Abstract
Purpose: Immunosuppressant and steroid are inevitable for graft survival after renal transplantation, and their usage is known to be a risk factor for mortality and morbidity after cardiac surgery. We evaluated the long-term clinical outcomes in patients who underwent cardiac surgery after renal transplantation.
Methods: We retrospectively reviewed 23 patients who underwent cardiac surgery after renal transplantation with maintained grafts at the time of the cardiac surgery in our institution between June 2000 and June 2018 (19 males, 4 females; mean age, 55 (38–81) years).
Results: The interval from renal transplantation to cardiac surgery was 80.0 ± 84.6 (0.25–298) months. The mean follow-up period after cardiac surgery was 78.3 (range: 1–216) months. Cumulative survival rates at 1, 5, 7, and 10 years were 95.7%, 95.7%, 87.7%, and 68.2%, respectively. Renal graft survival rates at 1 and 5 years were 86.1% and 79.9%, respectively.
Conclusions: This retrospective review suggests that cardiac surgery in kidney transplant patients can result in good survival rates. Thanks to dedicated postoperative and long-term management, approximately 80% of the renal grafts still maintained their function 5 years after cardiac surgery.
Keywords: renal transplantation, clinical outcomes, cardiac surgery, renal allografts, immunosuppression
Introduction
Annually, approximately 1600–1700 kidney transplantations are performed in Japan, and this number has been increasing.1) According to the most recent report, the 5-year patient and graft survival rates after renal transplantation are reportedly 97.4% and 94.5%, respectively, for living donor transplants and 92.7% and 87.3%, respectively, for cadaveric transplants.
Immunosuppressive medications as well as steroids are inevitable to avoid rejection after renal transplantation, and they are considered to be risk factors for postsurgical infections as well as for coronary arteriosclerosis.2) Especially after cardiac surgery, infection including mediastinitis and sepsis can be life-threatening. The study aimed to evaluate the long-term clinical outcomes in kidney transplant patients who undergo cardiac surgery.
Materials and Methods
Patients
We retrospectively reviewed 23 patients who underwent cardiac surgery after renal transplantation with maintained grafts at the time of the cardiac surgery in our institution between June 2000 and June 2018. Nineteen males and four females were included in the study, and their age was 55 years (range: 38–81 years).
The etiology for kidney disease was diabetic nephropathy (n = 4), chronic glomerulonephritis (n = 6), IgA nephropathy (n = 3), collagen disease nephropathy (n = 3), nephrosclerosis (n = 2), polycystic kidney (n = 1), membranoproliferative nephritis (n = 1), and unknown (n = 3). Five patients (22%) were diagnosed with diabetic mellitus including four patients who had kidney transplantation for diabetic nephropathy. The interval time from kidney transplantation until the surgery was 80 ± 84.6 months (range: 0.25–298 months) (Table 1).
Table 1. Patient characteristics.
| Number of patients | 23 |
| Age, years | 55 (38-81) |
| Gender, male/female | 19/4 |
| Diabetes | 5 (21.7) |
| Dyslipidemia | 8 (34.8) |
| Cause of renal transplantation | |
| Diabetic nephropathy | 4 (17.3) |
| Chronic glomerulonephritis | 6 (26.0) |
| IgA nephropathy | 3 (13.0) |
| Collagen disease nephropathy | 3 (13.0) |
| Nephrosclerosis | 2 (8.7) |
| Polycystic kidney | 1 (4.3) |
| Membranoproliferative nephritis | 1 (4.3) |
| Unknown | 3 (13.0) |
| Interval from renal transplantation to cardiac surgery, months | 80.0 ± 84.6 (0.25-298) |
Data presented as n(%) or as median (interquartile range) or as mean ± standard deviation (range).
The study was approved by the ethics committee of Tokyo Women’s Medical University.
Statistical analysis
The data were analyzed using EZR version 1.28 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R version 3.2.0 (The R Foundation for Statistical Computing, Vienna, Austria).3) Continuous variables are expressed as means ± standard deviation. Survival was estimated using Kaplan–Meier analysis.
