Abstract
Objective
To estimate the effectiveness of quadrivalent influenza vaccines during the 2018-2019 season for influenza A (H1N1) pdm09 and A (H3N2) in all age groups.
Methods
A test-negative case-control study was performed.
Patients
A total of 1,331 participants were divided into 4 groups (younger children: ≤6 years, older children: 7-15 years, younger adults: 16-64 years, and older adults: ≥65 years).
Results
For all children, the adjusted vaccine effectiveness (VE) was significant against any influenza [41.3% (95% confidence interval (CI): 19.7-57.2%)], total A [A (H1N1) pdm09 and (H3N2); 38.3% (95% CI: 15.1-55.1%)], and A [H3N2; 39.8% (95% CI: 13.8-57.9%)]. In younger children, the adjusted VE against any influenza was 44.8% (95% CI: 14.1-64.5%) and against total A was 43.8% (95% CI: 12.5-63.9%). For all adults, the adjusted VE was significant against any influenza was 42.3% (95% CI: 17.9-59.5%); total A, 39.3% (95% CI: 13.5-57.4%); A (H1N1) pdm09, 56.7% (95% CI: 19.1-76.8%); and A (H3N2), 33.2% (95% CI: 1.5-54.6%). In younger adults, the adjusted VE against any influenza was 43.4% (95% CI: 17.3-61.2%), total A, 41.7% (95% CI: 14.4-60.3%); A (H1N1) pdm09, 56.2% (95% CI: 14.9-77.5%); and A (H3N2), 34.5% (95% CI: 0.3-56.9%). In both older children and older adults, no significant VE was observed.
Conclusion
This study is the first to report on the VE against all types of influenza in all age groups using a rapid influenza diagnostic test. The VE varied with both age and influenza subtype.
Keywords: test-negative case-control study, quadrivalent influenza vaccine, rapid influenza diagnostic test, influenza A (H1N1) pdm09
Introduction
According to the recommendation by the World Health Organization (WHO), quadrivalent influenza vaccines replaced trivalent vaccines in the 2015-2016 season in Japan (1). A test-negative case-control study (TNS), which is a modified case-control study, was conducted; this study design has been validated and has become the most popular study design for estimating influenza vaccine effectiveness (VE) against influenza (2,3). For clinicians, a TNS is easier to conduct than a classic case-control study and can minimize confounding due to health care-seeking behavior in evaluating influenza VE (4).
In Japan, reports describing the efficacy of quadrivalent influenza vaccines using a TNS have been increasing (5-8). However, each of these studies was focused on only children or only adults. The rapid influenza diagnostic test (RIDT) is widely used for diagnosing influenza in Japan. However, a conventional RIDT cannot distinguish influenza A (H1N1) pdm09 from other subtypes of influenza A. Although there has been one report published describing trivalent VE that partially included influenza A (H1N1) pdm09 in children (9), no studies regarding quadrivalent VE that focus on all types of influenza, including influenza A (H1N1) pdm09, among all age groups have been published in Japan.
The present study estimated the effectiveness of quadrivalent influenza vaccines during the 2018-2019 season based on a TNS that distinguished influenza A (H1N1) pdm09 from other subtypes of influenza A in all age groups.
Materials and Methods
Patients
The subjects in this research were patients who underwent an RIDT in the Ando Clinic (Narashino City, Chiba, Japan) due to possible influenza infections during the 2018-2019 season. Patients with influenza-like illness (ILI) were informed of the concept of this study and divided into 4 age groups (younger children: ≤6 years, older children: 7-15 years, younger adults: 16-64 years, and older adults: ≥65 years) to consider the age effects.
In this study, the following clinical information was collected: sex, age, vaccination status for the quadrivalent influenza vaccine, comorbidities, month of ILI onset, and outcomes of RIDT-positive cases. Comorbidities were defined as the following conditions that might affect the immune status: chronic pulmonary, cardiovascular (excluding hypertension), renal, liver, hematologic, and neurological disorders (including febrile convulsion with multiple episodes), diabetes mellitus, autoimmune disorders, congenital anomaly, cancer, and pregnancy.
Eligibility criteria
1) Patients who underwent an RIDT due to an ILI during the 2018-2019 season. ILIs were defined as a suspected influenza infection, as evidenced by symptoms including a fever, acute onset, nasal discharge, sore throat, cough, arthralgia, and myalgia.
2) The interval from the time that the quadrivalent inactivated influenza vaccination was administered was ≥14 days and <5 months (10).
3) If patients had experienced multiple episodes:
a) For patients with any influenza-negative episodes, the episode during which the highest body temperature was observed was analyzed,
b) For patients with both influenza-positive and influenza-negative episodes, the positive episode was analyzed,
c) For patients with both influenza A- and B-positive episodes, both episodes were analyzed.
