Abstract
Objective
The influence of interferon (IFN)-free direct-acting antiviral (DAA) on hepatocellular carcinoma (HCC) recurrence remains unclear. Previous retrospective analyses revealed that the time interval between HCC curative treatment and IFN-free DAA induction is the critical factor affecting HCC recurrence. Thus, this study aimed to examine the influence of DAA therapy on HCC recurrence considering this interval.
Methods
Factors contributing to HCC recurrence were retrospectively analyzed using a landmark time analysis and time-dependent extended Cox proportional hazards model.
Patients
After screening 620 patients who were diagnosed with primary HCC from January 2001 to December 2016, 76 patients with early-stage (primary and solitary) disease who received curative treatment and were positive for serum hepatitis C virus RNA were included.
Results
HCC recurrence was observed in 8 of 17 (47.1%) patients who had received IFN-free DAA therapy and 45 of 59 (76.3%) who had not. No significant difference was seen between the IFN-free DAA (-) and IFN-free DAA (+) groups in the landmark time and time-dependent Cox proportional hazards model analyses. However, IFN-free DAA therapy tended to decrease the HCC recurrence rate after curative treatment for primary HCC in patients with chronic hepatitis. In addition, IFN-free DAA therapy tended to decrease the second HCC recurrence rate after treatment for the first HCC recurrence.
Conclusion
Our results, with a consideration of the time interval between HCC curative treatment and IFN-free DAA induction, showed that IFN-free DAA therapy was not associated with early-stage HCC recurrence after curative treatment.
Keywords: hepatitis C, hepatocellular carcinoma, direct-acting antivirals, recurrence, time interval
Introduction
Interferon (IFN)-free direct-acting antiviral (DAA) therapy has replaced IFN as the standard treatment for hepatitis C virus (HCV) infection based on its high efficacy and tolerability (1,2). It has also been actively used after curative treatment in patients with concurrent hepatocellular carcinoma (HCC). It is well-characterized that chronic inflammation due to HCV infection accelerates hepatic fibrosis, which promotes HCC development (3-5). Furthermore, several molecular studies have shown that the presence of HCV infection or HCV proteins themselves are involved in hepatic carcinogenesis (6-8). Therefore, the elimination of HCV by IFN-free DAA therapy is believed to suppress hepatic carcinogenesis, and its use in patients with HCC to prevent recurrence after curative treatment is acceptable.
However, a controversial Spanish study published in 2016 raised concerns that IFN-free DAA therapy may promote the progression of HCC (9). Since this report, many studies have investigated the influence of IFN-free DAA therapy on HCC progression. Several systematic reviews and meta-analyses have shown that IFN-free DAA therapy does not affect HCC recurrence (10-13). In contrast, single-center studies have shown that IFN-free DAA therapy may have either promotive or suppressive effects on HCC recurrence (14-18). Thus, the studies reported to date present conflicting data.
When investigating HCC recurrence, it is appropriate to analyze the period from the time of curative treatment to recurrence (19). However, several problems plague retrospective analyses on the effect of IFN-free DAA treatment on HCC recurrence. First, various factors must be considered at the primary HCC stage. Even after curative treatment, the risk of HCC recurrence is thought to differ among patients with single or multiple tumors and those with primary or recurrent tumors. Second, the interval between curative treatment and IFN-free DAA therapy is different. HCC recurrence rates are believed to differ according to this recurrence-free interval. When analyzing the effect of IFN-free DAA treatment on HCC recurrence, it is necessary to consider these factors and to adjust for them accordingly.
With the aforementioned gap in research, this study aimed to delineate the effect of IFN-free DAA therapy on HCC recurrence considering the above-mentioned factors. We limited the study to patients with primary and solitary HCC curatively treated with surgery, radiofrequency ablation (RFA), and stereotactic radiotherapy (SRT). Furthermore, to adjust for differences in the recurrence-free interval, we used a landmark time analysis and the time-dependent extended Cox proportional hazards model to retrospectively analyze the effect of IFN-free DAA therapy on HCC recurrence (20,21).
