Abstract
Purpose: Acute pulmonary embolism (PE) remains a significant cause of morbidity and requires prompt diagnosis and management. While non-surgical approaches have supplanted surgery as primary treatment, surgical pulmonary embolectomy (SPE) remains a vital option for select patients. We review the current management of acute PE, with a focus on surgical therapy.
Methods: A PubMed search was performed to identify literature regarding PE and treatment. Results were filtered to include the most comprehensive publications over the past decade.
Results: PE is stratified based on presenting hemodynamic status or degree of mechanical pulmonary arterial occlusion. Although systemic or catheter-guided fibrinolysis is the preferred first-line treatment for the majority of cases, patients who are not candidates should be considered for SPE. Studies demonstrate no mortality benefit of thrombolysis over surgery. Systemic anticoagulation is a mainstay of treatment regardless of intervention approach. Following surgical embolectomy, direct oral anticoagulants (DOACs) have been shown to reduce recurrence of thromboembolism.
Conclusions: Acute PE presents with varying degrees of clinical stability. Patients should be evaluated in the context of various available treatment options including medical, catheter-based, and surgical interventions. SPE is a safe and appropriate treatment option for appropriate patients.
Keywords: pulmonary embolism, fibrinolysis, catheter thrombolysis, surgical pulmonary embolectomy
Introduction
Acute pulmonary embolism (PE) is a significant cause of mortality worldwide, with over 100,000 deaths in 2018 alone.1) It is the third most common cause of cardiovascular death among hospitalized patients in the Western world following acute myocardial infarction and stroke.2,3) Early diagnosis and intervention are paramount as most deaths from acute PE occur within the first several hours to days, with over 70% of deaths occurring within the first hour.4,5) The most common risk factor for PE is a history of prior deep vein thrombosis (DVT).2) However, patients with underlying malignancy, those who have undergone a recent surgery, and those with history of hypercoagulability, such as factor V Leiden, also pose a greater risk of PE than the general population.6) These risk factors can be explained based on the pathogenesis of the DVT, commonly described using the Virchow’s Triad criteria: stasis of blood, hypercoagulability, and endothelial vessel wall injury. Other risk factors that have been linked to acute PE include history of prolonged immobilization, advanced age, obesity, smoking, stroke, congestive heart failure, respiratory failure, sepsis, irritable bowel disease, pregnancy, hormone replacement therapy, and oral contraceptive use.2)
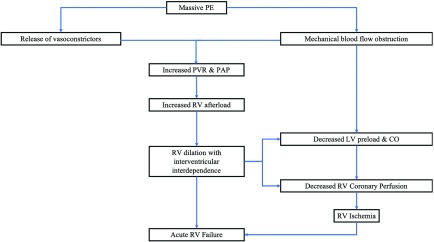
Although there are several classification systems, acute PE is defined as massive or submassive. The presence of any of the following criteria defines massive PE: hypotension or shock that results from right heart failure or cardiovascular collapse, a thrombus which occludes greater than 50% of the pulmonary artery (PA) cross-sectional area or occludes two or more lobar arteries, or if the patient is dependent on inotropic agents.2,6–9) The pathophysiology of massive PE, summarized in Fig. 1, comprises a series of events that, if not treated rapidly, can lead to hemodynamic instability, right heart failure, cardiogenic shock, cardiac arrest, and death within minutes to a few hours after the event.7,10–12) Massive PE accounts for approximately 4.5%–10% of all PE cases and carries substantial morbidity and mortality that exceeds 50%.1,2) Submassive PE is characterized by less severe clinical findings. These patients are often hemodynamically stable (systolic blood pressure >90 mmHg) but show signs of right heart strain, dysfunction, or injury on echocardiogram or by laboratory biomarkers.7) Common echocardiographic findings of submassive PE include right ventricular (RV) systolic dysfunction, RV dilation, or a RV/left ventricular (LV) diameter ratio of >0.9 on four chamber view. Laboratory studies found in submassive PE include BNP >90 pg/mL, N-terminal pro BNP >500 pg/mL, troponin I >0.4 ng/mL, or troponin T >0.1 ng/mL.9) RV injury can be demonstrated on electrocardiogram (ECG) with new right bundle branch block or anteroseptal ST elevations, ST depressions, or T-wave inversions.7)
Fig. 1. Series of events after massive PE causing acute right ventricular heart failure. CO: cardiac output; LV: left ventricular; PAP: pulmonary artery pressure; PE: pulmonary embolism; PVR: pulmonary vascular resistance; RV: right ventricular.