Exclusion criteria
1) Patients who had already experienced the same type of influenza infection during the 2018-2019 season.
2) Patients who had already been given a neuraminidase inhibitor due to negative results of the RIDT.
The diagnosis of influenza
Nasopharyngeal swabs were obtained from all patients and tested using ImunoAce™ Flu and Linjudge™ FluA/pdm (TAUNS Laboratories, Izunokuni, Japan). ImunoAce™ Flu can detect and differentiate between influenzas A and B, with high positive concordance (influenza A: 94.3%, influenza B: 100%) and negative concordance rates (influenza A: 95.4%, influenza B: 98.7%) as demonstrated by a viral isolation culture (11). Linjudge™ FluA/pdm can detect influenza A (H1N1) pdm09 with high positive concordance (96.3%) and negative concordance rates (98.4%), as demonstrated by a viral isolation culture (12). The diagnosis of influenza A (H1N1) pdm09 was made using both ImunoAce™ Flu, which detects positivity for influenza A, and Linjudge™ FluA/pdm, which also detects positivity. The diagnosis of other subtypes of influenza A was also made employing both ImunoAce™ Flu, which detects positivity for influenza A, and Linjudge™ FluA/pdm, which detects negativity. Since subtypes of influenza A (H3) other than A (H3N2) were not detected, the diagnosis of influenza A (H3N2) was made when ImunoAce™ Flu detected positivity for influenza A and Linjudge™ FluA/pdm detected negativity (13).
Vaccine
The quadrivalent influenza vaccine contained influenza A/Singapore/GP1908/2015 (IVR-180) (H1N1) pdm09, A/Singapore/INFIMH-16-0019/2016 (IVR-186) (H3N2), B/Phuket/3073/2013 (Yamagata lineage), and influenza B/Maryland/15/2016 (NYMC BX-69A) (Victoria lineage) viral strains.
At 2- to 4-week intervals, two doses (0.25 mL and 0.5 mL) of vaccine were administered to children 6 months to 2 years old and 3-12 years old, respectively. A single 0.5-mL vaccine dose was generally administered to patients ≥13 years old.
Test-negative case-control study
As previously described (7,8), VE was estimated using a test-negative case-control design. Patients who had ILIs and were RIDT-positive for influenza infection were considered cases, and those who had ILIs and were RIDT-negative for influenza infection were considered controls. VE was defined as [1 - odds ratio (OR)] ×100 (%); the OR was calculated as (the number of influenza-positive among vaccinated patients × the number of influenza-negative patients among unvaccinated patients) / (the number of influenza-negative among vaccinated patients × influenza-positive among unvaccinated patients). The OR was calculated using the Wald test. First, the crude VE was calculated and then adjusted for the sex, age group, presence of comorbidities, and month of onset of ILI (5-9). In each age group, the VE was adjusted for the sex, presence of comorbidities, and month of onset of ILI.
Statistical analyses
Student's t-test was used to compare continuous variables (i.e. time from the onset, age) between participants with and without influenza infection. Pearson's Chi-square test and Fischer's exact test were used to compare nominal variables (i.e. sex, comorbidities, and month of onset). When considering the VE by the number of vaccine doses in children, a multivariate logistic regression analysis was performed using the age, body temperature, time from onset, and vaccine doses as explanatory variables (7,8). The OR of vaccine doses was adjusted for other explanatory variables (age, body temperature, time from onset). The effect of the vaccine dose was analyzed in children 6 months to 12 years old because the vaccine doses varied from 0 to 2 times in this age group. Two-sided p values <0.05 were considered significant. Statistical analyses were performed using the JMPⓇ software program, ver. 14 (Statistical Analysis Software; SAS Institute, Cary, USA).
Ethics
This study was conducted according to the Declaration of Helsinki. Written informed consent was obtained from the patients, their parents, or both. Participants were recruited prospectively. The study design was approved by the Joint Institutional Review Board (approval number: 14000050.20181116-4704).
Results
Enrollment
From December 17, 2018, to May 31, 2019, 1,368 episodes of influenza were identified; 35 of these were excluded for the following reasons: 32 for overlapped episodes; 1 for uncertain vaccination, and 2 for intervals from the time of vaccination ≥5 months. Among the 713 patients <15 years old (children), 714 episodes were identified (1 patient had episodes of both influenza A and B). Among the 618 patients ≥15 years old (adults), 619 episodes were identified (1 patient had episodes of both influenza A and B). In total, 1,333 episodes and 1,331 patients were analyzed, with 2 patients having episodes of both influenza A and B.