Materials and Methods
Study design and subjects
This study was approved by the Graduate School of Nagasaki University's Research Ethics Committee. Informed consent was obtained from most of the study's participants in accordance with the Declaration of Helsinki, and for those from whom we did not have the opportunity to obtain informed consent, the retrospective nature of the study enabled us to provide information about the research on the hospital's web page and to guarantee these patients the option to refuse participation in the research.
A total of 620 patients who were diagnosed with primary HCC from January 2001 to December 2016 at the Department of Gastroenterology and Hepatology in Nagasaki University Hospital were screened. The diagnosis of HCC was based on the typical findings detected by ultrasonography (US), dynamic computed tomography (CT), magnetic resonance imaging (MRI), abdominal angiography, and/or biopsy findings. The inclusion criteria were as follows: (i) primary and solitary HCC without metastasis and vessel invasion, (ii) positivity for serum HCV RNA at treatment for primary HCC, and (iii) primary HCC treated with surgery, RFA, and SRT curatively. The exclusion criterion was a follow-up period after curative treatment of <6 months.
Curative treatment with surgery was confirmed by findings of resection with a comfortable margin and the absence of HCC recurrence within six months after treatment. Curative treatment with RFA was confirmed by tumor findings contained within the ablation area on either enhanced CT or MRI at one to two weeks after treatment and the absence of HCC recurrence within six months after treatment. Similarly, curative treatment with SRT was confirmed by tumor findings contained in the cautery region on enhanced CT or MRI at three to six weeks after treatment and absence of HCC recurrence within six months after treatment. HCC recurrence was defined as the appearance of enhancement in the arterial phase on either dynamic CT or MRI, and the recurrence-free period was calculated based on the interval between curative treatment and confirmation of recurrence by imaging.
HCC recurrence was investigated by reviewing patients' medical records up to August 2019. Underlying liver diseases, such as chronic hepatitis (CH) and liver cirrhosis (LC), were diagnosed comprehensively based on blood laboratory examinations and US, CT, MRI, and endoscopy findings. The Fib-4 index was calculated based on the blood examination data at the curative treatment for primary HCC (22). The history of antiviral therapy after treatment for primary HCC, including IFN-based and IFN-free DAA therapy, was also extracted from the medical records. Anti-HCV therapy was initiated based on the attending physicians' discretion. Regarding the components of the anti-HCV therapy, IFN-based anti-HCV therapy was given until August 2014, and IFN-free DAA therapy was given after September 2014.
To determine the influence of IFN-free DAA therapy on HCC recurrence, we compared the recurrence rate between patients who received IFN-free DAA therapy and those who did not. The factors contributing to HCC recurrence were also examined.
Statistical analyses
To compare the patients' baseline characteristics, categorical data were summarized by the frequency and rate and analyzed using Fisher's exact test, whereas numerical data were summarized by the median and analyzed using Wilcoxon's rank sum test.
To compare the recurrence rate after curative treatment in the presence or absence of IFN-free DAA therapy, a landmark time analysis was conducted to adjust for the difference in the recurrence-free interval after curative HCC treatment (23). The analysis was performed by setting the landmark time to 1 year and 2 years. To determine the factors contributing to HCC recurrence, a time-dependent, extended Cox proportional hazards model was developed. The time period between primary HCC treatment and HCC recurrence was set as the objective variable, while the age, sex, primary HCC diameter, underlying liver disease (CH and/or LC), treatment for primary HCC (surgery, RFA, or SRT), administration of IFN-free or IFN-based anti-HCV therapy after primary HCC treatment, and the presence of early enhancement of primary HCC were set as explanatory variables. The administration of IFN-free DAA therapy was set as the time-dependent explanatory variable (20, 21, 24). The time-dependent expanded Cox proportional hazards model was analyzed as follows:
h(t, X(t))= h0(t)exp [β1*Age (years)+β2*Gender (Man/Woman)+β3*HCC diameter (cm)+β4*Underlying liver disease (CH/LC)+β5*therapy (Operation/ RFA/ SRT)+β6*IFN-free DAA therapy (t)+β7*IFN-based therapy+β8*early phase enhancement of primary HCC]
The software programs SAS for Windows Release ver. 9.4 (SAS Institute, Cary, USA) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for the analyses. A two-tailed p<0.05 indicated a significant difference.
