Abstract
A 58-year-old man was referred for obstructive jaundice. Imaging modalities revealed the presence of multiple pancreatic tumors and the stenosis of the middle common bile duct due to a hypoenhanced localized tumor. The multiple pancreatic tumors were histopathologically diagnosed as autoimmune pancreatitis by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). To differentiate between IgG4-related sclerosing cholangitis (IgG4-SC) and cholangiocarcinoma, we diagnosed the biliary tumor as IgG4-SC by EUS-FNA because of insufficient pathological materials obtained in a transpapillary manner. We herein report a case of IgG4-SC diagnosed by EUS-FNA.
Keywords: fine-needle aspiration, IgG4, IgG4-related diseases, obstructive jaundice, pancreatitis, sclerosing cholangitis
Introduction
IgG4-related sclerosing cholangitis (IgG4-SC) is a type of sclerosing cholangitis linked to high levels of serum IgG4 due to unknown mechanisms (1). According to the clinical diagnostic criteria of IgG4-SC from 2012 (2), an IgG4-SC diagnosis is made using a combination of biliary imaging, hematological examinations, histopathological findings, and concomitance with IgG4-related disease (IgG4-RD) (3).
On biliary imaging, IgG4-SC appears as segmental or diffuse stenosis of the intra- and/or extrahepatic bile duct with thickening of the bile duct wall. Biliary imaging of IgG4-SC is classified into four characteristic types of features. The differential diagnosis of IgG4-SC from other diseases causing bile duct stenosis is necessary. Examples of other bile duct stenoses are primary sclerosing cholangitis (PSC), cholangiocarcinoma, chronic pancreatitis, and pancreatic cancer (3). IgG4-SC has characteristic cholangiogram features, including lower and perihilar biliary strictures with mild upstream dilatation. Furthermore, endoscopic ultrasonography (EUS) and intraductal ultrasonography (IDUS) occasionally show wall thickening of the bile duct in both the stenotic and non-stenotic portion. An IgG4-SC histopathological examination shows the marked infiltration of inflammatory cells with lymphocytes and IgG4-positive plasma cells along with storiform fibrosis in the submucosal area of the bile duct.
IgG4-SC is generally treated by the oral administration of steroids (4) following the exclusion of malignant tumors. However, an IgG4-SC histopathological diagnosis, which is made by obtaining a histological sample through a transpapillary biliary biopsy, is very difficult due to the small size of the pathological samples (5). We herein report a case of IgG4-SC with uncommon biliary imaging findings and middle common bile duct stenosis that was diagnosed using EUS-guided fine-needle aspiration (FNA; EUS-FNA).
Case Report
A 58-year-old man was referred to our hospital for jaundice. Laboratory data showed the elevation of some biliary enzymes, including the following: alkaline phosphatase (1752 IU/L; normal range, 106-322 IU/L), γ-glutamyl transpeptidase (726 IU/L; normal range, 13-64 IU/L), total bilirubin (7.4 mg/dL; normal range, 0.4-1.5 mg/dL), and direct bilirubin (4.5 mg/dL; normal range, 0-0.2 mg/dL). In addition, the carcinoembryonic antigen level was 4.7 ng/mL (normal range, 0-5.0 ng/mL), and the cancer antigen 19-9 level was mildly elevated (54.7 U/mL; normal range, 0-37 U/L). The serum IgG and IgG4 levels were elevated to 2023 mg/dL (normal range, 861-1,747 mg/dL) and 253 mg/dL (normal range, 5-117 mg/dL), respectively.