Patient characteristics
Patient characteristics are summarized in Table 1.
Table 1.
Patients’ Characteristics.
| a) Children (≤ 15 years) | ||||||
|---|---|---|---|---|---|---|
| n | Age (mean) | Sex (M:F) | Comorbidityb
n |
Body Temperaturec (°C) (mean±SE) | Time from onset (hours±SE) | |
| RIDT-positive | 372 | 7.3 | 220:152 | 25 | 39.0±0.0* | 20.3±0.8 |
| Influenza total Aa | 357 | 7.2 | 211:146 | 25 | 39.1±0.0* | 20.0±0.9 |
| Influenza A (H1N1) pdm09 | 133 | 7.3 | 77:56 | 8 | 39.2±0.1* | 22.3±1.5d |
| Influenza A (H3N2) | 224 | 7.2 | 134:90 | 17 | 39.0±0.1* | 18.6±1.1 |
| Influenza B | 15 | 9.5 * | 9:6 | 0 | 38.8±0.2 | 27.7±4.7 |
| RIDT-negative | 342 | 6.8 | 184:158 | 27 | 38.7±0.0 | 20.4±0.9e |
| b) Adults (≥ 16 years) | ||||||
| RIDT-positive | 277 | 40.7 | 123:154 | 33 | 38.7±0.1* | 25.7±1.1 |
| Influenza total Aa | 270 | 41.0 | 120:150 | 33 | 38.7±0.1 * | 25.6±1.1 |
| Influenza A (H1N1) pdm09 | 75 | 39.9 | 34:41 | 12 | 38.9±0.1 * | 26.8±2.3 |
| Influenza A (H3N2) | 195 | 41.4 * | 86:109 | 21 | 38.6±0.1* | 25.1±1.3 |
| Influenza B | 7 | 31.0 | 3:4 | 0 | 39.2±0.3 * | 30.1±7.7 |
| RIDT -negative | 342 | 38.5 | 167:175 | 42 | 38.3±0.1f | 23.8±1.0g |
* Statistically significant
RIDT: Rapid influenza diagnostic test
aInfluenza total A: Influenza A (H1N1) pdm09 and Influenza A (H3N2)
bComorbidities included: chronic disease of the lung (such as bronchial asthma), heart (such as ischemic heart disease), and kidney (such as chronic renal failure); endocrine metabolism disorder (such as diabetes mellitus); malignancy; autoimmune disorder (such as rheumatoid arthritis); neurological disease (such as epilepsy); pregnancy, and others (such as neurofibromatosis).
cSE: standard error
dThere is one value missing from the SE calculation (n=132).
eIn cases of Influenza B, the mean value±SE of the time from onset of RIDT-negative controls was 20.4±1.0.
fIn cases of Influenza both A (H1N1) pdm09 and B, the mean value±SE of the body temperature of RIDT-negative control was 38.3±0.0.
gIn cases of Influenza both A (H1N1) pdm09 and B, the mean value±SE of the time from onset of RIDT-negative control was 23.8±1.1.
In children, 372 episodes were RIDT-positive (cases), and 342 were RIDT-negative (controls). Regarding types of influenza, influenza A accounted for the greatest proportion (96.0%, 357/372). Among subtypes of influenza A, A (H3N2) was predominant with 224 cases (60.2%), whereas there were 133 cases (35.8%) of A (H1N1) pdm09. RIDT-positive patients were significantly older than those who were RIDT-negative in influenza B (mean age of RIDT-positive cases:RIDT-negative case=9.5:6.8 years old, p value=0.0116). The body temperature was significantly higher in RIDT-positive cases than in RIDT-negative cases except for influenza B. Comorbidities included bronchial asthma (n=46), epilepsy (n=1), and neurofibromatosis (n=1), among others.
In adults, 277 episodes were RIDT-positive (cases), and 342 were RIDT-negative (controls). Influenza A also accounted for the greatest proportion (97.5%, 270/277). Among influenza A subtypes, influenza A (H3N2) was predominant with 195 cases (70.4%), while there were 75 cases (27.1%) of influenza A (H1N1) pdm09. RIDT-positive patients were significantly older than those who were RIDT-negative in influenza A (H3N2) (mean age of RIDT-positive cases:RIDT-negative cases=41.4:38.5 years old, p value=0.0484). The body temperature was significantly higher in RIDT-positive cases than in RIDT-negative cases. Comorbidities included bronchial asthma (n=27), diabetes mellitus (n=14), and malignancy (n=6), among others.