Results
Characteristics of the study population
A total of 76 patients met the inclusion criteria and were included in the final analysis (Fig. 1). The baseline characteristics of the patients are presented in Table 1. IFN-free DAA therapy was administered to 17 patients (22.4%) during the follow-up period [IFN-free DAA (+) group] but not to the remaining 59 patients (77.6%) [IFN-free DAA (-) group]. Within the IFN-free DAA (+) group, 8 patients received daclatasvir and asunaprevir combination therapy for 24 weeks and 9 received sofosbuvir and ledipasvir combination therapy for 12 weeks. The median duration from curative treatment for primary HCC to induction of IFN-free DAA therapy was 0.72 (0.31-10.4) years. All patients completed their course of treatment and achieved a sustained virologic response (SVR). No significant differences in the sex, age at the diagnosis with primary HCC, underlying liver disease, or Fib4 index were found between the two groups. The diameter of the primary HCC in the IFN-free DAA (+) group was significantly less than that in the IFN-free DAA (-) group. Furthermore, no significant difference in the treatment for primary HCC was found between the groups. IFN therapy was administered to 8 of 59 patients (13.6%) in the IFN-free DAA (-) group and 1 of 17 (5.9%) in the IFN-free DAA (+) group during the follow-up period. Two patients in the IFN-free DAA (-) group achieved SVR by IFN therapy.
Figure 1.
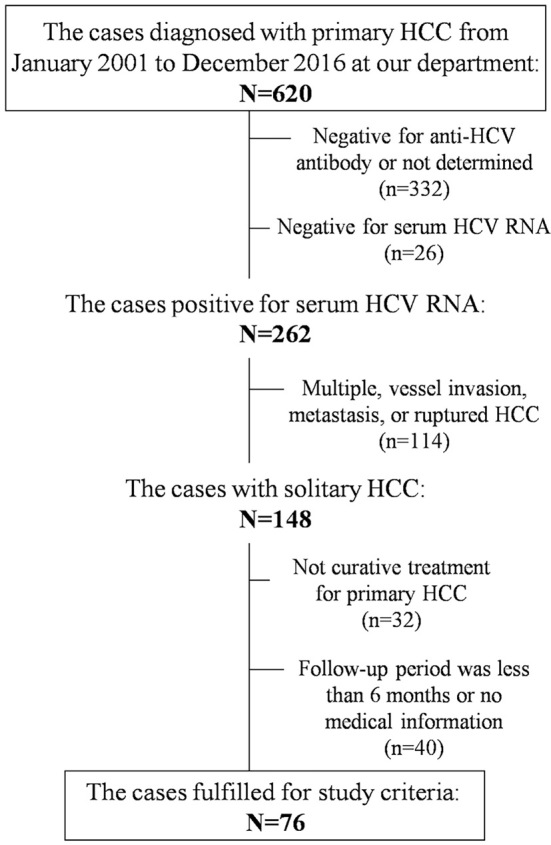
Flowchart illustrating the method of screening patients for the present study.
Table 1.
Characteristics of the Study Population.