Early recognition of acute PE allows for earlier diagnostic and therapeutic management. While the presentation of acute PE can ranges from asymptomatic to sudden death, nearly 81% of patients will present with dyspnea, 70% with tachycardia, and 50% with hypoxia.6) Other common initial symptoms include pleuritic chest pain, syncope, hypotension, and hypocapnia.4) Contrast-enhanced computed tomography (CT) and transesophageal echocardiography (TEE) have 90%–95% sensitivity and 100% specificity for diagnosis of acute PE.13) TEE has the added benefit of being able to simultaneously assess both RV function and intracardiac thrombi. Ultrasound of the lower extremities should also be performed to identify DVT. CT pulmonary angiography is considered the gold standard of diagnosis for acute PE, although contrast-enhanced CT is the more commonly performed diagnostic test.8)
Therapeutic options for PE have been investigated for over a century, yet an established therapy algorithm has not been established due to rapid advances in surgical and non-surgical approaches. Currently available treatment strategies for acute PE include systemic anticoagulation, catheter-based fibrinolysis, systemic fibrinolysis, and surgical pulmonary embolectomy (SPE). This review provides an overview of the current management of acute PE, with a focus on surgical options.
Methods
We performed a search using the PubMed/MEDLINE database with the key terms “acute pulmonary embolism,” “massive pulmonary embolism,” “submassive pulmonary embolism,” “surgical pulmonary embolectomy,” and “fibrinolysis and pulmonary embolectomy.” We aimed to narrow our reference list to those which included multi-institutional studies when feasible. Several single-institution studies were included if they included either a substantial number of patients or presented novel treatment options or algorithms.
Therapeutic Approaches
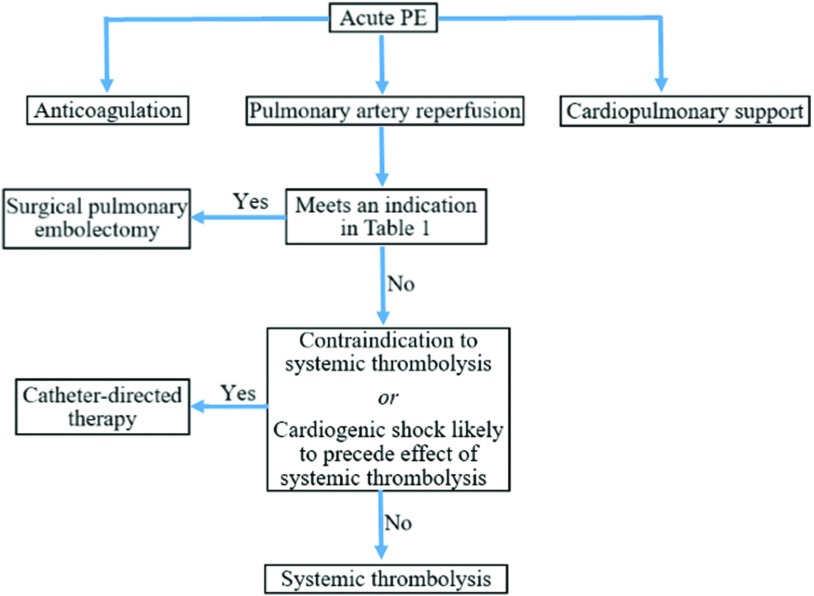
The treatment approach for acute PE should always consists of three major components: cardiopulmonary support, anticoagulation to prevent extension and recurrence, and reperfusion of the PA. Cardiopulmonary support should first be initiated with methods such as supplemental oxygen and inotropic agents. If the RV fails to respond appropriately with inotropes, then the initiation of more aggressive adjunctive measures such as surgery or extracorporeal membrane oxygenation (ECMO) should be strongly considered. Anticoagulation, usually with heparin, should be initiated as soon as PE is suspected unless the patient has a strong contraindication. Finally, reperfusion of the PA should be achieved expeditiously (Fig. 2).4,9)
Fig. 2. Acute PE reperfusion algorithm. PE: pulmonary embolism.