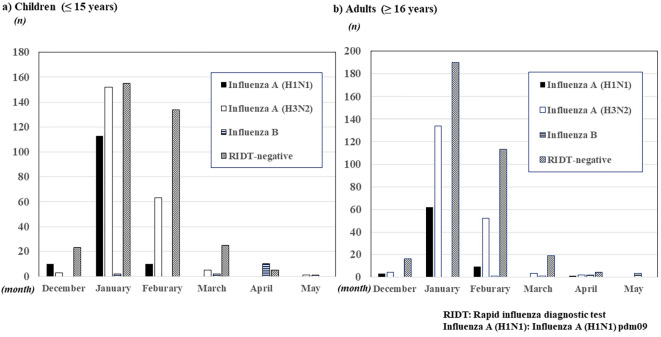
The distribution of the influenza epidemic is shown in Figure. In both children and adults, its distribution was nearly the same. Both influenza A (H1N1) pdm09 and A (H3N2) epidemics peaked starting in January 2018 and began to decline in February. After March, both subtypes of influenza A were rarely observed. In addition, no evident influenza B epidemic was observed; however, sporadic influenza B cases were observed after March.
Figure.
The distribution of influenza infection during the 2018-2019 season.
VE
The VE is shown in Table 2.
Table 2.
Vaccine Effectiveness during the 2018-2019 Season.
| a) Children (≤ 15 years) | |||||
|---|---|---|---|---|---|
| 1) Total | n | Vaccinated, Unvaccinated cases | Vaccinated, Unvaccinated controls | Crude VE (95% CI) | Adjusted VEb(95% CI) |
| Any Influenza | 714 | 139, 233 | 175, 167 | 43.1 % (23.3 to 57.8) * | 41.3% (19.7 to 57.2) * |
| Influenza total A | 699 | 137, 220 | 175, 167 | 40.6 % (19.7 to 56.0) * | 38.3% (15.1 to 55.1) * |
| Influenza A (H1N1) pdm09 | 475 | 50, 83 | 175, 167 | 42.5 % (13.4 to 61.8) * | 34.5% (-2.3 to 58.1) |
| Influenza A (H3N2) | 566 | 87, 137 | 175, 167 | 39.4 % (14.7 to 57.0) * | 39.8% (13.8 to 57.9) * |
| 2) ≤ 6 yearsa | |||||
| Any Influenza | 348 | 75, 98 | 103, 72 | 46.5 % (18.1 to 65.0) * | 44.8% (14.1 to 64.5) * |
| Influenza total A | 347 | 75, 97 | 103, 72 | 46.0% (17.2 to 64.7) * | 43.8% (12.5 to 63.9) * |
| Influenza A (H1N1) pdm09 | 235 | 22, 38 | 103, 72 | 59.5% (25.9 to 77.9) * | 48.3% (-0.9 to 73.5) |
| Influenza A (H3N2) | 287 | 53, 59 | 103, 72 | 37.2% (-1.3 to 61.1) | 38.4% (-0.4 to 62.2) |
| 3) 7-15 years | |||||
| Any Influenza | 366 | 64, 135 | 72, 95 | 37.4% (4.1 to 59.2) * | 36.2% (-0.2 to 59.4) |
| Influenza total A | 352 | 62, 123 | 72, 95 | 33.5% (-2.5 to 56.8) | 30.2% (-11.1 to 56.2) |
| Influenza A (H1N1) pdm09 | 240 | 28, 45 | 72, 95 | 17.9% (-44.1 to 53.2) | 15.3% (-56.9 to 54.2) |
| Influenza A (H3N2) | 279 | 34, 78 | 72, 95 | 42.5% (4.6 to 65.3) * | 40.0% (-2.2 to 64.8) |
| b) Adults (≥ 16 years) | |||||
| 1) Total | |||||
| Any Influenza | 619 | 76, 201 | 135, 207 | 42.0 % (18.4 to 58.8) * | 42.3% (17.9 to 59.5) * |
| Influenza total A | 612 | 75, 195 | 135, 207 | 41.0 % (16.9 to 58.2) * | 39.3% (13.5 to 57.4) * |
| Influenza A (H1N1) pdm09 | 417 | 15, 60 | 135, 207 | 61.7 % (29.7 to 79.1) * | 56.7% (19.1 to 76.8) * |
| Influenza A (H3N2) | 537 | 60, 135 | 135, 207 | 31.9% (1.0 to 53.1) * | 33.2% (1.5 to 54.6) * |
| 2) 16-64 years | |||||
| Any Influenza | 555 | 61, 182 | 119, 193 | 45.6% (21.4 to 62.4) * | 43.4% (17.3 to 61.2) * |
| Influenza total A | 548 | 60, 176 | 119, 193 | 44.7% (19.8 to 61.9) * | 41.7% (14.4 to 60.3) * |
| Influenza A (H1N1) pdm09 | 381 | 13, 56 | 119, 193 | 62.3% (28.2 to 80.3) * | 56.2% (14.9 to 77.5) * |
| Influenza A (H3N2) | 479 | 47, 120 | 119, 193 | 36.5% (4.5 to 57.7) * | 34.5% (0.3 to 56.9) * |
| 3) ≥ 65 years | |||||
| Any Influenza | 64 | 15, 19 | 16, 14 | 30.9% (-85.2 to 74.2) | 32.5% (-82.4 to 75.0) |
| Influenza total A | 64 | 15, 19 | 16, 14 | 30.9% (-85.2 to 74.2) | 32.5% (-82.4 to 75.0) |
| Influenza A (H1N1) pdm09 | 36 | 2, 4 | 16, 14 | 56.3% (-176.2 to 93.1) | 59.7% (-176.6 to 94.1) |
| Influenza A (H3N2) | 58 | 13, 15 | 16, 14 | 24.2% (-112.9 to 73.0) | 25.7% (-110.2 to 73.7) |
VE: vaccine effectiveness, CI: confidence interval
Influenza total A: Influenza A (H1N1) pdm09 and Influenza A (H3N2)
n: total numbers of cases
* Statistically significant
a Eighteen patients aged ≤ 11 months were included.