| Number (%) or Median (range) | IFN-free DAA (-) (n=59) |
IFN-free DAA (+) (n=17) |
p value | |||
|---|---|---|---|---|---|---|
| Sex: male/female | 33 (55.9)/26 (44.1) | 9 (52.4)/8 (47.1) | 0.953 | |||
| Age at diagnosis with primary HCC, years | 72.0 (55-86) | 74.0 (50-85) | 0.557 | |||
| Underlying liver disease, CH/LC | 24 (40.7)/35 (59.3) | 5 (29.4)/12 (70.6) | 0.576 | |||
| Fib4 index | 4.90 (1.19-17.8) | 5.46 (1.45-15.1) | 0.837 | |||
| Diameter of the primary HCC, cm | 2.0 (0.7-8.0) | 1.4 (0.6-3.4) | 0.004 | |||
| Diameter of the primary HCC, ≤2.0 cm/>2.0 cm | 34 (57.6)/25 (42.4) | 16 (94.1)/1 (5.9) | 0.012 | |||
| Early phase enhancement of primary HCC, (-)/(+) | 6 (10.1)/53 (89.8) | 5 (29.4)/12 (70.6) | 0.118 | |||
| Therapy for primary HCC | ||||||
| Surgery | 21 (35.6) | 5 (29.4) | 0.319 | |||
| RFA | 35 (59.3) | 9 (52.9) | ||||
| SRT | 3 (5.1) | 3 (17.6) | ||||
| IFN therapy induction during follow up period, (-)/(+) | 51 (86.4)/8 (13.6) | 16 (94.1)/1 (5.9) | 0.662 | |||
| IFN-free DAA therapy regimen | ||||||
| DCV/ASV | 8 (47.1) | |||||
| SOF/LDV | 9 (52.9) | |||||
| Interval between HCC treatment and IFN-free DAA therapy, years | 0.72 (0.31-10.4) |
Categorical data are presented as numbers (percentages) of patients and numerical data as median (range).
CH: chronic hepatitis, DCV/ASV: daclatasvir and asunaprevir combination therapy, HCC: hepatocellular carcinoma, IFN-free DAA: interferon free direct-acting antiviral therapy, LC: liver cirrhosis, RFA: radiofrequency ablation, SOF/LDV: sofosbuvir and ledipasvir combination therapy, SRT: stereotactic radiotherapy
Influence of IFN-free DAA therapy on recurrence after curative treatment for primary HCC
HCC recurrence was observed in 45 of 59 (76.3%) patients in the IFN-free DAA (-) group and in 8 of 17 (47.1%) in the IFN-free DAA (+) group. The median periods from curative treatment for primary HCC to recurrence in the IFN-free DAA (-) and IFN-free DAA (+) groups were 1.72 and 3.10 years, respectively.
Fig. 2 shows the landmark time analysis of the recurrence rates in the two groups. The landmark time of Fig. 2A was set at one year. Sixty-one patients had no HCC relapse for one year after the curative treatment. Among them, eight patients were treated with IFN-free DAA therapy during that time. Similarly, the landmark time of Fig. 2B was set at two years. Thirty-nine patients had no HCC relapse for two years after the curative treatment. Among them, seven patients were treated with IFN-free DAA therapy during that time. Furthermore, no significant difference was found between the IFN-free DAA (-) and IFN-free DAA (+) groups in the landmark time analysis set at 1 year and 2 years.
Figure 2.
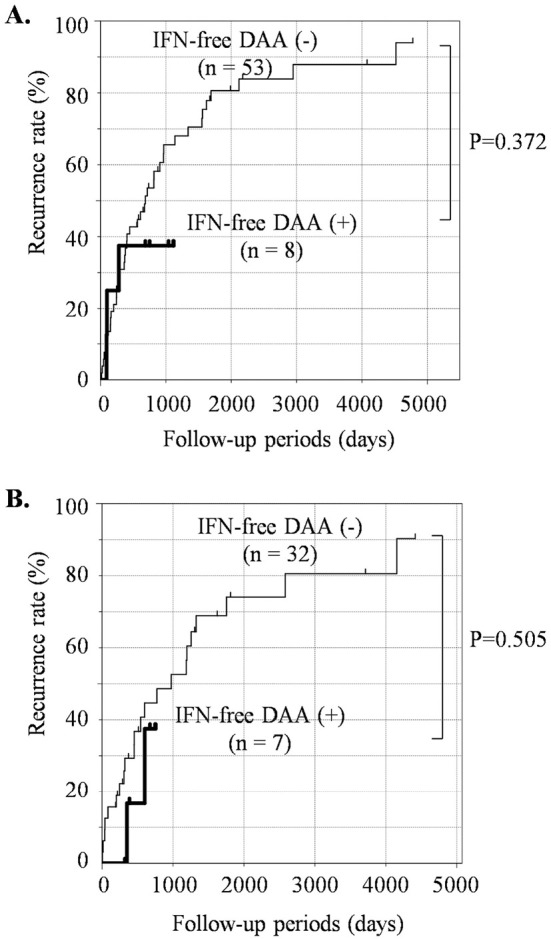
Cumulative HCC recurrence rate after curative treatment for primary and solitary HCC in the presence or absence of IFN-free DAA therapy (landmark time analysis). The HCC recurrence rate in patients who had received IFN-free DAA therapy [IFN-free DAA (+), bold line] or had not [IFN-free DAA (-), thin line] was analyzed by a landmark time analysis set at 1 year (A) and 2 years (B). HCC: hepatocellular carcinoma, IFN-free DAA: interferon-free direct-acting antiviral therapy
