Abstract
Patients with chronic limb-threatening ischemia often have multiple segmental diseases requiring revascularization. However, there is no defined milestone to indicate sufficient endovascular treatment (EVT). Using multiple near-infrared spectroscopic oximeters, we intraoperatively monitored regional tissue oxygen saturation (rSO2) to perform target region oxygenation-based EVT for a patient with chronic limb-threatening ischemia. Stent placement at the superficial femoral artery and angioplasty of the tibioperoneal trunk enabled rSO2 in the target ischemic regions (dorsal foot and heel) to be >50% for ulcer healing. We herein describe target region oxygenation-based EVT with rSO2 monitoring as an effective strategy for performing the minimum requisite procedures.
Keywords: Endovascular treatment, Regional oxygen saturation monitoring, Near-infrared spectroscopic oximeter, Chronic limb-threatening ischemia, Foot ulcer
Patients with chronic limb-threatening ischemia (CLTI) secondary to peripheral artery disease (PAD) often require multiple revascularizations.1 Currently, no definite intraoperative characteristic exists for the extent of revascularization required for ulcer healing during endovascular treatment (EVT).
It is important to increase perfusion and regional oxygenation saturation (rSO2) by revascularization for ischemic wound healing. However, these are rarely monitored during the revascularization procedure because of lack of reliable measuring devices. Near-infrared spectroscopy devices noninvasively measure rSO2, which is the oxygenated hemoglobin/total hemoglobin ratio; they have not been used during EVT except in a few studies.2 We previously reported the utility of a newly invented finger-mounted tissue oximeter (Toccare; Astem Co, Ltd, Kawasaki, Japan) designed to measure rSO2 up to a depth of 5 mm below the probe for evaluating perfusion at the toe and dorsal foot of PAD patients.3 We routinely use the device in an office setting in daily practice. Here, we present a case in which rSO2 was continuously monitored as a milestone using multiple oximeters at the target regions during EVT. This concept, which we named target region oxygenation-based EVT (TOE), was applied in a patient with CLTI. Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Case report