b VE adjusted for age group, sex, month of onset of influenza infection (December vs. January vs. February and later), comorbidity in total children; VE adjusted for sex, month of onset of influenza infection (December vs. January vs. February and later) and comorbidity in each age group of children.
c VE adjusted for age group, sex, month of onset of influenza infection (December vs. January vs. February vs. March and later), comorbidity in total adults; VE adjusted for sex, month of onset of influenza infection (December vs. January vs. February vs. March and later), comorbidity in the younger adult group (16–64 years); VE adjusted for sex, month of onset of influenza infection (December and January vs. February and later) and comorbidity in the older adult group (≥ 65 years).
Among all children, the adjusted VE was significant against any influenza, 41.3% [95% confidence interval (CI): 19.7-57.2%]; influenza total A, 38.3% (95% CI: 15.1-55.1); and influenza A (H3N2), 39.8% (95% CI: 13.8-57.9%). When children were divided into 2 age groups (younger children: ≤6 years old, and older children: 7-15 years old), the VE was only significant in younger children; the adjusted VE was 44.8% (95% CI: 14.1-64.5%) against any influenza and 43.8% (95% CI: 12.5-63.9%) against influenza total A. When influenza total A was divided into influenza A (H1N1) pdm09 and A (H3N2), no significant VE was observed: influenza A (H1N1) pdm09, 48.3% (95% CI: −0.9-73.5%); and influenza A (H3N2), 38.4% (95% CI: −0.4-62.2%) (Table 2a).
Among all adults, the adjusted VE was significant against any influenza, 42.3% (95% CI: 17.9-59.5%); influenza total A, 39.3% (95% CI: 13.5-57.4%); influenza A (H1N1), 56.7% (95% CI: 19.1-76.8%); and A (H3N2), 33.2% (95% CI: 1.5-54.6%). When adults were divided into 2 age groups (younger adults: 16-64 years old, and older adults: ≥65 years old), the VE was only significant in younger adults; the adjusted VE was 43.4% (95% CI: 17.3-61.2%) against any influenza, 41.7% (95% CI: 14.4-60.3%) against influenza total A, 56.2% (95% CI: 14.9-77.5%) against influenza A (H1N1) pdm09, and 34.5% (95% CI: 0.3-56.9%) against influenza A (H3N2) (Table 2b). The VE against influenza B could not be estimated because of the small number of cases.
Vaccine doses
The relationship between the vaccine dose and adjusted ORs of the incidence of influenza is shown in Table 3. Vaccination doses correlated with decreasing rates of incidence of influenza, such as influenza total A and influenza A (H1N1) pdm09, in younger children (6 months to 6 years old). The adjusted OR was significant against any influenza, being 0.76 (95% CI: 0.60-0.96) in patients who had received 1 dose and 0.58 (95% CI: 0.36-0.93) in those who had received 2 doses (p value=0.0240); against influenza total A, being 0.76 (95% CI: 0.60-0.97) in patients who had received 1 dose and 0.58 (95% CI: 0.36-0.94) in those who had received 2 doses, (p value=0.0272); and against influenza A (H1N1) pdm09, being 0.69 (95% CI: 0.49-0.98) in patients who had received 1 dose and 0.48 (95% CI: 0.24-0.96) in those who had received 2 doses (p value=0.0383). However, in older children (7-12 years old), the adjusted OR was not significant against any type of influenza (Table 3a). A dose-dependent response relationship was suggested for the VE in younger children. However, no significant difference in the incidence of influenza existed between patients who received one dose and those who received two doses (Table 3b). The VE against influenza B could not be estimated because of the small number of cases.