Results
Table 2 summarizes the surgical procedure. The surgical procedures were coronary artery bypass grafting (n = 9), and two cases combined coronary artery bypass grafting with valvular surgery and right atrium tumor resection, isolated valvular surgery (n = 7), aortic surgery (n = 4), pulmonary artery thrombectomy (n = 1). The operation time was 357 ± 140 min (range: 186–627 min), cardiopulmonary bypass time was 175 ± 76 min (range: 53–312 min), and cross-clamp time was 124 ± 46 min (range: 59–195 min).
Table 2. Surgical procedure.
| Type of cardiac surgery | |
| Coronary artery bypass grafting | 9 (39.1) |
| Valve | 7 (30.4) |
| Aortic | 4 (17.4) |
| Combined | |
| Coronary artery bypass grafting + Valve | 1 (4.3) |
| Coronary artery bypass grafting + right atrium tumor resection | 1 (4.3) |
| Pulmonary | 1 (4.3) |
| Operation time, min | 357 ± 140 (186-627) |
| Cardiopulmonary bypass time, min | 175 ± 76 (53-312) |
| Cross-clamp time, min | 124 ± 46 (59-195) |
Data presented as n(%) or as mean ± standard deviation (range).
Table 3 summarizes the mortality and morbidity. The early mortality was 4.3%, while one patient died of sepsis on the 23rd postoperative day after coronary artery bypass grafting.
Table 3. Mortality and morbidity.
| In-hospital mortality | 1 (4.3) |
| Extubation, hours | 61 ± 129 (3-552) |
| ICU stay, days | 7.6 ± 11.3 (2-53) |
| Hospital stay, days | 25.9 ± 23.4 (6-121) |
| Morbidity | |
| Re-operation for bleeding | 2 (8.7) |
| Renal insufficiency requiring hemodialysis | 3 (13.0) |
| Surgical site infection | 0 |
| Pneumoniae | 1 (4.3) |
| Respiratory failure requiring tracheotomy | 1 (4.3) |
| Stroke | 1 (4.3) |
| Paraplegia | 1 (4.3) |
| Late death | 3 (13.0) |
| Renal allograft loss | 3 (13.0) |
Data presented as n(%) or as mean ± standard deviation (range).
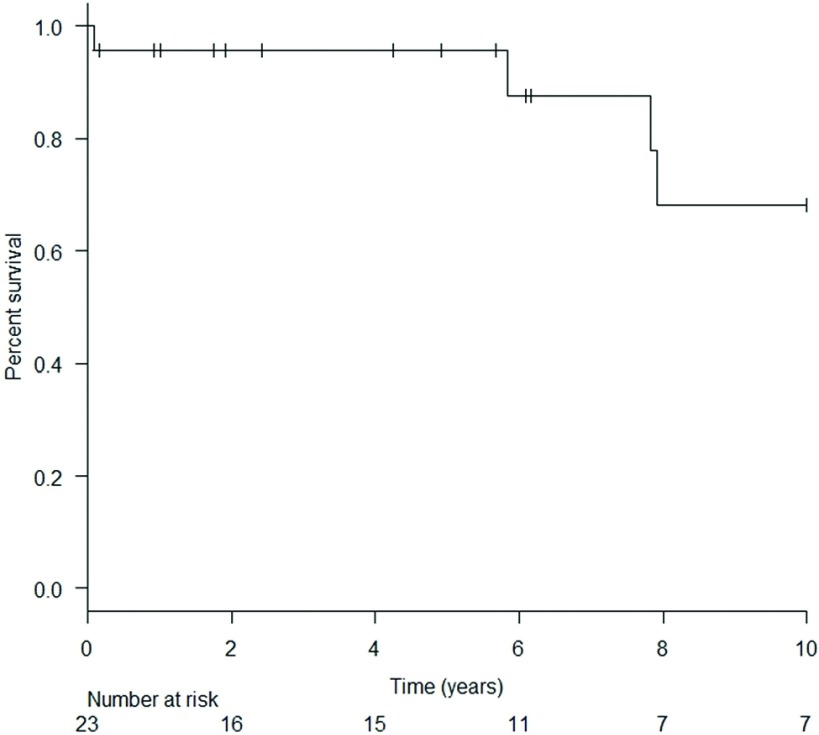
Three patients required temporary hemodialysis perioperatively and there was no surgical site infection. The mean follow-up period after cardiac surgery was 78.3 (range: 1–216) months. Cumulative survival rates at 1, 5, 7, and 10 years were 95.7%, 95.7%, 87.7%, and 68.2%, respectively (Fig. 1). In all, there were three late deaths due to sepsis (n = 2) and leukemia (n = 1).