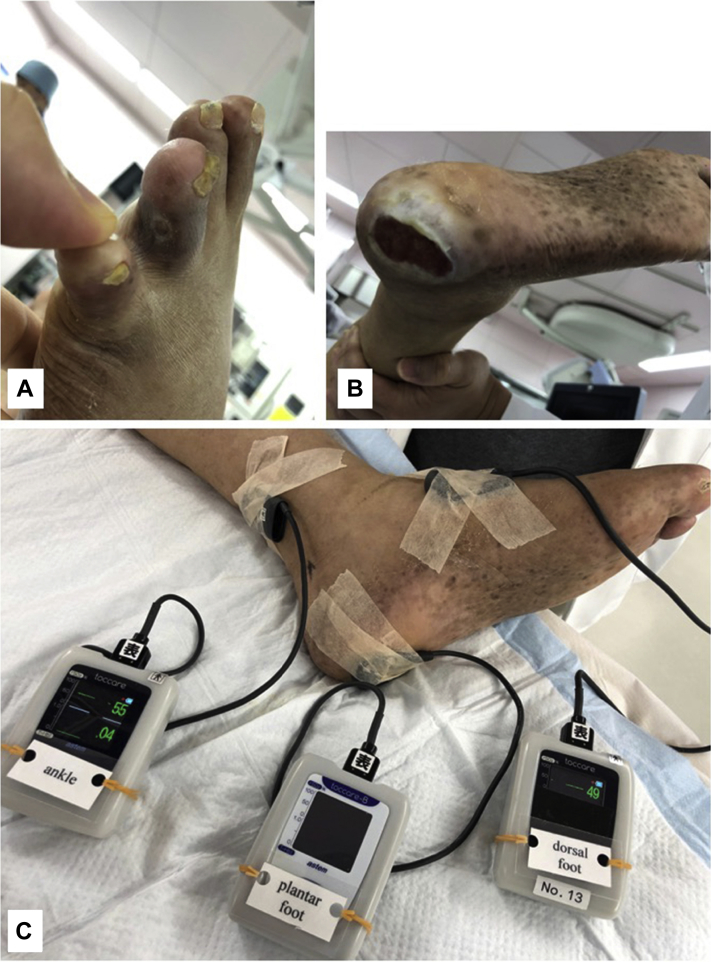
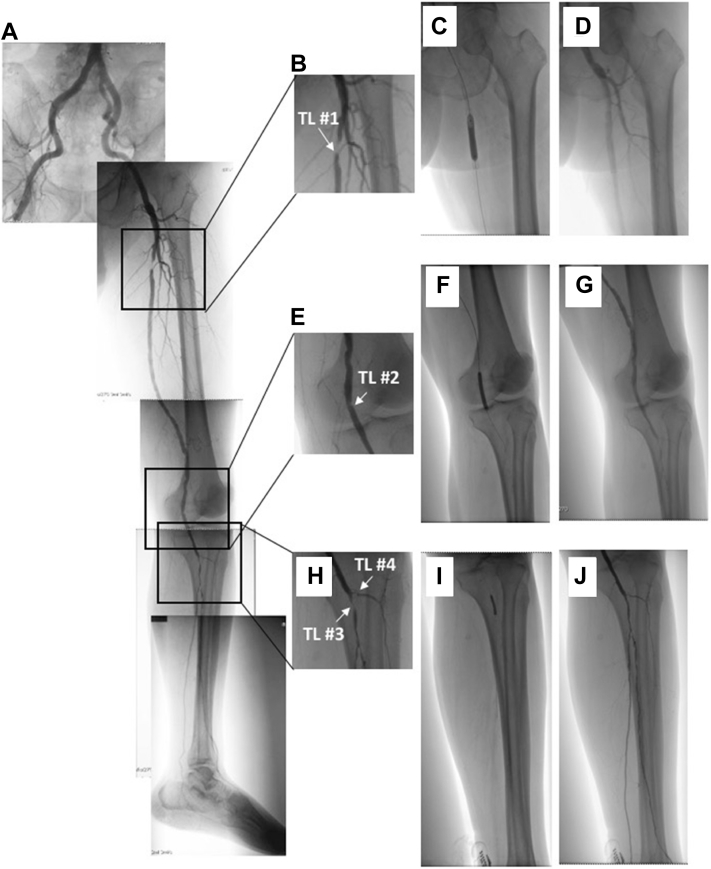
A 71-year-old man was admitted with a 4-month history of painful ulcers in the left second toe and heel (Fig 1, A and B). He had diabetes and was undergoing hemodialysis. The right ankle-brachial index was 0.35, and the transcutaneous oxygen tension (TcPO2) at the right dorsal foot was 8 mm Hg. Given the limb-threatening situation due to ischemia, EVT was planned to revascularize the ischemic regions (toe and heel). Before the start of EVT, three oximeter sensor probes were placed at the selected target oxygenation regions: ankle, dorsal, and plantar regions (Fig 1, C). They were a finger-mounted type of sensor attached with adhesive tape.3, 4, 5 Angiography (Fig 2, A) revealed multiple stenotic lesions, including lesions in the proximal superficial femoral artery (SFA, target lesion #1; Fig 2, B), popliteal artery (target lesion #2; Fig 2, C), tibioperoneal trunk (TPT, target lesion #3; Fig 2, D), and anterior tibial artery (target lesion #4; Fig 2, D). The pedal arch was scarcely visible.
Fig 1.
A, Ulcer on the second toe. B, Ulcer on the heel. C, Intraoperative monitoring of tissue oxygen saturation using three oximeters with the probes attached to the ankle, dorsal foot, and plantar regions.
Fig 2.
A, Angiogram of the left foot. B, Severe stenosis in the proximal superficial femoral artery (SFA) as the target lesion (TL) #1. C, Angioplasty at the popliteal artery; mild stenosis as TL #2. D, Severe stenosis at the tibioperoneal trunk (TPT) as TL #3, and moderate stenosis at the anterior tibial artery as TL #4. E, Angioplasty at TL #1. F, Stent placement at TL #1. G, Angioplasty at TL #2. H, Postangioplasty angiogram showing no stenosis at TL #2. I, Angioplasty at TL #3. J, Postangioplasty angiogram showing revascularized TPT (TL #3) and residual stenosis at TL #4.