More than 70% of patients with massive PE receive advanced therapies for reperfusion of the PA, including systemic thrombolysis, catheter-directed thrombolysis (CDT), and SPE. Most patients can be successfully treated with non-surgical therapeutic interventions; the most frequently used is systemic thrombolysis, which has shown to decrease mortality rates to 2.4% in patients suffering from a massive PE.12) Systemic thrombolysis, however, carries concerns about bleeding, unnecessary systemic exposure of the thrombolytic agent, and length of time before treatment effectiveness (usually hours). Systemic thrombolysis generally requires tissue plasminogen activator (t-PA) infusion of 50–100 mg intravenously (IV) over 1–2 hours and carries a 20% risk of major bleeding and a 2%–5% risk of hemorrhagic stroke.14) These limitations, along with technological advances, led to the development of CDT which can target thrombolytic agent administration directly into the pulmonary arterial system. Indications for CDT in massive PE include contraindications to or failed systemic thrombolysis, or cardiogenic shock that is likely to cause death before systemic thrombolysis can take effect.
CDT is generally performed by infusing t-PA at a rate of 0.5–1 mg/h under ultrasound or fluoroscopic guidance.14–17) Kuo et al. conducted a prospective multicenter study of CDT for acute PE in 101 patients. In this study, clinical success was defined as stabilization of hemodynamics, improvement of pulmonary hypertension or right-sided heart strain, and survival to hospital discharge. In all, 28 of the 101 patients suffered from massive PE and immediate CDT achieved an 85.7% clinical success rate in this population with no major hemorrhagic complications.17) Piazza and colleagues reported the results of the SEATTLE II study, a prospective, multicenter trial of CDT involving 150 patients throughout 22 states.14) All patients had massive or submassive PE less than 14 days old with a RV to LV diameter ratio of at least 0.9. At 48 hours after CDT infusion, there was a 25% decrease in the RV/LV diameter, a 30% decrease in PA obstruction, and a 30% decrease in PA systolic pressure. No patients suffered fatal or intracranial hemorrhage, but there was a 10% incidence of major bleeding events within 30 days after the procedure. These outcomes suggest that at experienced centers, CDT may be an appropriate and effective primary treatment strategy for acute PE.
Surgical Pulmonary Embolectomy
Historical perspective
Friedrich Trendelenburg was the first to report SPE in 1908 at the 37th annual Congress of the German Surgical Association.18) He operated on three patients with acute PE and successfully extracted the thrombus, but no patient survived. His initial approach to SPE involved exposing the entire heart, making an incision in the conus arteriosus of the RV, and aspirating emboli from the PA with a veterinary syringe. His technique evolved to a small opening in the chest directly over the PA, occluding the aorta and main PA, and extracting emboli with forceps. In 1924, Martin Kirschner performed the first successful SPE on a 38-year-old woman who had collapsed on postoperative day 3 after an inguinal hernia repair.18) For historical context, heparin was first used for prophylaxis and treatment of venothromboembolism (VTE) in 1936.4) Over the course of the century, as technique improved, so did patient outcomes. Arguably, the most important development was that of the cardiopulmonary bypass (CPB) machine. The first successful SPE utilizing CPB was in 1961, more than 100 years after Trendelenburg’s initial attempts.18)
Indications
The most recent guidelines from the American Heart Association and European Society of Cardiology outline indications for SPE (Table 1), including hemodynamic instability, failed thrombolysis, contraindications to thrombolysis, patent foramen ovale, thrombus-in-transit in the right-sided cardiac chambers, and patients who are predicted to die before realizing the benefits of thrombolytics.9,19) Special considerations must be made for patients who are in the immediate postoperative period as these patients are at an increased risk of developing DVT and subsequent PE. In particular, recent intracranial or spinal surgery within the past 2 months, any surgical operation within the past 10 days, or recent intracranial hemorrhage are also indications for SPE versus thrombolytic therapy due to the high risk of hemorrhage. Pregnant and postpartum patients due to their high levels of estrogen are hypercoagulable and are therefore at an increased of DVT and acute PE. The use of thrombolysis in this population poses a risk of uterine hemorrhage, and therefore SPE may be indicated. Three case reports have been published on SPE during pregnancy which resulted in 100% maternal survival and 25% fetal/neonatal mortality.4)
Table 1. Indications for surgical pulmonary embolectomy.