Table 3.
Vaccine Effectiveness by the Number of Vaccine Doses in Children.
| a) Multivariate logistic regression analysis | ||||
|---|---|---|---|---|
| 1) Total | ||||
| Adjusted odds ratiosa(95% CI) | ||||
| Zero doses | One dose | Two doses | p value | |
| Any Influenza | 1.0 | 0.76 (0.63-0.91) | 0.57 (0.40-0.83) | 0.0033 * |
| Influenza total A | 1.0 | 0.78 (0.64-0.94) | 0.60 (0.42-0.88) | 0.0081 * |
| Influenza A (H1N1) pdm09 | 1.0 | 0.76 (0.58-0.98) | 0.57 (0.34-0.97) | 0.0378 * |
| Influenza A (H3N2) | 1.0 | 0.78 (0.63-0.96) | 0.60 (0.39-0.92) | 0.0201* |
| 2) ≤ 6 yearsb | ||||
| Any Influenza | 1.0 | 0.76 (0.60-0.96) | 0.58 (0.36-0.93) | 0.0240 * |
| Influenza total A | 1.0 | 0.76 (0.60-0.97) | 0.58 (0.36-0.94) | 0.0272 * |
| Influenza A (H1N1) pdm09 | 1.0 | 0.69 (0.49-0.98) | 0.48 (0.24-0.96) | 0.0383 * |
| Influenza A (H3N2) | 1.0 | 0.79 (0.61-1.03) | 0.62 (0.37-1.07) | 0.0850 |
| 3) 7-12 years | ||||
| Any Influenza | 1.0 | 0.76 (0.56-1.03) | 0.57 (0.31-1.05) | 0.0725 |
| Influenza total A | 1.0 | 0.80 (0.59-1.09) | 0.64 (0.35-1.19) | 0.1560 |
| Influenza A (H1N1) pdm09 | 1.0 | 0.90 (0.59-1.36) | 0.80 (0.35-1.84) | 0.6067 |
| Influenza A (H3N2) | 1.0 | 0.75 (0.52-1.07) | 0.56 (0.27-1.15) | 0.1114 |
| b) Pearson’s Chi-square test and Fischer’s exact test | ||||
| 1) Total | ||||
| Influenza infection (Yes: No) | ||||
| One dose | Two doses | p value | ||
| Any Influenza | 38:46 | 87:119 | 0.6393 | |
| Influenza total A | 36:46 | 87:119 | 0.7960 | |
| Influenza A (H1N1) pdm09 | 9:46 | 34:119 | 0.4394† | |
| Influenza A (H3N2) | 27:46 | 53:119 | 0.3461 | |
| 2) ≤ 6 yearsb | ||||
| Any Influenza | 16:22 | 59:81 | 0.9967 | |
| Influenza total A | 16:22 | 59:81 | 0.9967 | |
| Influenza A (H1N1) pdm09 | 2:22 | 20:81 | 0.2423† | |
| Influenza A (H3N2) | 14:22 | 39:81 | 0.4778 | |
| 3) 7-12 years | ||||
| Any Influenza | 22:24 | 28:38 | 0.5716 | |
| Influenza total A | 20:24 | 28:38 | 0.7536 | |
| Influenza A (H1N1) pdm09 | 7:24 | 14:38 | 0.7959† | |
| Influenza A (H3N2) | 13:24 | 14:38 | 0.4062 | |
CI: confidence interval
Influenza total A: Influenza A (H1N1) pdm09 and Influenza A (H3N2)
* Statistically significant
† Fischer’s exact test
aAdjusted for age (years), body temperature (°C), time from onset (hours)
bEighteen patients aged ≤ 11 months were included.
Outcomes
In children, among the 372 RIDT-positive cases, 99.5% [370/372] of the patients were prescribed neuraminidase inhibitors. The antivirals prescribed included oseltamivir [199], laninamivir octanoate hydrate [69], zanamivir hydrate [62], baloxavir marboxil [30], and peramivir hydrate [10]. The outcomes of 352 cases were traceable (vaccinated:not vaccinated=134:218). All patients recovered successfully.