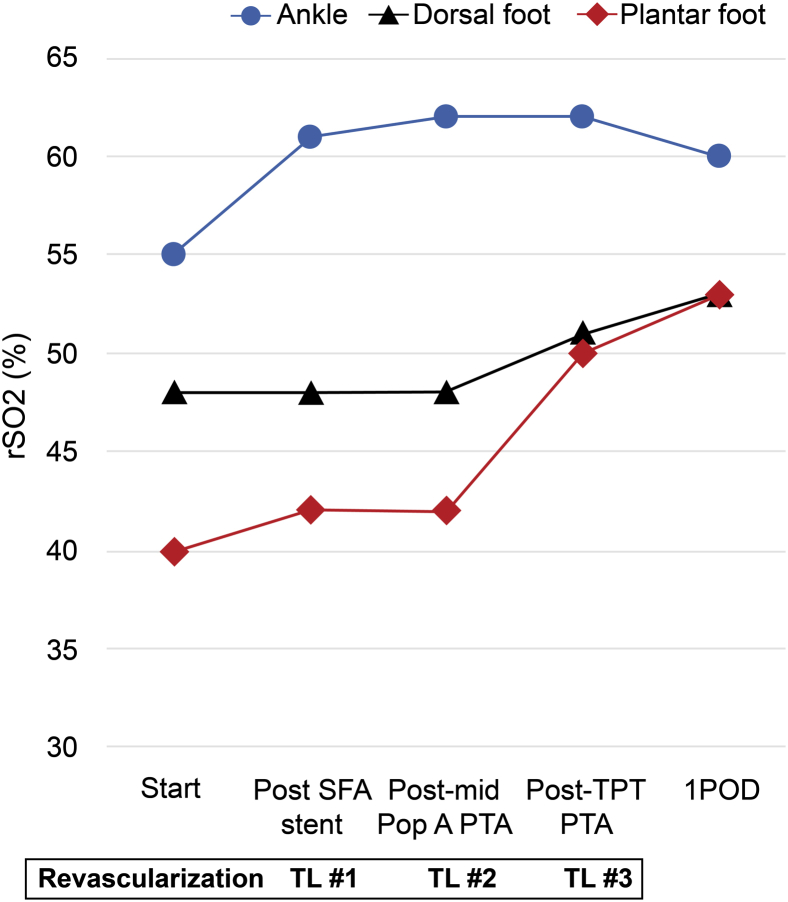
To revascularize the four target lesions (Fig 2, B-D), EVT was performed as follows: a 6F retrograde puncture was made in the right common femoral artery, a 6F 45-cm Destination guiding sheath (Terumo, Tokyo, Japan) was passed into the left common femoral artery, and revascularization was performed from the proximal regions. The rSO2 was recorded every 5 minutes after each procedure. The TOE end point was rSO2 >50%.4 Proximal SFA angioplasty was followed by stent placement (6- × 60-mm SMART; Cordis, Bridgewater, NJ; Fig 2, E and F) because the lesion was severely calcified. Next, angioplasty of the proximal popliteal lesion (Fig 2, C) was successfully performed with a 5- × 40-mm Armada balloon (Abbott Vascular, Abbott Park, Ill; Fig 2, G and H). After that, we performed angioplasty of the TPT with a 3- × 20-mm Coyote balloon (Boston Scientific, Marlborough, Mass; Fig 2, I and J). Although the SFA angioplasty increased rSO2 from 55% to 61% in the ankle (Fig 3), rSO2 did not show any significant increase after the SFA stent placement and midpopliteal artery angioplasty at either the dorsal foot or the plantar region (Fig 3). After successful EVT at the TPT stenosis, rSO2 at both dorsal foot and plantar regions finally increased to >50% (Fig 3). At that point, we ended EVT, leaving stenosis at the proximal anterior tibial artery, fibular artery, and pedal arch. The patient had an uneventful postoperative course. On postoperative day 1, rSO2 values of the dorsal foot and plantar regions were elevated to 53%. The patient's pain was alleviated, and he was discharged on the same day. Two months after EVT, the TcPO2 at the left dorsal foot was 48 mm Hg, and the ulcers in both the second toe and heel of the patient were completely healed.
Fig 3.
The changes in regional tissue oxygenation saturation (rSO2) during endovascular treatment (EVT). The blue circle represents the value of the oximeter sensor when it is placed at the ankle. The black triangle represents the value of the oximeter sensor when it is placed at the dorsal foot. The red diamond represents the value of the oximeter sensor when it is placed at the plantar region. POD, Postoperative day; Pop A, popliteal artery; PTA, percutaneous transluminal angioplasty; SFA, superficial femoral artery; TL, target lesion; TPT, tibioperoneal trunk.
Discussion
The number of PAD patients has exceeded 200 million globally.6 The number of CLTI patients has increased because of the growing incidence of diabetes and renal insufficiency. In the United States, there are about 3500 new cases of CLTI annually.7 Recently, advancement in EVT has enabled revascularization of high-risk patients with complex arterial diseases. However, devices such as balloons and stents mainly focus not on the rate of wound healing but on target lesion revascularization as the successful end point for the devices in numerous clinical trials after recanalization of a single arterial lesion. The prognosis of the ischemic ulcer after EVT depends on the cumulative effect of multiple procedures, particularly patent pedal arch, which seems to be a key factor for wound healing.8 However, the EVT parameter that facilitates ulcer healing has not yet been identified.