Influence of IFN-based therapy on recurrence after curative treatment for primary HCC
There have been many reports about the influence of IFN-based therapy on HCC recurrence after curative treatment for primary HCC (25-27). In this study, nine patients received IFN-based therapy after curative treatment for primary HCC, and only two patients achieved SVR. Among them, one patient with chronic hepatitis did not develop HCC recurrence after IFN-based therapy. The other patient with liver cirrhosis developed HCC at 6.5 years after curative treatment (4.5 years after the IFN treatment); however, a second instance of recurrence was not observed in the subsequent 2.4 years. In contrast, HCC continued to recur during the follow-up period in patients who did not achieve SVR by IFN-based therapy, except for in one patient who subsequently received IFN-free DAA therapy. No recurrence has been observed in patients who received both IFN-based and IFN-free DAA therapies. On comparing the recurrence rates after curative treatment for primary HCC by the landmark time analysis set at 1 year, no significant difference in the recurrence rate was found between patients treated with IFN-based therapy and those without IFN-based therapy (p=0.319, data not shown).
The analysis of risk factors for HCC recurrence after curative treatment
We analyzed the factors contributing to HCC recurrence using a time-dependent expanded Cox proportional hazards model that considers the time to induction of IFN-free DAA therapy. Multivariate analysis results are shown in Table 2. No significant factors, including a history of IFN-free DAA therapy, were found among the study participants.
Table 2.
Multivariate Analysis of Risk Factors Associated with HCC Recurrence.
| Variable | Hazard ratio (95% CI) | p value | ||||
|---|---|---|---|---|---|---|
| Sex | female | 0.856 (0.445-1.648) | 0.642 | |||
| Age | year | 1.007 (0.962-1.053) | 0.774 | |||
| Background | LC | 1.383 (0.660-2.895) | 0.390 | |||
| Diameter of primary HCC | cm | 1.126 (0.487-2.605) | 0.782 | |||
| Early phase enhancement of primary HCC | (+) | 1.372 (0.456-4.128) | 0.574 | |||
| Therapy for primary HCC | RFA | 0.937 (0.394-2.229) | 0.884 | |||
| SRT | 1.379 (0.349-5.594) | 0.637 | ||||
| IFN-based therapy | (+) | 0.921 (0.332-2.550) | 0.874 | |||
| IFN-free DAA therapy | (+) | 0.917 (0.358-2.344) | 0.856 |
CI: confidence interval, HCC: hepatocellular carcinoma, IFN-free DAA: interferon-free direct-acting antiviral therapy, LC: liver cirrhosis, RFA: radiofrequency ablation, SRT: stereotactic radiotherapy
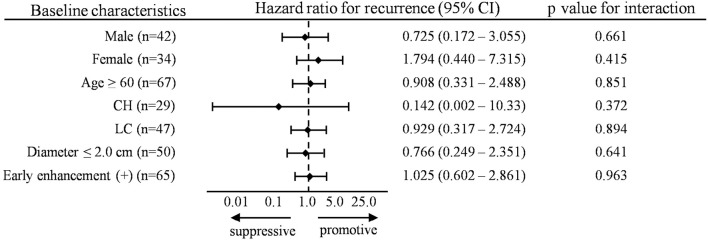
In addition, we analyzed the influence of IFN-free DAA therapy on HCC recurrence according to the subgroup analysis using a time-dependent extended Cox proportional hazards model. As shown in Fig. 3, IFN-free DAA therapy was not significantly associated with HCC recurrence after curative treatment in any of the subgroups. However, IFN-free DAA therapy in the presence of CH exhibited a trend towards decreased HCC recurrence rates (hazard ratio=0.142, p=0.372).