Fig. 1. Long-term survival rate of patients undergoing cardiac surgery after renal transplantation.
Three patients experienced kidney graft loss after the cardiac surgery and subsequently required regular hemodialysis. The renal graft survival rates at 1 and 5 years were 86.1% and 79.9%, respectively.
Mean serum creatinine levels preoperatively and at discharge were 1.61 ± 0.81 and 1.76 ± 1.02 mg/dL, respectively. Those at 1, 2, and 5 years after the cardiac surgery excluding those who required regular hemodialysis were 1.28 ± 0.38, 1.19 ± 0.29, and 1.39 ± 0.42 mg/dL, respectively.
Discussion
This retrospective review of 23 kidney transplant patients with maintained grafts who underwent cardiac surgery suggested that cardiac surgery resulted in reasonably good postoperative outcomes and survival rates for this patient group. The cumulative survival rates were 95.7%, 95.7%, 87.7%, and 68.2% at 1, 5, 7, and 10 years after cardiac surgery, respectively. Dedicated long-term management by kidney and transplant physicians is mandatory for renal graft survival, and thanks to their contribution, approximately 80% of renal grafts survived for up to 5 years in this study.
Developments in renal transplantation have resulted in increased numbers of cases and remarkable improvements in post-transplantation survival and graft survival rates. This has enabled a larger number of transplant recipients to receive cardiac surgery. This means the necessity of dedicated management for these patients to address immune suppression-related infections, steroid-induced tissue fragility, and the development of atherosclerosis.4,5) Infection rates of 5%–19% have been reported for patients who underwent cardiac surgery after renal transplantation.6–8) Farag et al. reported that postoperative infection was the most common fatal complication, accounting for 39% of deaths in this patient group.9) In the present study, of the four patients that died, three died due to infection-related complications, whereas no patient out of those three had diabetes mellitus, suggesting that diabetic mellitus was not associated with postoperative death in this study. One underwent coronary artery bypass grafting and died from sepsis due to pneumoniae 23 days after surgery. The other two late death causes were both aortic surgery ones, suffering from infection of the aortic arch artificial graft 70 months after surgery and a bedsore after paraplegia resulting from graft replacement of thoraco-abdominal aorta 95 months after surgery. The cause of the remaining one non-infection-related death was acute bone marrow leukemia 94 months after surgery.
After cardiac surgical intervention for kidney transplant patients, dedicated renal management of hemodynamics with dopamine and karipeptide is reported to be essential.8,10) Serum creatinine levels showed no remarkable deterioration from the preoperative level during 5 years of follow-up excluding three patients who came to require regular hemodialysis. Three of the patients (13%) experienced renal graft loss 4, 11, and 24 months after cardiac surgery. Jukic et al. reported similar results with 4 out of 14 cases (31%) requiring hemodialysis and two (15%) experiencing the renal graft loss.11)
The routine regimen after kidney transplant was a combination of tacrolimus hydrate or cyclosporine, mycophenolate mofetil, and steroid in our institution. In perioperative immunosuppressant management, patients in whom difficult to take oral administration after the surgery, tacrolimus hydrate or cyclosporine and steroid were administered intravenously and administration of mycophenolate mofetil was temporary discontinued. After confirming the bowel movement, all the medications were administered orally or through the gastric tube.