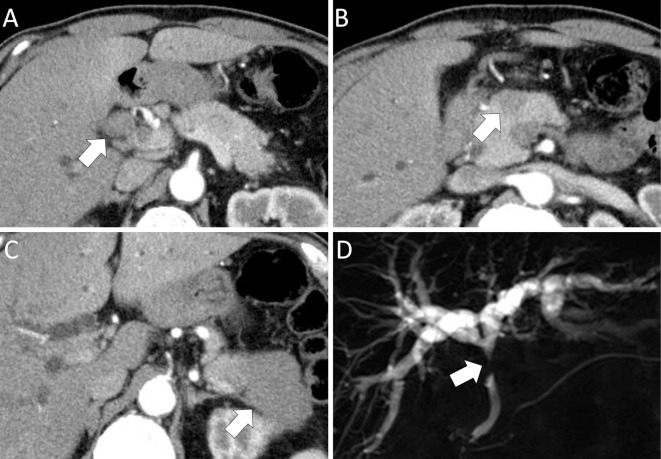
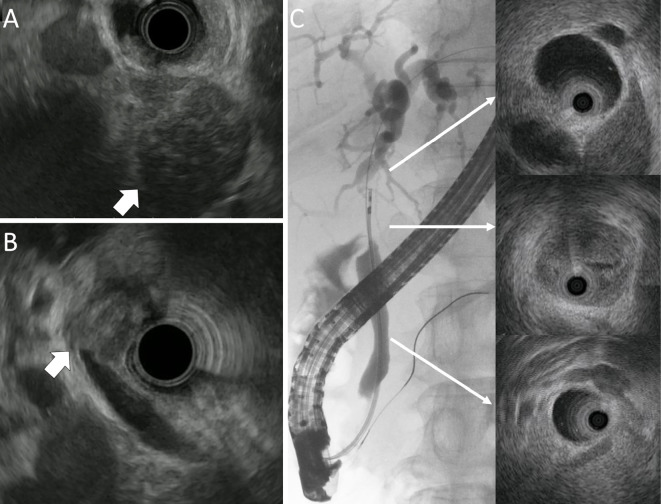
Contrast-enhanced computed tomography (CE-CT) revealed the presence of intrahepatic bile duct dilation and a hypoenhanced 20 mm localized tumor at the junction of the cystic and common bile duct (Fig. 1A). In addition, CE-CT also revealed several localized hypoenhanced tumors in the head and tail of the pancreas (Fig. 1B, C). Magnetic resonance cholangiopancreatography (MRCP) demonstrated stenosis of the middle common bile duct (Fig. 1D). The accumulation of fluorodeoxyglucose (FDG) was detected by FDG-positron emission tomography in multiple organs, including the pancreas, middle common bile duct, parotid glands, abdominal lymph node, and retroperitoneal tissue. EUS revealed the presence of multiple pancreatic hypoechoic tumors (Fig. 2A) and a localized tumor in the junction of the cystic and common bile duct (Fig. 2B). However, EUS and IDUS were unable to detect spreading of the bile duct's wall thickening (Fig. 2C). Based on these findings, the bile duct features were deemed to be uncommon for an IgG4-SC, therefore IgG4-RD with cholangiocarcinoma was suspected.
Figure 1.
CE-CT and MRCP findings of the bile duct and the pancreas. (A) CE-CT: hypoenhanced tumor localized on the junction of the cystic and common hepatic ducts (arrow). (B) CE-CT: hypoenhanced tumor in the head of the pancreas (arrow). (C) CE-CT: hypoenhanced tumor in the tail of the pancreas (arrow). (D) MRCP: intrahepatic bile duct dilation and common bile duct stenosis (arrow). CE-CT: contrast-enhanced computed tomography, MRCP: magnetic resonance cholangiopancreatography
Figure 2.
EUS and IDUS findings. (A) EUS: hypoechoic tumor in the head of the pancreas (arrow). (B) EUS: localized tumor at the junction of cystic and common bile duct (arrow). (C) IDUS: localized tumor at the junction of cystic and common bile duct. The absence of wall thickening in the intrahepatic duct and distal common bile duct was observed. EUS: endoscopic ultrasonography, IDUS: intraductal ultrasonography
The histopathological findings of EUS-FNA for the pancreatic tail tumor showing marked lymphoplasmacytic inflammation (IgG4/IgG-ratio: 50%) and dense fibrosis were confirmed a diagnosis of autoimmune pancreatitis (AIP). However, differentiation between cholangiocarcinoma and IgG4-SC was impossible because insufficient histopathological material had been obtained by transpapillary methods using brush cytology and a forceps biopsy during endoscopic retrograde cholangiopancreatography (ERCP) to confirm the diagnosis, despite the processes being repeated two times and four biopsy specimens being obtained for each procedure.
