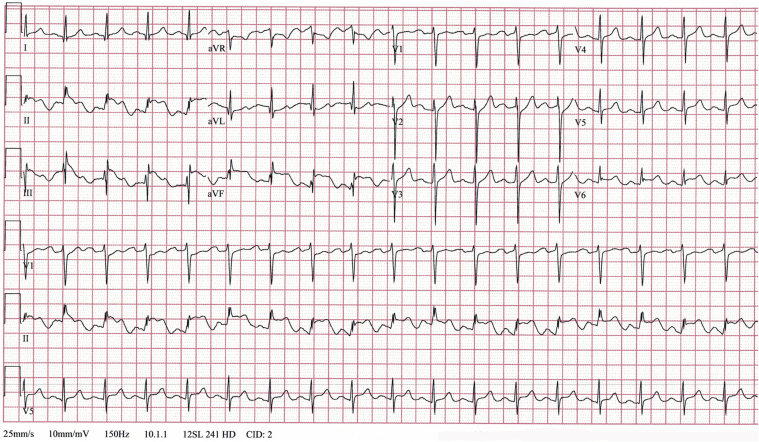
Abstract
A 29-year-old man tested positive for COVID-19 and developed acute respiratory distress syndrome. While mechanically ventilated, his electrocardiogram showed inferior ST-segment elevations, with normal serial cardiac troponin I and transthoracic echocardiograms. He was treated conservatively, with complete clinical recovery and resolution of his electrocardiographic abnormalities. (Level of Difficulty: Beginner.)
Key Words: acute myocardial infarction, COVID-19, myocardial injury, ST-segment elevation
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; CT, computed tomography; cTnI, cardiac troponin I; CVD, cardiovascular disease; ECG, electrocardiogram
Graphical abstract
A 29-year-old man tested positive for COVID-19 and developed acute respiratory distress syndrome. While mechanically ventilated, his electrocardiogram…
History of Presentation
A 29-year-old, morbidly obese man (body mass index, 42 kg/m2) presented to an emergency care center after 8 days of fever (up to 103°F), myalgias, nonproductive cough, sore throat, and malaise. He had been in contact with a family member with upper respiratory symptoms who had recently traveled by airplane. While receiving symptomatic treatment, the patient returned to the urgent care facility twice, with reports of worsening dyspnea. His physical examination revealed a young man in obvious respiratory distress, with mild peripheral cyanosis, and corresponding vital signs: respiratory rate, 29 breaths/min; oxygen saturation, 89% with supplemental oxygen flow rate of 4 l/min; temperature, 98.8°F; blood pressure, 116/88 mm Hg; and heart rate, 104 beats/min. Lung auscultation revealed bilateral fine crackles and no evidence of consolidation, whereas the rest of his examination was normal. While in the emergency department, the patient required mechanical ventilation for rapidly progressing respiratory failure.
Learning Objectives
-
•
Early and rapid testing is critically necessary in patients with suspected COVID-19 to prevent severe evolution.
-
•
ECG ST-segment elevations in inferior leads have been described in several COVID-19 patients, with variable clinical significance.
-
•
An accurate evaluation of the true incidence of acute myocardial injury related to COVID-19 requires a standardized definition, which should include a combination of ECG changes, biochemical markers, and imaging abnormalities.
Past Medical History
The patient had no significant medical history. He never smoked. There was no family history of cardiovascular disease (CVD).
Differential Diagnosis
An early viral panel polymerase chain reaction was negative for multiple respiratory viruses. A severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) nucleic acid amplification test returned a positive result 5 days after admission. In the absence of any cardiovascular risk factors, the cause of the ST-segment elevations was initially interpreted as evidence of coronavirus disease-2019 (COVID-19)–related myocarditis.