This was carefully managed with the cooperation of renal specialists, resulting in the 5-year patient survival of 95.7% and over 80% of the transplanted kidneys surviving at 5 years after the cardiac surgery. These clinical outcomes of ours are acceptable, considering the report from Japan’s kidney transplantation registry, which showed survival rates at 5 years after renal transplantation of 97.4% for living donor transplants and 92.7% for cadaveric transplants, with 5-year graft survival rates of 94.5% for living donor transplants and 87.3% for cadaveric transplants.1)
Limitations
The present study had some limitations. This was single-center, retrospective and non-randomized study with relatively small number of patients. The immune status including management of immunosuppressant and steroid as well as cardiac function of the patients were not necessarily uniform in this study.
Conclusion
This retrospective review suggests that cardiac surgery in kidney transplant patients can be performed with acceptable postoperative and long-term results, including cumulative survival rates of 95.7%, 95.7%, 87.7%, and 68.2% at 1, 5, 7, and 10 years after cardiac surgery, respectively. Thanks to dedicated postoperative and remote-phase management, approximately 80% survival of the renal grafts was achieved at 5 years after the cardiac surgery.
Disclosure Statement
None of the authors has a conflict of interest to disclose.
Acknowledgment
Clinical management for the patients and advices by the department of Urology and department of Surgery, Kidney Center, Tokyo Women’s Medical University are greatly appreciated.
References
- 1).Annual progress report from the Japanese Renal Transplant Registry: number of renal transplantations in 2016 and follow-up survey. Japanese Society for Clinical Renal Transplantation, The Japan Society for Transplantation; 2017. [Google Scholar]
- 2).Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol 2000; 16: 505-11. [PubMed] [Google Scholar]
- 3).Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Matsumiya G, Nakano S, Shirakura R, et al. Aortic valve replacement in a kidney transplant recipient. Nihon Kyobu Geka Gakkai Zasshi 1994; 42: 956-60. (in Japanese) [PubMed] [Google Scholar]
- 5).Zamora JL, Burdine JT, Karlberg H, et al. Cardiac surgery in patients with end-stage renal disease. Ann Thorac Surg 1986; 42: 113-7. [DOI] [PubMed] [Google Scholar]
- 6).Ono M, Wolf RK, Angouras DC, et al. Short- and long-term results of open heart surgery in patients with abdominal solid organ transplant. Eur J Cardiothorac Surg 2002; 21: 1061-72. [DOI] [PubMed] [Google Scholar]
- 7).Zhang L, Garcia JM, Hill PC, et al. Cardiac surgery in renal transplant recipients: experience from Washington Hospital Center. Ann Thorac Surg 2006; 81: 1379-84. [DOI] [PubMed] [Google Scholar]
- 8).Mitruka SN, Griffith BP, Kormos RL, et al. Cardiac operations in solid-organ transplant recipients. Ann Thorac Surg 1997; 64: 1270-8. [DOI] [PubMed] [Google Scholar]
- 9).Farag M, Nikolic M, Arif R, et al. Cardiac surgery in patients with previous hepatic or renal transplantation: a pair-matched study. Ann Thorac Surg 2017; 103: 1467-74. [DOI] [PubMed] [Google Scholar]
- 10).Fujii H, Kaku R, Ohashi I, et al. Human atrial natriuretic peptide increased urinary production in patients with immunosuppressant-induced acute renal failure after transplantation. J Jpn Soc Intensive Care Med 2001; 8: 355-9. [Google Scholar]
- 11).Basic-Jukic N, Ivanac-Jankovic R, Biocina B, et al. Cardiovascular surgery after renal transplantation—indications, complications and outcome. Ren Fail 2015; 37: 1020-1. [DOI] [PubMed] [Google Scholar]