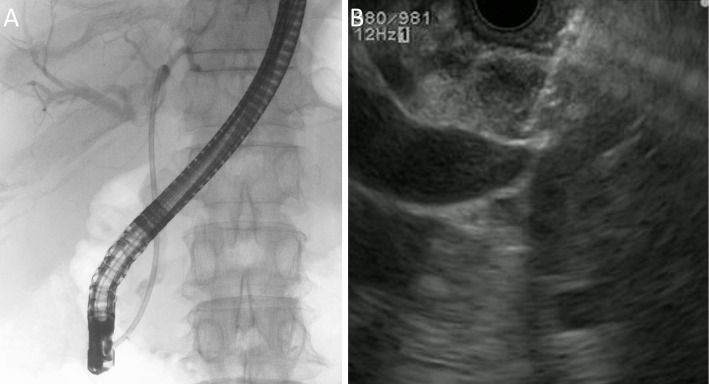
EUS-FNA was then attempted in an effort to obtain sufficient pathological material from the bile duct lesion of the middle common bile duct following the insertion of an endoscopic biliary stent (EBS) (Fig. 3). EUS-FNA was carefully performed as 3 punctures with a stylet slow-pull technique using a 22-gauge Endoscopic Ultrasound Aspiration Needle (Expect™ Needle; Boston Scientific, Marlborough, USA) to avoid penetrating the bile duct lumen and ensure there were no adverse events related to the procedure.
Figure 3.
EUS-FNA after endoscopic biliary stenting. (A) Endoscopic biliary stenting. (B) EUS-FNA. EUS-FNA: endoscopic ultrasonography-guided fine-needle aspiration
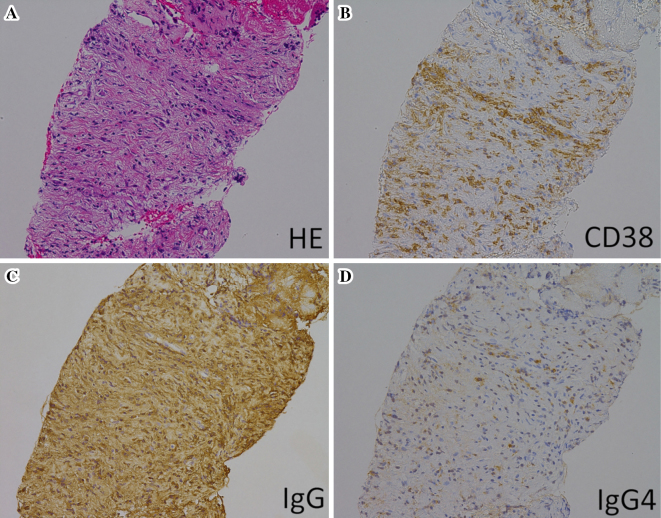
The EUS-FNA histopathological findings revealed marked lymphoplasmacytic inflammation and fibrosis. Infiltration of IgG- and IgG4-positive plasma cells (IgG4/IgG-ratio: 30-40%) was observed by immunohistochemistry (Fig. 4). The biliary lesion was finally diagnosed as IgG4-SC. Therefore, the patient was treated with prednisolone (PSL) at a dose of 40 mg/day, which was reduced by 5 mg per week to a dose of 20 mg/day. After 4 weeks of steroid treatment, imaging findings revealed an improvement in both the bile duct and pancreatic tumors (Fig. 5). Subsequently, the PSL dose was decreased by 2.5 mg per month to a dose of 5 mg/day. The patient has been treated with a continuous maintenance dose of PSL (5 mg/day) for 5 years without recurrence.
Figure 4.
Histopathological and immunochemistry findings. (A) Hematoxylin and Eosin staining and immunohistochemistry for (B) CD38, (C) IgG, (D) IgG4. All Figures are shown at 200×.
Figure 5.