| Massive or submassive PE with any of the following: |
| Contraindication to thrombolytic therapy |
| - History of intracranical hemorrhage |
| - Intracranial malignancy, mass, or aneurysm |
| - Cerebrovascular accident with the past 3 months |
| - Major surgery within the past 1 month |
| - Brain or spinal surgery within the past 2 months |
| Failed thrombolytic therapy |
| Patent foramen ovale |
| Pregnancy |
| Right heart failure or cardiogenic shock |
| Thrombus-in-transit within the right sided heart chambers |
PE: pulmonary embolism
Approach
Patients with acute PE are at risk of sudden decompensation during induction of general anesthesia due to loss of vascular tone and subsequent hypotension and loss of cardiac output. This risk can be addressed or mitigated by either having bilateral groins prepped for rapid vascular access or initiating ECMO prior to induction.7) The incision of choice is midline sternotomy with central aortic and bicaval venous cannulation. CBP is initiated with an activated clotting time of >480 seconds and normothermia or mild systemic hypothermia. Cardioplegic arrest and aortic cross clamping are only indicated if other cardiac procedures are to be performed, such as the retrieval of an intracardiac thrombus which can be visualized with intraoperative TEE.
Once on CPB, a longitudinal incision is made in the main PA with extension into the left proximal PA. The incision may be extended into the proximal right PA as necessary. Stay sutures on the edges of the arteriotomy assist with visualization exposure. The thrombus is usually immediately visible and can be extracted using forceps, suction, or a Fogarty embolectomy catheter. To ensure optimal clot retrieval, a videoscope may be utilized to visualize more distal arteries. In complex cases, additional procedures to remove peripheral clots may include retrograde perfusion whereby the left atrium is opened and oxygenated pump blood or saline is infused into the pulmonary veins which expels clots through the incision in the PA. The limitation of this maneuver is the requirement for cardioplegic arrest. Once all visible thrombus is removed, the pulmonary arteriotomy is closed and the patient weaned off CPB. If additional support is needed due to residual RV dysfunction, mechanical circulatory support such as veno-arterial ECMO or a temporary right ventricular assist device (RVAD) can be initiated. Placement of an inferior vena cava (IVC) filter is considered standard of care following SPE due to the known increased risk of recurrent PE in these patients, although the timing of this procedure (whether intraoperative at the time of SPE or postoperatively) is debated.20–22)
Outcomes
The International Cooperative Pulmonary Embolism Registry was the first multinational study to report the clinical outcomes of acute PE and identify factors associated with death.23) The study reported an overall 3-month mortality rate of 17.4% in a cohort of 2454 patients diagnosed with an acute PE. Kilic et al. examined the outcomes of patients undergoing SPE and found an overall mortality of 27.2%, although this study did not stratify according to type of PE.24)
The Surgical Pulmonary Embolectomy as Routine therapy (SPEAR) working group reported an 11.7% in-hospital postoperative mortality, of which 23.7% had massive PE and 9.1% had submassive PE.25) Of interest, 13.1% had CPR before SPE with a significant difference between the massive (34.2%) and the submassive (8.5%) PE groups. Massive PE was associated with significantly worse postoperative outcomes including blood product transfusion (76.3% vs. 36.4% for submassive) and prolonged mechanical ventilatory requirement (42.1% vs 25%). 8.4% of patients required re-exploration for hemorrhage without a significant difference between the massive and the submassive PE groups. The SPEAR working group concluded that SPE is a safe procedure at high-volume centers that can be used more frequently to treat patients presenting with an acute massive PE or acute submassive PE.