Figure 3.
Subgroup analyses of the influence of IFN-free DAA therapy on recurrence after curative treatment. Subgroup analyses of the hazard ratio of the influence of IFN-free DAA therapy on HCC recurrence by a time-dependent extended Cox proportional hazards model. HCC: hepatocellular carcinoma, IFN-free DAA: interferon-free direct-acting antiviral therapy
Influence of IFN-free DAA therapy on second HCC recurrence after curative treatment for primary HCC
Some previous studies reported that anti-HCV therapy following HCC treatment prevented a second HCC recurrence but not a first recurrence (25,27-31). We therefore analyzed the period until the second recurrence after treatment for first HCC recurrence in our study population.
The period until the second recurrence after treatment for first HCC recurrence was analyzed using the Kaplan-Meier method in patients followed for more than six months after the treatment for the first recurrence. As shown in Fig. 4A, no significant difference was found between the IFN-free DAA (-) and (+) groups (p=0.068). Similarly, no significant difference was noted in those who had received curative treatment for the first HCC recurrence (p=0.115) (Fig. 4B). However, IFN-free DAA therapy tended to reduce the frequency of a second HCC recurrence.
Figure 4.
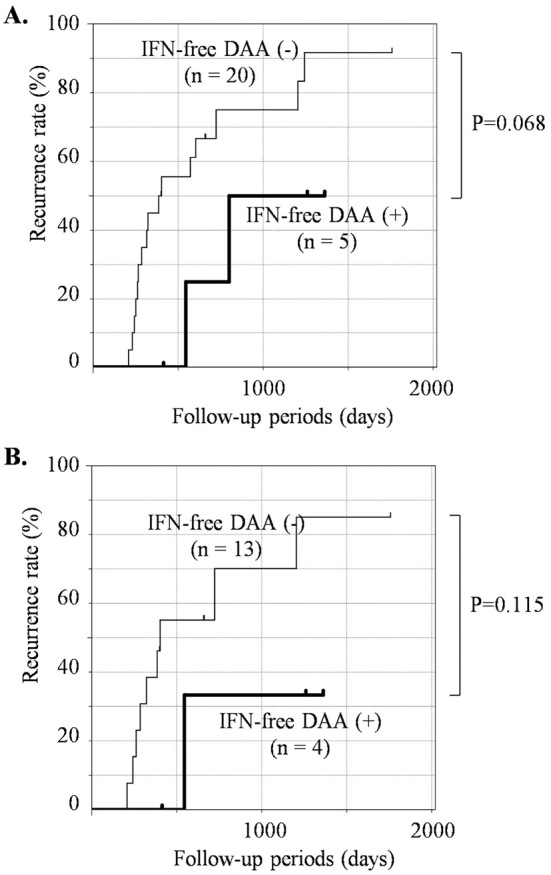
Cumulative second HCC recurrence rate after treatment for the first HCC recurrence in the presence or absence of IFN-free DAA therapy. The cumulative second HCC recurrence rate after treatment for the first HCC recurrence was analyzed by a Kaplan-Meier analysis (A) in all patients with a first HCC recurrence (n=25) and (B) in the patients who had received curative treatment for the first HCC recurrence (n=17). The patients who received IFN-free DAA therapy [IFN-free DAA (+) group] are represented as a bold line, and those who had not received [IFN-free DAA (-) group] are presented as a thin line. HCC: hepatocellular carcinoma, IFN-free DAA: interferon-free direct-acting antiviral therapy
Discussion
In the present study, we analyzed the effect of IFN-free DAA therapy on HCC recurrence in patients with primary, solitary tumors that had been treated curatively. Although several studies have investigated this issue, to our knowledge, none have limited their analysis to patients with primary, solitary HCC. Our results showed that IFN-free DAA therapy did not have a significant effect on early-stage HCC recurrence. However, IFN-free DAA therapy tended to reduce the HCC recurrence rate after curative treatment for primary HCC in patients with CH and a second instance of HCC recurrence after treatment for the first HCC recurrence.
