Abstract
The coronavirus disease 2019 (COVID-19) pandemic is posing insurmountable challenges to healthcare systems globally. Cancer therapy is complex, and outcomes are centered on timing. Many oncology societies and health ministries have issued guidelines for cancer care to enable oncologists and patients to navigate the crisis. Lessons learned should inform care models for future pandemics.
Keywords: cancer, COVID-19, pandemic, research, chemotherapy, immunotherapy
Unprecedented Challenges Need Unprecedented Solutions
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in COVID-19, is posing insurmountable challenges to healthcare systems globally. Cancer therapy is complex, regimented, and outcomes are centered on timing. COVID-19 throws a gauntlet to an already strained systemi. Given the extrinsic factors (e.g., resource constraints and high morbidity/mortality) of the COVID-19 pandemic and the intrinsic factors of patients with cancer (e.g., high-risk population, lower immune system, and many at advanced stage of disease), practitioners face great responsibilities in ensuring timely, appropriate, compassionate, and justified cancer care, while protecting themselves from becoming infected with COVID-19 [1]. The reported prevalence of cancer among patients with COVID-19 (1%) appears to be higher than its prevalence in the overall population [2]. Early data from two small, heterogeneous populations [3] showed that 39–54% of patients with cancer were reported to have a severe event (admission to intensive care unit, or death) when infected with COVID-19 [2,4]. Receiving antitumor therapy or surgery within 2–4 weeks of developing symptoms [2,4] predicted worse outcomes.
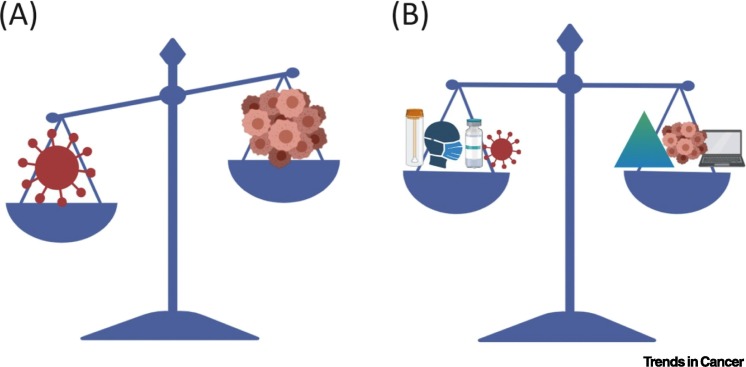
Oncology often requires a complex set of clinic visits, infusion sessions, surgical stays, radiation therapy appointments, hospital admissions, laboratory blood draws, and imaging studies. Furthermore, patients with cancer need caregiver support. Collectively, caring for patients with cancer requires a large number of personal contact points, which means many potential opportunities for viral transmission. The challenges imposed by COVID-19 impact every aspect of care, starting with diagnosis all the way to end-of-life care for patients, which raises concerns about patients receiving suboptimal care (Figure 1 ). To enable oncologists to navigate the COVID-19 public health crisis, all major oncology societies [e.g., American Society of Clinical Oncology (ASCO)ii, European Society of Medical Oncology (ESMO)iii, and American Society of Hematology (ASH)iv] and health ministries have formed task-forces and issued resources and guidelines [5]. The ASCO, ESMO, and ASH websites provide a comprehensive set of ‘frequently asked questions and answers’ based on best-level evidence available. In many cases, since there is no evidence, guidelines are based on consensus and committee recommendations. Most of them refer to CDC guidelines, which are constantly being updated. Comprehensive cancer centers have also published their own strategies to maintain cancer care during the COVID-19 outbreak. Here, we summarize potential solutions to these challenges, incorporating some of the major society guidelines (Figure 1).
Figure 1.
Restoring the Balance for Cancer Care in the Coronavirus Disease 2019 (COVID-19) Era.
(A) COVID-19 challenges the existing paradigm for cancer care. (B) Expanded testing, social distancing, vaccine programs and new therapies for COVID19, telemedicine, and prioritizing certain cancer therapies are suggested modalities to restore the balance of care.
Impact on Diagnosis and Workup
With no specific vaccine or specific antiviral therapy for COVID-19 currently available in the clinic, the best we can do for patients with cancer is to prevent them from contracting COVID-19. As a community, we should aim to slow down the spread of COVID-19 and ‘flatten the curve’. Slower spread would avoid overwhelming the health system and allow high-risk patients with cancer to receive necessary routine medical services. To achieve this goal, elective imaging, diagnostic biopsies, and/or procedures have to be prioritized for certain patients (e.g., symptomatic patients in a metastatic setting, and certain histologies in localized disease settings [6]) to avoid compromising chances of cure.