As PAD patients are usually frail and cannot tolerate lengthy procedures, EVT has been considered to be the first choice for such patients rather than open bypass/revascularization. However, PAD patients often have kidney dysfunction; thus, contrast medium should be used minimally, with either a more dilute contrast medium or a small volume. Hence, only the necessary revascularization procedure for ulcer healing should be performed. According to our previous study, rSO2 >50% is almost equivalent to skin perfusion pressure >50 mm Hg or TcPO2 >50 mm Hg,3 which is considered adequate for ulcer healing.9 Furthermore, our retrospective study indicated that all patients with critical limb ischemia who underwent EVT with rSO2 >50% in the dorsal foot on postoperative day 1 showed improved ulcer healing.4 Therefore, we aimed to perfuse the target region up to rSO2 >50%. This would save time and costs. In the case that rSO2 >50% is not achieved, other EVT strategies or vascular reconstruction procedures, such as bypass surgery, should be considered. The oximeter used to perform TOE in this study was efficient. Unlike an ankle-brachial index, skin perfusion pressure, or TcPO2 that consume time and lack data reliability,10 the newly invented oximeter can measure rSO2 within seconds and can be used for real-time monitoring. Although the rSO2 was monitored continuously, its measurements were recorded every 5 minutes after each EVT procedure because it took a few minutes for the value to stabilize. There is also a possibility of vasospasm or embolism, which may decrease the rSO2; this decrease may be resolved after the procedure. The oximeter can reflect the dermal or subdermal tissue oxygenation. Unlike with our oximeter measuring rSO2 up to a depth of 5 mm below the probe, depth exceeding 15 to 20 mm in conventional near-infrared spectroscopy devices may affect the rSO2 values because of the thin subdermal layer of the foot and may influence the outcome of ulcer healing. With multiple oximeters and the concept of the TOE, we may be able to perform EVT to the extent necessary for individual CLTI patients. However, it may be better to place the sensor probe at the site of one of the ulcerations on the toe or on the surface of open ulcers directly rather than on the dorsal foot, especially when the pedal arch is absent.
Conclusions
With multiple oximeters and the concept of TOE, it is feasible to monitor the effect of each EVA procedure on tissue perfusion. TOE may become a standard strategy to perform EVT; however, further studies are needed to identify the suitable location for sensor probes for monitoring the rSO2 and its threshold levels for wound healing.
Footnotes
This work was supported by the project “Development of Medical Devices and Systems for Advanced Medical Services” from the Division of Medical Device Research in the Japan Agency for Medical Research and Development, Japan (ID: 19he1602005h0003 awarded to N.U.).
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;70:662. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boezeman R.P., Becx B.P., van den Heuvel D.A., Ünlü Ç., Vos J.A., de Vries J.P. Monitoring of foot oxygenation with near-infrared spectroscopy in patients with critical limb ischemia undergoing percutaneous transluminal angioplasty: a pilot study. Eur J Vasc Endovasc Surg. 2016;52:650–656. doi: 10.1016/j.ejvs.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Yata T., Sano M., Kayama T., Naruse E., Inuzuka K., Unno N. Utility of a finger-mounted tissue oximeter with near-infrared spectroscopy to evaluate limb ischemia in peripheral arterial disease patients. Ann Vasc Dis. 2019;12:36–43. doi: 10.3400/avd.oa.18-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayama T., Unno N., Yata T., Naruse E., Sano M., Inuzuka K. Predictive value of tissue oxygenation saturation for ulcer healing after endovascular treatment in patients with critical limb ischemia—utility of a newly-invented finger-mounted tissue oximeter with near-infrared spectroscopy. Eur J Vasc Endovasc Surg. 2019;58:e792–e793. [Google Scholar]
- 5.Kanayama N., Niwayama M.E. Examiner’s finger-mounted fetal tissue oximetry. J Biomed Opt. 2014;19:067008. doi: 10.1117/1.JBO.19.6.067008. [DOI] [PubMed] [Google Scholar]
- 6.Fowkes G., Rudan D., Rudan I., Aboyans V., Denenberg J., McDermott M.M. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 7.Nehler M.R., Duval S., Diao L., Annex B.H., Hiatt W.R., Rogers K. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 8.Jung H.W., Ko Y.G., Hong S.J., Ahn C.M., Kim J.S., Kim B.K. Editor's choice—the impact of endovascular pedal artery revascularization on wound healing in patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2019;58:854–863. doi: 10.1016/j.ejvs.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Andersen C.A. Noninvasive assessment of lower extremity hemodynamics in individuals with diabetes mellitus. J Vasc Surg. 2010;52(Suppl):76S–80S. doi: 10.1016/j.jvs.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Cao P., Eckstein H.H., De Rango P., Setacci C., Ricco J.B., de Donato G. Chapter II: diagnostic methods. Eur J Vasc Endovasc Surg. 2011;42:S13–S32. doi: 10.1016/S1078-5884(11)60010-5. [DOI] [PubMed] [Google Scholar]