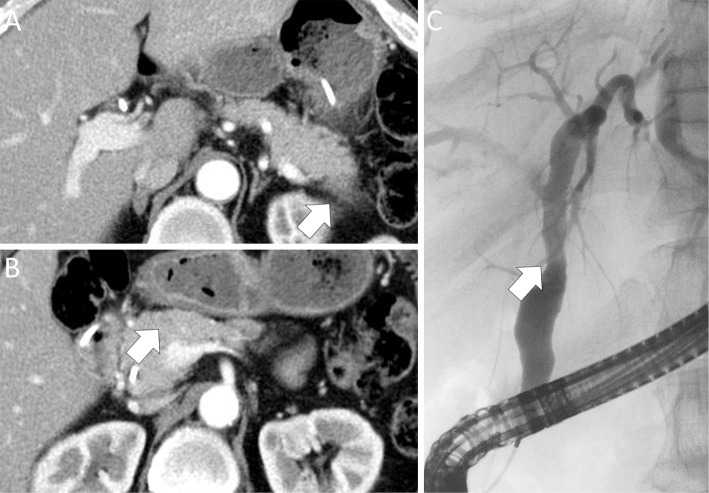
CE-CT and ERC findings after steroid therapy. (A) CE-CT: downsizing of the hypoenhanced tumor in the tail of the pancreas (arrow). (B) CE-CT: downsizing of the hypoenhanced tumor in the head of the pancreas (arrow). (C) ERC: improvement of bile duct stenosis (arrow). CE-CT: contrast-enhanced computed tomography, ERC: endoscopic retrograde cholangiography
Discussion
IgG4-SC is characterized by a distinct biliary stricture with serum IgG4 elevation and recognized as a biliary manifestation of IgG4-RD. Based on cholangiogram features, IgG4-SC is classified into four types of biliary stricture (6). Type 1 IgG4-SC is a localized biliary stricture in the distal common bile duct and is the most typical type of cholangiogram. Type 2 IgG4-SC shows diffuse strictures of intrahepatic and extrahepatic bile ducts. It requires a differential diagnosis from PSC. Furthermore, type 2 is subclassified by the presence (Type 2a) or absence (Type 2b) of prestenotic dilatation of the intrahepatic duct. Type 3 IgG4-SC is characterized by stricture in the perihilar hepatic lesion and distal common bile duct. Type 4 IgG4-SC has stricture only in the perihilar hepatic lesion. A national survey in Japan reported the following frequencies for the four types of IgG4-SC: type 1, 64%; type 2a, 5%; type 2b, 8%; type 3, 10%; type 4, 10% (4). Furthermore, findings of bile duct wall thickening were identified along all parts of the bile duct. The cholangiogram findings of the present case were not classified into any type of IgG4-SC due to the localized biliary stricture being observed only at the junction of the cystic and common bile duct, with no spreading of the wall thickening elsewhere. These findings were similar to cholangiocarcinoma.
The clinical practice guidelines for IgG4-SC provide some algorithms for the diagnosis of IgG4-SC, depending on the type of cholangiogram (5). The diagnostic exclusion of other biliary diseases, such as cholangiocarcinoma or PSC, is important. In particular, cholangiocarcinoma and IgG4-SC had to be distinguished in the present case because patients with AIP may develop carcinoma in several organs based on paraneoplastic syndrome (7). However, diagnostic confirmation is not possible when the histological material obtained through transpapillary collection during ERCP is insufficient (8-13). In order to differentiate IgG4-SC from cholangiocarcinoma, samples from the epithelium and a bile duct submucosal lesion are needed. Of note, specific IgG4-SC histological findings are located in the submucosal layer. A definitive diagnosis of IgG4-SC by a bile duct biopsy therefore requires the collection of samples containing the bile duct stroma.
Ghazale et al. reported the pathological diagnosis of IgG4-SC in 14 of 16 patients (88%) by a bile duct biopsy (14). However, Kawakami et al. (15), Naitoh et al. (16), and Hirano et al. (17) reported the diagnosis of IgG4-SC by a bile duct biopsy in 15 of 29 patients (52%), 3 of 17 patients (18%), and 0 of 5 patients (0%), respectively, which cannot be considered a good outcome. The poor ability to diagnose IgG4-SC by a bile duct biopsy may be attributed to the fact that endoscopic bile duct samples are often small, and collecting samples containing the bile duct stroma using biopsy forceps is challenging.