The precise effects of IFN-free DAA therapy on HCC recurrence after curative treatment remain unclear. Some retrospective studies have shown that the progression of hepatic fibrosis, tumor-specific factors at the time of curative treatment (such as numbers and size), and number of previous treatments sessions are associated with the HCC recurrence rate after IFN-free DAA therapy (17,18,32). In this study, we limited the analysis to patients with primary, solitary HCC cases that had been treated curatively to minimize the influence of tumor-specific factors. Therefore, although the sample size was limited, the analysis focused more on the effect of IFN-free DAA therapy itself on HCC recurrence than other studies.
In addition, in a systematic review and meta-analysis of 24 studies (1,820 subjects) conducted by Saraiya (33), the risk of HCC recurrence after IFN-free DAA therapy was higher in patients with a short interval between curative treatment for HCC and IFN-free DAA therapy initiation than those with a long interval. Therefore, assessing the timing of IFN-free DAA therapy after HCC curative treatment is extremely important when retrospectively analyzing the effect of IFN-free DAA therapy on HCC recurrence (19). To take this factor into account, we used a landmark time analysis and time-dependent extended Cox proportional hazards model with this between-treatment interval as the time-dependent variable and showed that IFN-free DAA therapy did not have a significant influence on early-stage HCC recurrence. If conventional Kaplan-Meier and Cox proportional hazards model analyses had been used, we would have concluded that IFN-free DAA decreased the HCC recurrence (data not shown). This discrepancy may be explained by bias during selection for the induction of IFN-free DAA therapy. In other words, IFN-free DAA therapy is more easily given to patients who have survived and remained recurrence-free for a long time and are at a low risk of recurrence. Attention must be given to conventional Kaplan-Meier and Cox proportional hazards model analyses when retrospectively analyzing the effect of IFN-free DAA therapy on HCC recurrence.
In the stratified analysis, the presence of CH exhibited a trend toward decreased HCC recurrence rates. This may be due to a halt in genetic mutations after IFN-free DAA therapy in the context of CH, in contrast to LC, where such mutations would have already accumulated. In addition, despite the small sample size, the second recurrence rate after treatment for the first HCC recurrence tended to be lower in patients who had been treated with IFN-free DAA therapy after curative treatment for primary HCC than those without IFN-free DAA therapy (Fig. 4). This is similar to the reports that IFN treatment after curative HCC treatment did not affect the first HCC recurrence rate but did have a suppressive effect on the second HCC recurrence rate (25,27,28,30).
However, some limitations associated with this study should be noted. First, IFN-free DAA therapy was initiated based on the attending physicians' discretion. In addition, selection bias cannot be denied for cases receiving IFN-free DAA therapy considered to have a reduced risk of HCC recurrence. Second, as we limited the analysis to patients with primary, solitary tumors, the sample size was relatively small. A prospective study based on large, evenly matched patient groups (in terms of tumor size) is required to definitively determine the effect of IFN-free DAA therapy on HCC recurrence. However, conducting such a study prospectively may face ethical issues due to the efficacy of IFN-free DAA therapy in inhibiting liver function deterioration, leading to an improved HCC prognosis.
Conclusion
IFN-free DAA therapy did not contribute to early-stage HCC recurrence after curative treatment according to a time-dependent extended Cox proportional hazards model analysis. However, a potential promotive effect cannot be definitively excluded, and future studies with larger numbers of patients are required. The time interval between curative treatment for HCC and IFN-free DAA therapy must be taken into account in retrospective analyses, which can be achieved using a landmark time analysis and time-dependent extended Cox proportional hazards models.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank our colleagues at the Department of Gastroenterology and Hepatology, Graduate School of Biomedical Sciences, Nagasaki University, for their kind cooperation and support.