Kon and associates reported the outcomes of SPE in North America and identified 1075 SPE cases among 310 centers.26) The study population was not stratified based on massive and submassive PE, but on presentation status of no cardiogenic shock, cardiogenic shock without arrest, and cardiogenic shock with cardiac arrest. The cardiac arrest group carried the highest mortality of 44.4%, followed by the shock without arrest group with 23.7%, and the no shock group with 7.9%. Kalra and colleagues performed a recent meta-analysis that demonstrated an in-hospital all-cause mortality of 26.3% for SPE. Among this population, 36.0% had preoperative contraindications to systemic thrombolysis, 33.9% suffered preoperative cardiac arrest, and 27% required the use of ECMO.27) Not surprisingly, long-term mortality was greatest among those who suffered preoperative cardiac arrest and those who required preoperative ECMO. The investigators suggested SPE as an appropriate management option in patients who show signs of RV dysfunction without hemodynamic instability as SPE can rapidly reduce RV strain and interrupt the progression to cardiogenic shock.
Other reports examining SPE outcomes from various time periods and of varying study sample size demonstrate overall operative mortality ranging from 4.2% to 30%.12,28–30) Numerous studies have shown improvements in SPE outcomes over time with significant 30%–70% decreases in mortality rate from the 1960s to the 2000s.28,29) It is likely that the relatively higher mortality of SPE is attributable to the fact that patients undergoing SPE are, by definition, higher risk and higher acuity than those who undergo non-surgical management. Several investigators have shown no survival advantage of thrombolytic therapy over SPE.2,4,5,15,16) Significant hemorrhagic complications occur in nearly 25% of thrombolysis patients compared to 15% in SPE.5,15,31) Recurrent PE is a known complication of all treatment modalities, but tend to occur more frequently following thrombolysis (21%) than SPE (7.7%).5,15,16) Regardless of treatment strategy, IVC filter placement should be performed either during the index procedure or shortly thereafter.
Postoperative management
Postoperative management following SPE can often be challenging due to the inherent critical nature of these patients. Bleeding, hypoxemia, RV dysfunction, and ongoing cardiogenic shock must be treated aggressively balancing the need for volume infusion with aggressive right heart support. Inotropes and mechanical ventilatory support are routinely used, although there should be a low threshold to initiate mechanical circulatory support such as ECMO. RV dysfunction in these patients often resolves with time and adequate support, as long as the mechanical obstruction has been removed. Acute kidney injury is also common in these patients due to hypoperfusion due to impaired cardiac output preoperatively or during CPB during surgery.8)
Postoperative anticoagulation is a necessary component of postoperative management and serves to prevent recurrent PE.5) Studies indicate that with appropriate postoperative anticoagulation, the risk of recurrent PE is less than 5%.5) While low molecular weight heparin bridged to warfarin was once the treatment of choice, direct oral anticoagulants (DOACs) are now recommended as the first-line therapy for most patients.7,32) DOACs including dabigatran, apixaban, rivaroxaban, and edoxaban have been shown to be non-inferior to warfarin in a series of large meta-analyses including the RE-COVER, RE-COVER II, EINSTEIN-DVT, EINSTEIN-PE, AMPLIFY, and Hokusai-VTE trials.33) In addition, DOACs have many potential advantages over warfarin, including rapid onset, decreased risk of bleeding, fewer food and pharmaceutical interactions, and fewer monitoring requirements.32–34)
Prognosis
While the morbidity and mortality associated with PE is greatest in the short term, these risks extend beyond the acute period in many patients. The overall 90-day mortality for acute PE is estimated at 15%.32) Prognosis varies greatly and may be predicted based on individual patient characteristics. The Pulmonary Embolism Severity Index (PESI) and its simplified version (sPESI) are externally validated and simple tools which may be used to predict 30-day mortality in patients suffering from acute PE.34) These tools rely on basic information which can be obtained on history and physical exam.34) PESI emphasizes history of underlying malignancy and cardiopulmonary disease which are the most common long-term causes of death in acute PE patients.35) Patients who suffer from PE in the setting of underlying neoplasia have the worst prognosis.35)
Another reported predictor of prognosis is pulmonary artery pressure (PAP) at the time of diagnosis. Mean PAP ≥30 mmHg is associated with a higher risk for pulmonary hypertension, ≥40 mmHg is associated with a 70% mortality rate, and >50 mmHg has a 90% mortality rate.32,35) Patients who survive an acute PE require close follow-up for the development of chronic thromboembolic pulmonary hypertension (CTEPH) and subsequent right heart failure.32)
Conclusion
Acute PE remains a highly morbid condition that requires prompt diagnosis and treatment. Several treatment modalities are available, ranging from systemic anticoagulation in patients who have no signs of right heart dysfunction to systemic thrombolysis, catheter-directed therapy, and surgical embolectomy in patients with submassive and massive PE. Non-surgical approaches remain first-line therapy for most cases of PE, although select patient subgroups should be referred up front for surgical intervention as primary treatment. Surgical outcomes have improved substantially in the past decades and now offer a safe and appropriate treatment option that can reduce the mortality and morbidity associated with acute PE.