In adults, among the 277 RIDT-positive cases, 99.6% [276/277] of the patients were prescribed neuraminidase inhibitors. The antivirals prescribed included oseltamivir [109], baloxavir marboxil [75], laninamivir octanoate hydrate [40], zanamivir hydrate [34], and peramivir hydrate [18]. The outcomes of 249 cases were traceable (vaccinated:not vaccinated=68:181, respectively). Although all patients ultimately recovered, four were admitted to other hospitals (one each for an asthma attack, complications of pneumonia, a severe headache, and pregnancy safety assessment). None of the admitted patients had been vaccinated.
Discussion
To our knowledge, this study is the first to report the VE against all types of influenza in all age groups using an RIDT. This study showed the significant VE in both younger children and younger adults and the difference in the VE between influenza A (H1N1) pdm09 and A (H3N2). Although the sample size was small, these results are comparable to those of previous reports obtained overseas using reverse transcription polymerase chain reaction (RT-PCR) (14-16).
During the 2018-2019 season, the influenza B epidemic was delayed and small in scale in Japan (13). The proportions of influenza A (H1N1) pdm09, influenza A (H3N2), and influenza B were 38%, 56%, and 6%, respectively (13), which were similar than in our own study. Since the analysis objectives for the VE mostly concerned influenza A, it was essential to distinguish between influenza A subtypes.
In younger children (≤6 years old), although significant VE was observed against any influenza (44.8%; 95% CI: 14.1-64.5%) and influenza total A (43.8%; 95% CI: 12.5-63.9%), no significant VE was found against either influenza A (H1N1) pdm09 (48.3%; 95% CI: −0.9-73.5%) or influenza A (H3N2) (38.4%; 95% CI: −0.4-62.2%). In contrast, in older children (7-15 years old), no significant VE was observed. This may be due to severe pandemics in elementary schools, the decline in the VE inherently associated with increasing age (5), and the decline in the VE with increasing age due to a low rate of vaccinations (7).
This study showed that significant VE was observed against any influenza (43.3%; 95% CI: 17.3-61.2%), influenza total A (41.7%; 95% CI: 14.4-60.3%), influenza A (H1N1) pdm09 (56.2%; 95% CI: 14.9-77.5%), and influenza A (H3N2) (34.5%; 95% CI: 0.3-56.9%) in younger adults (16-64 years old). Although the VE against influenza A (H1N1) pdm09 was slightly lower than previously reported, the results of this study, namely the high VE against influenza A (H1N1) pdm09 and low VE against influenza A (H3N2), are comparable to those findings obtained in previous studies conducted during the 2018-2019 season and a review (14-17). In contrast, in older adults (≥65 years old), no significant VE was observed. The immune response decreases among older adults, since the immune function gradually declines with age, including a decreased antibody response following vaccination (18-20). In older adults, both the initial immune response and the antibody response have been reported to have waned compared to those responses in younger adults during a single season (21,22). These factors may explain the VE variation among age groups.
Several limitations associated with the present study warrant mention. First, there may have been bias due to sample sizes; the markedly small sizes may have affected the results of this test-negative case-control study. However, the study builds a solid basis for future research using larger sample sizes. Second, since this study was conducted in a single clinic, sampling bias was unavoidable. However, because the patients were treated by the same physician, treatment was consistent across all cases. Third, since 2.9% of Linjudge™ FluA/pdm shows weak positive reaction for influenza A (H3N2) (information provided on the package insert), the diagnosis of influenza A (H1N1) pdm09 is not completely accurate. However, the diagnoses of influenza A (H1N1) pdm09 were made only when Linjudge™ FluA/pdm showed evident positive reactions. Fourth, since this study includes a relatively small number of influenza A (H1N1) pdm09 cases, the VE against influenza A (H1N1) pdm09 might have been slightly lower than previously reported (14-17). Fifth, we were unable to estimate the VE against influenza B in the 2018-2019 season because of the small number of cases. Finally, the RIDT is not 100% accurate. The precise identification of the influenza virus was not performed, such as by virus isolation or RT-PCR; however, a previous study showed no significant differences between the results estimated by an RIDT and RT-PCR data (23). In a previous study, a different brand of RIDT was used (RapidTesta Flu II; Sekisui Medical, Japan). Therefore, those results may not be applicable to the present study. However, in this study, the RIDT showed a comparable or higher concordance rate with viral isolation cultures compared to previous reports (ImunoAce™ Flu/positive concordance rates: influenza A, 94.3%; influenza B, 100%; and negative concordance rates: influenza A, 95.4%; influenza B, 98.7%; Linjudge™ FluA/pdm/positive concordance rate: 96.3%, and negative concordance rate: 98.4%; RapidTesta Flu II/positive concordance rates: influenza A, 96.5%; influenza B, 100%; and negative concordance rates: influenza A, 94.7%; influenza B, 95.7%) (11,12). Although a confirmation study must be conducted, the results of this study may be equivalent to those of studies using RT-PCR.