Investigations
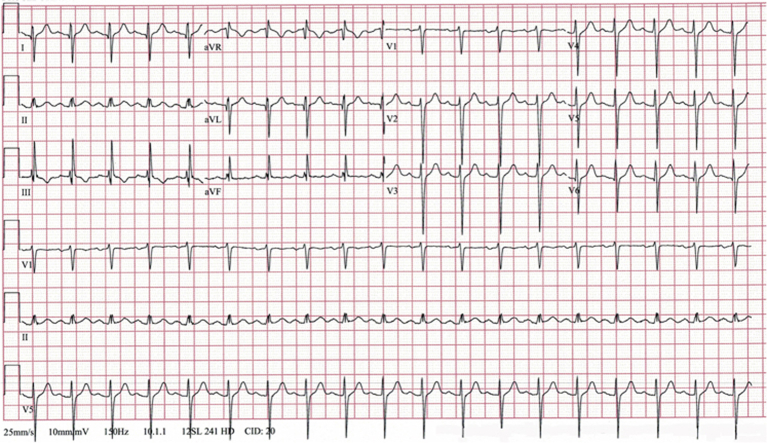
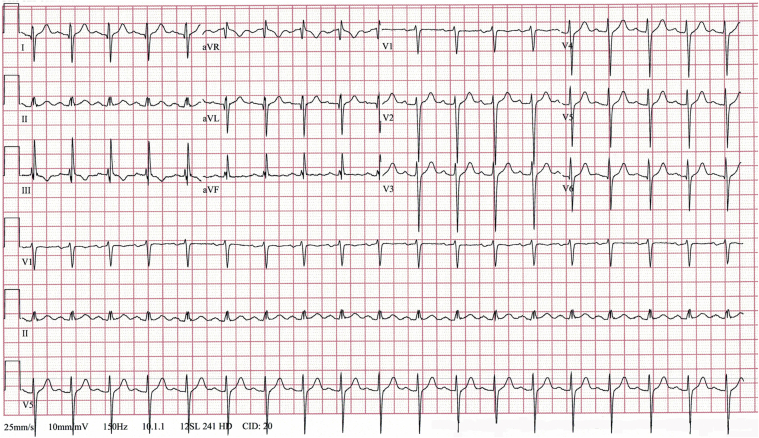
A chest computed tomography (CT) study showed bilateral, multifocal airspace opacities. An initial ECG (Figure 1) showed sinus tachycardia and marked right QRS axis deviation. Over the following 48 h, while the patient was acidotic (pH, 7.27; base excess, −4 mmol/l), he developed marked ST-segment elevations in leads II, III, aVF, and V6 (Figure 2). Serial cardiac troponin I (cTnI) and myoglobin levels remained normal (<0.02 ng/ml, and 55.0 ng/ml, respectively). The creatine kinase-myocardial band (CK-MB) was initially elevated (2,034 U/l), and it normalized over the subsequent 72 h. Repeated transthoracic echocardiograms showed normal heart chamber size, no regional wall motion abnormalities, and no pericardial effusion.
Figure 1.
Initial Electrocardiogram
The electrocardiogram shows sinus tachycardia of 112 beats/min and marked QRS right axis deviation of 127°.
Figure 2.
Electrocardiogram After 48 h of Mechanical Ventilation
The electrocardiogram shows sinus tachycardia of 107 beats/min and marked ST-segment elevation in leads II, III, avF, and V6.
Management
Given the very low pre-test probability for coronary artery disease and the absence of coronary calcifications on the chest CT scan, a coronary CT angiogram was not indicated, and the patient was not referred for invasive coronary angiography. The patient was treated conservatively, without thrombolytic agents or initiation of the acute coronary syndrome management protocol.
Discussion
The COVID-19 pandemic represents the largest worldwide health care challenge to date. Limited but rapidly emerging data have documented the role of CVD in increasing both the risk of infection and the severity of its clinical presentation (1, 2, 3, 4). In particular, CVD is associated with a sharp increase in overall mortality, which reaches almost 20% of patients hospitalized (5). However, although such an association can be anticipated to a certain degree (on the basis of existing data from previous outbreaks of influenza and severe acute respiratory syndrome), the incidence of myocardial injury in COVID-19 infection appears to be higher (6). Furthermore, the definition of COVID-19–associated “myocardial injury” lacks standardization and is based primarily on elevated (and highly variable) serum levels of cardiac-specific troponins as the single most common defining markers. This myocardial injury has been associated with evidence of left ventricular systolic dysfunction and arrhythmia (2,5,7,8).
In contrast, our case demonstrates the presence of major, isolated ECG abnormalities in a patient without any other commonly accepted evidence of “myocardial injury.” This includes normal results of serial measurements of cTnI and repeated transthoracic echocardiogram studies and no arrhythmia other than sinus tachycardia. Accepting electrocardiographic ST-segment elevation as representative of “myocardial injury” may be conceptually tempting for the diagnosis of cardiac involvement related to COVID-19 infection. However, the cautious practitioner will remember the poor specificity of ST-segment elevation, which is encountered in a variety of conditions that mimic acute myocardial infarction. To complicate the matter further, serum cTnI can also be elevated in a variety of noncardiac conditions, including sepsis and critical illness.