To obtain sufficient bile duct submucosal tissue for the diagnosis of IgG4-SC in the present case, we performed EUS-FNA from the biliary tumor. With EUS-FNA, we obtained a sufficient sample, including submucosa with stroma, and were able to make a diagnosis of IgG4-SC. The histological diagnosis of the bile duct was important in order to avoid an easy steroid trial.
Importantly, recent reports have shown that EUS-FNA is useful for the diagnosis of bile duct diseases. A systematic review and meta-analysis study demonstrated that the sensitivity and specificity of EUS-FNA for the diagnosis of biliary malignant stricture were 80% and 97%, respectively, suggesting that EUS-FNA has a high diagnostic ability for malignancy in biliary strictures (18). Although EUS-FNA for biliary diseases carries some concerns associated with adverse events, such as bile juice leakage and tumor seeding, some reports have described the safety of EUS-FNA for biliary malignant stricture and indicated that there have been almost no adverse events reported in association with EUS-FNA for extrahepatic cholangiocarcinoma (19). These reports suggest the usefulness of EUS-FNA for the diagnosis of bile duct diseases. However, the diagnostic role of EUS-FNA for IgG4-SC is unclear. To avoid complications of EUS-FNA in the bile duct, we performed EUS-FNA following the insertion of an EBS.
The findings of the present case suggest that EUS-FNA could be an effective diagnostic modality for IgG4-SC when it demonstrates wall thickness and/or mass. Further studies are needed to clarify EUS-FNA’s role and associated complications in the diagnosis of biliary diseases.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This work was supported by Department of Anatomic Pathology the Tohoku University Graduate School of Medicine.
References
- 1. Hamano H, Kawa S, Uehara T, et al. . Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis? Gastrointest Endosc 62: 152-157, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Ohara H, Okazaki K, Tsubouchi H, et al. . Clinical diagnostic criteria of IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 19: 536-542, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Umehara H, Okazaki K, Masaki Y, et al. . Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD). Mod Rheumatol 22: 21-30, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka A, Tazuma S, Okazaki K, et al. . Clinical features, response to treatment, and outcomes of IgG4-related sclerosing cholangitis. Clin Gastroenterol Hepatol 15: 920-926, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Kamisawa T, Nakazawa T, Tazuma S, et al. . Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci 26: 9-42, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas 32: 229, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Shiokawa M, Kodama Y, Yoshimura K, et al. . Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 108: 610-617, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Kitajima Y, Ohara H, Nakazawa T, et al. . Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol 22: 1615-1620, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Pugliese V, Conio M, Nicolò G, et al. . Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc 42: 520-526, 1995. [DOI] [PubMed] [Google Scholar]
- 10. Sugiyama M, Atomi Y, Wada N, et al. . Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol 91: 465-467, 1996. [PubMed] [Google Scholar]
- 11. Farrell RJ, Jain AK, Brandwein SL, et al. . The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc 54: 587-594, 2001. [DOI] [PubMed] [Google Scholar]
- 12. Ponchon T, Gagnon P, Berger F, et al. . Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc 42: 565-572, 1995. [DOI] [PubMed] [Google Scholar]
- 13. Volmar KE, Vollmer RT, Routbort MJ, et al. . Pancreatic and bile duct brushing cytology in 1000 cases: review of findings and comparison of preparation methods. Cancer 108: 231-238, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Ghazale A, Chari ST, Zhang L, et al. . Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 134: 706-715, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Kawakami H, Zen Y, Kuwatani M, et al. . IgG4-related sclerosing cholangitis and autoimmune pancreatitis: histological assessment of biopsies from Vater's ampulla and the bile duct. J Gastroenterol Hepatol 25: 1648-1655, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Naitoh I, Nakazawa T, Ohara H, et al. . Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol 44: 1147-1155, 2009. [DOI] [PubMed] [Google Scholar]
- 17. Hirano K, Tada M, Isayama H, et al. . Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc 71: 85-90, 2010. [DOI] [PubMed] [Google Scholar]
- 18. Sadeghi A, Mohamadnejad M, Islami F, et al. . Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc 83: 290-298, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Onoyama T, Matsumoto K, Takeda Y, et al. . Endoscopic ultrasonography-guided fine needle aspiration for extrahepatic cholangiocarcinoma: a safe tissue sampling modality. J Clin Med 8: 417, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]