Disclosure Statement
The authors have no conflict of interest to report.
References
- 1).LeVarge B, Wright C, Rodriguez-Lopez J. Surgical management of acute and chronic pulmonary embolism. Clinical Chest Med 2018; 39: 659-67. [DOI] [PubMed] [Google Scholar]
- 2).Yavuz S, Toktas F, Goncu T, et al. Surgical embolectomy for acute massive pulmonary embolism. Int J Clin Exp Med 2014; 7: 5362-75. [PMC free article] [PubMed] [Google Scholar]
- 3).Keeling WB, Leshnower BG, Lasajanak Y, et al. Midterm benefits of surgical pulmonary embolectomy for acute pulmonary embolus on right ventricular function. J Thorac Cardiovasc Surg 2016; 152: 872-8. [DOI] [PubMed] [Google Scholar]
- 4).Fukuda I, Daitoku K. Surgical embolectomy for acute pulmonary thromboembolism. Ann Vasc Dis 2017; 10: 107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Reyes G, Tamura A, Guerrero J, et al. Surgical treatment of a massive pulmonary embolism after a double cardiac arrest. Rev Esp Cardiol 2007; 60: 883-9. [PubMed] [Google Scholar]
- 6).Secemsky E, Chang Y, Jain CC, et al. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med 2018; 131: 1506-14.e0. [DOI] [PubMed] [Google Scholar]
- 7).Saxena P, Smail H, McGriffin D. Surgical techniques of pulmonary embolectomy for acute pulmonary embolism. Oper Tech Thorac Cardiovasc Surg 2017; 21: 80-8. [Google Scholar]
- 8).Elassal AA, Jabbad HH, Al-Ibrahim KI. Rescue surgical pulmonary embolectomy for acute massive pulmonary embolism. J Egypt Soc Cardio Thorac Surg 2016; 24: 166-72. [Google Scholar]
- 9).Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123: 1788-830. [DOI] [PubMed] [Google Scholar]
- 10).Askari K, Sneij W, Krick S, et al. Going with the flow: saddle pulmonary embolism complicated by severe hypoxemia without shock. Ann Am Thorac Soc 2017; 14: 1479-84. [DOI] [PubMed] [Google Scholar]
- 11).Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 2000; 48: 23-33. [DOI] [PubMed] [Google Scholar]
- 12).Neely RC, Byrne JG, Gosev I, et al. Surgical embolectomy for acute massive and submassive pulmonary embolism in a series of 115 patients. Ann Thorac Surg 2015; 100: 1245-51. [DOI] [PubMed] [Google Scholar]
- 13).Akay TH, Sezgin A, Ozkan S, et al. Successful surgical treatment of massive pulmonary embolism after coronary bypass surgery. Tex Heart Inst J 2006; 33: 498-500. [PMC free article] [PubMed] [Google Scholar]
- 14).Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. JACC Cardiovasc Interv 2015; 8: 1382-92. [DOI] [PubMed] [Google Scholar]
- 15).Gulba DC, Schmid C, Borst HG, et al. Medical compared with surgical treatment for massive pulmonary embolism. Lancet 1994; 343: 576-7. [DOI] [PubMed] [Google Scholar]
- 16).Aymard T, Kadner A, Widmer A, et al. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy--should surgical indications be revisited? Eur J Cardiothorac Surg 2013; 43: 90-4; discussion 94. [DOI] [PubMed] [Google Scholar]
- 17).Kuo WT, Banerjee A, Kim PS, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015; 148: 667-73. [DOI] [PubMed] [Google Scholar]