To our knowledge, this is the first reported COVID-19 patient to demonstrate acute, dynamic ST-segment elevations in inferior leads that were, in our opinion, neither ischemic nor representing true “myocardial injury” as defined by biochemical markers or echocardiographic changes. We did not interpret the isolated elevation of low-specificity CK-MB as evidence of a diagnosis of myocarditis in the context of severe illness, sepsis, and acidosis, with normal myoglobin. Right ventricular supply-demand mismatch was also ruled out by normal serial cTnI serum values. We believed that the hypothesis of nonischemic ECG changes was further supported by the absence of gross coronary calcifications on the chest CT, a finding associated with very high negative predictive value for coronary artery disease. Therefore, we attributed the ECG changes to acute, severe right ventricular strain in the setting of COVID-19–related acute respiratory distress syndrome, a phenomenon described by anecdotal reports of pulmonary embolism.
Worldwide, as of this writing, there are only 2 reports of COVID-19 patients who presented with acute ST-segment elevations, and for both patients the ECG changes were present in the inferior leads (9,10). In each case, a diagnosis of myocarditis was supported by elevated cardiac troponins, a moderate decrease of left ventricular ejection fraction, and the absence of flow-limiting coronary artery disease by invasive coronary angiography. In 1 of these cases, the diagnosis of myocarditis was complemented by the presence of myocardial edema by cardiac magnetic resonance imaging, as a marker of acute myocardial injury (9). Our patient, although critically ill and mechanically ventilated, maintained hemodynamic stability and a normal left ventricular ejection fraction, and this patient had no evidence of cardiac arrhythmias. As such, we felt comfortable recommending medical management and chose not to pursue invasive coronary angiography or any additional imaging tests, including a coronary CT angiogram. We perceived these tests to be not indicated in this clinical context and to have insufficient potential to alter further management decisions. Moreover, any additional imaging tests would have unnecessarily increased the health care staff exposure, a risk that we have actively tried to diminish.
Follow-Up
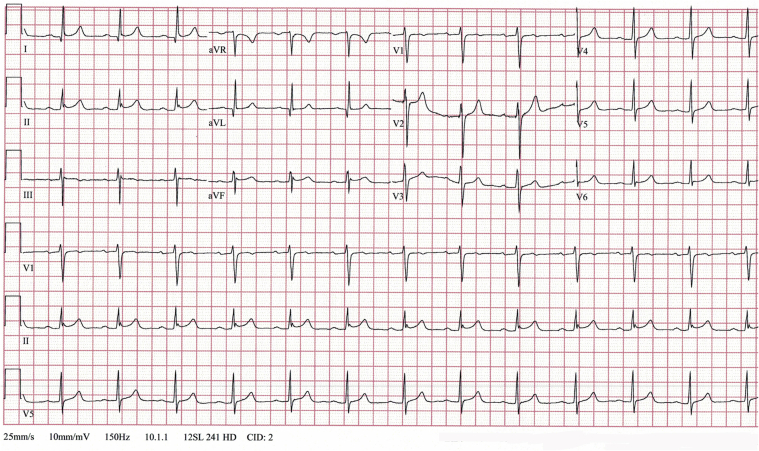
The evolution of our patient was favorable with vigorous correction of his metabolic abnormalities. An ECG 48 h later showed nearly complete resolution of the ST-segment elevations (Figure 3). Following continuous improvement of his respiratory status, he was successfully extubated. Two weeks after hospital discharge, the patient was interviewed by telephone and confirmed a complete clinical recovery.
Figure 3.
Follow-Up Electrocardiogram
The electrocardiogram shows sinus rhythm of 77 beats/min, normalization of the QRS axis at 7°, and nearly complete resolution of ST-segment elevation.
Conclusions
COVID-19 infection is associated with a high rate of cardiac complications, resulting in acute systolic heart failure and arrhythmias, which contribute to a marked increase in mortality. ECG changes may mimic an acute myocardial infarction, and these changes must be interpreted in the clinical context for a consistent diagnosis and to determine the true incidence of “myocardial injury” related to COVID-19 infection.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Bonow R.O., Fonarow G.C., O’Gara P.T. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1105. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang V., Jin Z. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0934. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:e200950. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M., Safavi-Naeini P.S., Solomon S.D. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term complications. Eur Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1017. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inciardi R., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020:ehaa190. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]