Impact on Treatment and Surveillance
How do we manage patients with cancer and COVID-19? Currently, there is no evidence to support changing or withholding chemotherapy or immunotherapy in these patients. However, since patients with cancer are uniquely vulnerable to the virus due to a weaker immune system, it is reasonable to withhold or postpone therapy until the patient is asymptomatic. Moreover, similarities between treatment related-adverse events and COVID-19 symptoms (e.g., fever, pneumonitis, and colitis) are challenging in patients receiving active cancer treatment. Since we do not know whether patients on immunotherapy are at a higher risk for pneumonitis or cytokine storm, streamlining COVID-19 testing with shorter turnaround time and using steroid-sparing strategies in managing immune-related adverse events can help mitigate the impact of COVID-19.
Other strategies to treat patients with cancer during the COVID-19 pandemic include splitting healthcare teams [7], implementing tele-multidisciplinary tumor boards, minimizing myelosuppressive agents, expanding the use of personal protective equipment (PPE), limiting visitors, scanning patients thermally, using oral therapy options when applicable, delaying therapy when in an adjuvant or palliative setting, and allowing certain deviations or violations in protocol patients. Splitting healthcare teams is a strategy where the staff is geographically segregated into two teams: clinical (each clinic team has its own registration, triage, venipuncture, consultation room, and lavatories) and nonclinical [7] to facilitate contact tracing and minimize cross-contamination. Furthermore, limited surgical and medical staffing has led to prioritization of surgery based on histology [1], neoadjuvant therapy, and curability of disease. In a similar fashion, radiation oncologists can modify the course of radiation to reduce the number of visits. In addition, shortages in blood products in certain parts of the USA mean that oncologists should minimize the use of myelosuppressive regimens and lower the threshold for transfusion when possible. In the USA, the Food and Drug administration (FDA) revised its recommendations to address the unprecedented challenge to blood supply due to COVID-19v. In the end, clinical decisions should be individualized to consider factors, such as the risk of cancer relapse if treatment is modified, the number of cycles already completed, and the patient's tolerance of therapy.
In diseases such as acute myeloid leukemia, where intensive chemotherapy is considered emergent, testing all patients for COVID-19 before the initiation of induction and delaying or de-intensifying treatment for patients who test positive for COVID-19 has been recommended. Healthcare workers taking care of patients with cancer must be uninfected with COVID-19, pass screening for COVID-19 (questionnaire and thermal scan), and follow strict PPE guidelines. Several efforts have aimed at addressing the increasing shortage of PPEs, including increasing supply, rationing, and reutilization (± sterilization). A challenge facing providers is that being asymptomatic does not necessarily mean one is not currently infected with COVID-19 [8] and cannot infect cancer patients. Therefore, viral testing with PCR could allow for periodic staff monitoring ensuring that staff are infection free. Prioritizing healthcare workers on high-risk units (bone marrow transplant, and acute leukemia) could address the caveat of limited testing availability. Another aspect of testing is serological testing (i.e., IgG and IgM antibodies), which hypothetically detects established immunity [9,10] but faces several challenges, including selection of the right testing antigen, the ability of antibodies to neutralize the virus, and their ability to prevent reinfection. Over 70 test developers claim to have developed an effective test. However, to date, the FDA has issued only one Emergency Use Authorization for a qualitative COVID-19 serological testingvi. Using the analogy of hepatitis B vaccination and surface antibody testing, maturation of antibody testing will help the development of an effective vaccine.
Patients with cancer frequently travel long distances for access to major cancer centers. Location of the patient and the cancer center, and travel all pose serious risk. They may require quarantine if they are exposed to COVID-19. In addition, patients with cancer are known to have intermittent and prolonged shedding of respiratory viruses [11]. To avoid risk of harm to themselves, they may require a longer period of quarantine compared with the general population. Stopping travel and working with local oncologists to treat patients at a local hospital could avoid unnecessary exposure to COVID-19. Other potential solutions would be to reschedule surveillance imaging and rely on telemedicine for clinical surveillance (i.e., clinical history). In addition to analyzing patients with cancer who are infected with COVID-19, the collateral damage of COVID-19 in those patients who were not infected with COVID-19, yet might have received suboptimal care due to having restaging scans rescheduled, cancelled, or postponed, is worth analyzing for future reference.