- 18).Meyer JA. Friedrich Trendelenburg and the surgical approach to massive pulmonary embolism. Arch Surg 1990; 125: 1202-5. [DOI] [PubMed] [Google Scholar]
- 19).Konstantinides SV. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3145-6. [DOI] [PubMed] [Google Scholar]
- 20).Aklog L, Williams CS, Byrne JG, et al. Acute pulmonary embolectomy: a contemporary approach. Circulation 2002; 105: 1416-9. [DOI] [PubMed] [Google Scholar]
- 21).Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg 2005; 129: 1018-23. [DOI] [PubMed] [Google Scholar]
- 22).Greelish JP, Leacche M, Solenkova NS, et al. Improved midterm outcomes for type A (central) pulmonary emboli treated surgically. J Thorac Cardiovasc Surg 2011; 142: 1423-9. [DOI] [PubMed] [Google Scholar]
- 23).Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353: 1386-9. [DOI] [PubMed] [Google Scholar]
- 24).Kilic A, Shah AS, Conte JV, et al. Nationwide outcomes of surgical embolectomy for acute pulmonary embolism. J Thorac Cardiovasc Surg 2013; 145: 373-7. [DOI] [PubMed] [Google Scholar]
- 25).Keeling WB, Sundt T, Leacche M, et al. Outcomes after surgical pulmonary embolectomy for acute pulmonary embolus: a multi-institutional study. Ann Thorac Surg 2016; 102: 1498-502. [DOI] [PubMed] [Google Scholar]
- 26).Kon ZN, Pasrija C, Bittle GJ, et al. The incidence and outcomes of surgical pulmonary embolectomy in North America. Ann Thorac Surg 2019; 107: 1401-8. [DOI] [PubMed] [Google Scholar]
- 27).Kalra R, Bajaj NS, Arora P, et al. Surgical embolectomy for acute pulmonary embolism: systematic review and comprehensive meta-analyses. Ann Thorac Surg 2017; 103: 982-90. [DOI] [PubMed] [Google Scholar]
- 28).Takahashi H, Okada K, Matsumori M, et al. Aggressive surgical treatment of acute pulmonary embolism with circulatory collapse. Ann Thorac Surg 2012; 94: 785-91. [DOI] [PubMed] [Google Scholar]
- 29).Stein PD, Alnas M, Beemath A, et al. Outcome of pulmonary embolectomy. Am J Cardiol 2007; 99: 421-3. [DOI] [PubMed] [Google Scholar]
- 30).Hartman AR, Manetta F, Lessen R, et al. Acute surgical pulmonary embolectomy: a 9-year retrospective analysis. Tex Heart Inst J 2015; 42: 25-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Loyalka P, Ansari MZ, Cheema FH, et al. Surgical pulmonary embolectomy and catheter-based therapies for acute pulmonary embolism: A contemporary systematic review. J Thorac Cardiovasc Surg 2018; 156: 2155-67. [DOI] [PubMed] [Google Scholar]
- 32).Kruger PC, Eikelboom JW, Douketis JD, et al. Pulmonary embolism: update on diagnosis and management. Med J Aust 2019; 211: 82-7. [DOI] [PubMed] [Google Scholar]
- 33).Eldredge JB, Spyropoulos AC. Direct oral anticoagulants in the treatment of pulmonary embolism. Curr Med Res Opin 2018; 34: 131-40. [DOI] [PubMed] [Google Scholar]
- 34).Howard L. Acute pulmonary embolism. Clin Med (Lond) 2019; 19: 243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Guerreiro I, Magalhães H, Coelho S, et al. Long term prognosis of acute pulmonary embolism. Eur J Intern Med 2019; 34: 131-40. [DOI] [PubMed] [Google Scholar]