References
- 1. Saab S, Le L, Saggi S, Sundaram V, Tong MJ. Toward the elimination of hepatitis C in the United States. Hepatology 67: 2449-2459, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Flisiak R, Pogorzelska J, Flisiak-Jackiewicz M. Hepatitis C: efficacy and safety in real life. Liver Int 37 (Suppl): 26-32, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 127: S35-S50, 2004. [DOI] [PubMed] [Google Scholar]
- 4. El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 36: S74-S83, 2002. [DOI] [PubMed] [Google Scholar]
- 5. Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J Hepatol 51: 810-820, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Irshad M, Gupta P, Irshad K. Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection. World J Hepatol 9: 1305-1314, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tornesello ML, Buonaguro L, Izzo F, Buonaguro FM. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget 7: 25087-25102, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rusyn I, Lemon SM. Mechanisms of HCV-induced liver cancer: what did we learn from in vitro and animal studies? Cancer Lett 345: 210-215, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 65: 719-726, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 67: 1204-1212, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer 140: 1042-1049, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol 9: 262-270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konjeti VR, John BV. Interaction between hepatocellular carcinoma and hepatitis C eradication with direct-acting antiviral therapy. Curr Treat Options Gastroenterol 16: 203-214, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Yamashita N, Tanimoto H, Shimoda S, Komori A, Nomura H. Rapidly growing hepatocellular carcinoma recurrence during direct-acting antiviral treatment for chronic hepatitis C. Clin J Gastroenterol 11: 497-500, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Warzyszynska K, Jonas M, Wasiak D, Kosieradzki M, Malkowski P. Accelerated hepatocellular carcinoma recurrence rate after postoperative direct-acting antivirals treatment - preliminary report. Clin Exp Hepatol 3: 194-197, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villani R, Facciorusso A, Bellanti F, et al. DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during anti-HCV treatment. PLoS One 11: e0167934, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeda K, Kawamura Y, Kobayashi M, et al. Direct-acting antivirals decreased tumor recurrence after initial treatment of hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci 62: 2932-2942, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Mashiba T, Joko K, Kurosaki M, et al. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One 13: e0194704, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guarino M, Vigano L, Ponziani FR, et al. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig Liver Dis 50: 1105-1114, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 20: 145-157, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Ngwa JS, Cabral HJ, Cheng DM, et al. A comparison of time dependent Cox regression, pooled logistic regression and cross sectional pooling with simulations and an application to the Framingham Heart Study. BMC Med Res Methodol 16: 148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46: 32-36, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Klein JP, Logan B, Harhoff M, Andersen PK. Analyzing survival curves at a fixed point in time. Stat Med 26: 4505-4519, 2007. [DOI] [PubMed] [Google Scholar]
- 24. Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med 376: 1332-1340, 2017. [DOI] [PubMed] [Google Scholar]
- 25. Saito T, Chiba T, Suzuki E, et al. Effect of previous interferon-based therapy on recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int J Med Sci 11: 707-712, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeong SC, Aikata H, Katamura Y, et al. Effects of a 24-week course of interferon-alpha therapy after curative treatment of hepatitis C virus-associated hepatocellular carcinoma. World J Gastroenterol 13: 5343-5350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagihara H, Nouso K, Kobayashi Y, et al. Effect of pegylated interferon therapy on intrahepatic recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. Int J Clin Oncol 16: 210-220, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Shiratori Y, Shiina S, Teratani T, et al. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med 138: 299-306, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 44: 1543-1554, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Miyatake H, Kobayashi Y, Iwasaki Y, et al. Effect of previous interferon treatment on outcome after curative treatment for hepatitis C virus-related hepatocellular carcinoma. Dig Dis Sci 57: 1092-1101, 2012. [DOI] [PubMed] [Google Scholar]
- 31. Sakae M, Kubo S, Takemura S, et al. Effect of interferon therapy on first and second recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. Hepatol Res 42: 564-573, 2012. [DOI] [PubMed] [Google Scholar]
- 32. Cabibbo G, Petta S, Calvaruso V, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct-acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther 46: 688-695, 2017. [DOI] [PubMed] [Google Scholar]
- 33. Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with meta-analysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther 48: 127-137, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]