In conclusion, this study is the first to focus on the VE of the inactivated quadrivalent influenza vaccine against all types of influenza, including both influenza A (H1N1) pdm09 and A (H3N2), in all age groups using the RIDT through the 2018-2019 season in Japan. The VE was shown to vary with both age and influenza subtype. Distinguishing between influenza A (H1N1) pdm09 and influenza A (H3N2) when estimating the VE can be particularly valuable during seasons with few cases of influenza B, such as the 2018-2019 season. Without the method outlined herein, only the VE against influenza total A can be estimated. Furthermore, by using this method, the estimation of the VE by influenza A subtype can be performed in practically any clinic. At present, annual studies estimating the VE against influenza are performed separately by individual hospitals in Japan. With the participation of hospitals and clinics all over Japan, we can generate a national annual report estimating the influenza VE against all types of influenza and in all age groups, as is done overseas.
The author states that he has no Conflict of Interest (COI).
References
- 1.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2013 southern hemisphere influenza season [Internet]. [cited 2019 May 23]. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2013_south/en/
- 2.Skowronski DM, Masro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005-2006 season of dual A and B vaccine mismatch in Canada. Vaccine 25: 2842-2851, 2007. [DOI] [PubMed] [Google Scholar]
- 3.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomized placebo-controlled clinical trials. Euro Surveill 18: 20585, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine 35: 4796-4800, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Sugaya N, Shinjoh M, Nakata Y, et al. Three-season effectiveness of inactivated influenza vaccine in preventing influenza illness and hospitalization in children in Japan, 2013-2016. Vaccine 36: 1063-1071, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Seki Y, Onose A, Murayama T, Koide C, Sugaya N. Influenza vaccine showed a good preventive effect against influenza-associated hospitalization among elderly patients, during the 2016/17 season in Japan. J Infect Chemother 24: 873-880, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Ando S. Effectiveness of quadrivalent influenza vaccine based on the test-negative control study in children during the 2016-2017 season. J Infect Chemother 24: 782-788, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Ando S. Effectiveness of current and repeated vaccination with quadrivalent influenza vaccine during the 2017/18 season in Japan. Jpn J Antibiot 72: 143-155, 2019. [Google Scholar]
- 9.Shinjoh M, Sugaya N, Yamaguchi Y, et al. Effectiveness of trivalent inactivated influenza vaccine in children estimated by a test-negative case-control design study based on influenza rapid diagnostic test results. PLoS One 10: e0136539, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nerome K. Vaccine Handbook Researcher's Associates National Institute of Health. Maruzen, Tokyo, 1996: 91-102. [Google Scholar]
- 11. TAUNS Laboratories, Inc. Operating method of ImunoAce™ Flu. Shizuoka, Japan [Internet]. 2018 [cited 2019 May 29]. Available from: http://imunoace.jp/pdf/flu/imunoace_flu_packageinsert_c01.pdf (in Japanese)
- 12. Influenza study group, Japan Physicians Association. A manual for medical treatment of influenza (for the 2018-2019 season). 13th ed. Japan Physicians Association, 2018: 9-12 (in Japanese). [Google Scholar]
- 13.National Institute of Infectious Diseases. About influenza virus in this winter (2018/19 season). Tokyo, Japan [Internet]. 2019 [cited 2019 Aug 17]. Available from: https://www.niid.go.jp/niid/images/idsc/disease/influ/fludoco1819.pdf (in Japanese)
- 14.Doyle JD, Chung JR, Kim SS, et al. Interim estimates of 2018-2019 seasonal influenza vaccine effectiveness-United States, February 2019. MMWR 68: 135-139, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissling E, Rose A, Emborg HD, et al. Interim 2018/19 influenza vaccine effectiveness: six European studies, October 2018 to January 2019. Euro Surveill 24: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skowronski DM, Leir S, Sabaiduc S, et al. Interim estimates of 2018/19 vaccine effectiveness against influenza A(H1N1) pdm09, Canada, January 2019. Euro Surveill 24: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systemic review and meta-analysis of test-negative design studies. Lancet Infect Dis 16: 942-951, 2016. [DOI] [PubMed] [Google Scholar]
- 18.McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 30: 2060-2067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24: 1159-1169, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Beyer WE, Palache AM, Bailjet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine 7: 385-394, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, I-Cheng MC. Do antibody response to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 35: 212-221, 2017. [DOI] [PubMed] [Google Scholar]
- 22.Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, Van Wormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine 33: 246-251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki M, Minh le N, Yoshimine H, et al. Vaccine effectiveness against medically attended influenza in Japan. 2011-2012 season. PLoS One 9: e88813, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]