Impact on End-of-Life Care
Sensitive discussions of end-of-life, progression of disease, and advance directives are critical in oncology and, because patients with COVID-19 are not allowed to have visitors, it becomes vital to document advanced care planning for each patient, sometimes aided by the use of telehealth. Now is the time to ensure that patients do not receive care they would not want if they become too severely ill to make their own decisions [12]. Use of videoconferencing for bad news, rather than using the phone, allows for better body language interpretation and effective communication. To compensate for the lack of in-person physician–patient encounters, implementing frequent telemedicine visits with very short-term follow-up will likely help avoid miscommunication.
Impact on Cancer Research
An indirect impact of COVID-19 on cancer care is its impact on travel and the need for social distancing. Basic science labs are closed, with all experiments halted. Hundreds of cancer clinical trials have been placed on hold by sponsors and academic institutions. Translational science to advance bench to bedside and back research will suffer major setbacks. Major cancer conferences have been cancelled and/or rescheduled. Hence, data that are critical in advancing cancer care and research may be delayed. With overflowing hospitals and strained healthcare workers, newly diagnosed and existing patients with cancer may not get the right treatment at the right time. As an interim solution, several institutes and societies are holding virtual conferences so that findings can still be discussed and disseminated. In addition, collaborators are discussing future studies via online platforms. However, remote access and virtual meetings may not be able to replace the research is being lost by closing wet labs and halting clinical trials.
COVID19, Cancer, and Telemedicine: A Silver Lining
Despite the challenges detailed earlier, the COVID-19 experience is poised to alter how patients with cancer are cared for now and in the future. This crisis has opened new doors for a telemedicine initiative in an unprecedented way. The rapid adoption of telehealth visits, virtual check-in services, e-visits, remote care management, and remote patient monitoring aid in patient care management not only for patients living in remote locations, but also for patients with cancer who are immunosuppressed and have to social distance. Furthermore, COVID-19 in cancer research is advancing at a breakneck speed, and tele-multidisciplinary tumor boards have broadened collaborations across the globe, for challenging cancer cases as well as for research. In addition, analyzing the ‘real-world’ outcomes of patients who received modified and/or alternate cancer care schedules (e.g., chemotherapy, surgery, and radiation) will allow us to identify patient cohorts who may benefit from modified treatment protocols. Results of such analyses could be then validated through prospective studies. Next-generation clinical operations could use all the invaluable lessons learned when switching from ‘pandemic mode’ to ‘normal operations’ to improve efficiency, flexibility, anticipate risks, and enhance resilience.
Concluding Remarks
The dynamic healthcare response to COVID-19 highlights the focus on protecting patients with cancer and healthcare workers as well as reducing the impact of COVID-19 on the community. Although the current emphasis is on managing COVD19, the focus in the near future centers on the recovery plan and restoration of the balance of cancer care in the era of COVID-19 and beyond (Figure 1). Undoubtedly, data gathered from caring for patients with cancer during COVID-19 will help create care models and predictors for high-risk populations during future epidemiological outbreaks or pandemics.
Resources
iwww.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19)iiwww.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19iiiwww.esmo.org/covid-19-and-cancerivhttps://hematology.org/covid-19vwww.fda.gov/regulatory-information/search-fda-guidance-documents/revised-recommendations-reducing-risk-human-immunodeficiency-virus-transmission-blood-and-bloodviwww.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-serological-testsReferences
- 1.Masumi U. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J. Natl. Compr. Cancer Netw. 2020 doi: 10.6004/jnccn.2020.7560. Published online March 20, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Liang W. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia Y. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21 doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. Published online March 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You B. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21:619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutikov A. A war on two fronts: cancer care in the time of COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/M20-1133. Published online March 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngoi N. A segregated-team model to maintain cancer care during the COVID-19 outbreak at an academic center in Singapore. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.306. Published online March 31. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009316. Published online April 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen C. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson L. Comparison of respiratory virus shedding by conventional and molecular testing methods in patients with haematological malignancy. Clin. Microbiol. Infect. 2016;22:380.e1–380.e7. doi: 10.1016/j.cmi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis J.R. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19) JAMA. 2020 doi: 10.1001/jama.2020.4894. Published online March 27, 2020. [DOI] [PubMed] [Google Scholar]