Abstract
Background
New dressings purport to reduce surgical wound complications after total hip arthroplasty (THA). This study compared delayed wound healing rates and reoperations between 2 increasingly popular dressings: a silver-impregnated occlusive (standard) dressing and a 2-octyl cyanoacrylate adhesive with polyester mesh.
Methods
This retrospective cohort study reviewed 431 consecutive THAs performed by 2 surgeons between January 2017 and May 2019. One hundred and eight were excluded for not using standard or mesh dressings. A final 323 cases were separated into 2 cohorts: mesh (n = 186) and standard dressings (n = 137). Standard dressings were removed at 1 week. Mesh persisted until nonadherent, approximately 3-4 weeks. The surgeon assessed delayed wound healing at the 2-week postoperative visit. Secondary outcomes include deep infection and return to the operating room for a wound-related diagnosis. Differences were determined using the chi-square test.
Results
There were no demographic, comorbidity, or surgical differences between groups. There were 22 total cases of delayed wound healing with 7 (3.8%) in the mesh group and 15 (10.9%) in the standard dressing group (P = .01). There were no significant differences in reoperations (2 [1.1%] vs 2 [1.5%], P = .76) or deep infections (2 [1.1%] vs 1 [0.7%], P = .75).
Conclusions
Mesh dressings are a safe and reliable dressing type for THA and were associated with a decrease in early wound healing complications when compared with standard, silver-impregnated occlusive dressings in this retrospective series. The mesh tension sharing properties and longer duration of occlusive protection may explain this difference.
Level of Evidence
Level III.
Keywords: Total hip arthroplasty, Wound-healing complications, Dressings
Introduction
Prosthetic joint infections (PJIs) and surgical site infections (SSIs) are devastating complications of total hip arthroplasty (THA) that significantly increase the morbidity, reoperation rate, and cost burden of THAs. The rate of THA infections is reported between 0.5% and 0.88% of all operations [[1], [2], [3], [4]]. Patients with PJI after THA have a nearly double hospital length of stay and hospitalization charges as their noninfected counterparts [3]. Furthermore, PJI and SSI contribute to increased management costs for surgeons who must follow up these patients at more frequent intervals in the postoperative period and to increased stress for patients and families.
Wound closure and preparation strongly influence rates of wound complications and PJI in both primary and revision total joint arthroplasty [[5], [6], [7]]. Delayed wound healing is a leading and potentially preventable risk factor for PJI and SSI [4,8]. No standard of care exists for postoperative total hip dressings and optimal forms of wound management after arthroplasty continue to be examined. A successful THA requires a relatively active recovery and therefore may present a higher tension wound environment. Owing to this higher tension environment, wound dehiscence and ischemia are possible complications after surgery [9].
Several innovative dressings have been developed to improve wound healing after arthroplasty surgery. Skin glue, such as 2-octyl cyanoacrylate adhesive (Dermabond, Ethicon, Somerville, NJ), and n-butyl-2-cyanoacrylate adhesive (SwiftSet, Covidien, Dublin, Ireland) have been used regularly to seal the epidermis and limit ingress and egress of fluid through a surgical wound. In addition, unique surgical dressings have been created such as the Aquacel Ag (Aquacel® Ag Surgical Cover Dressing, ConvaTec, Berkshire, UK), a silver-impregnated, occlusive, hydrofiber-based dressing that can protect a surgical wound for 7 days. Aquacel Ag has been shown in multiple studies to result in fewer wound infections and improved wound healing in comparison to traditional gauze-based dressings, likely because of its occlusive and antimicrobial properties [[10], [11], [12]]. Recently, a novel dressing has been developed combining the aforementioned dressing concepts to both protect and share tension across a surgical wound. The dressing is a combination of the skin glue 2-octyl cyanoacrylate adhesive and a polyester mesh (Dermabond Prineo, Ethicon, Somerville, NJ), which has been shown to decrease time to closure, wound edge ischemia, decrease wound drainage, improve cosmetic appearance, and decrease tensile stress on the wound [[13], [14], [15], [16], [17]].
An Aquacel Ag dressing with or without n-butyl-2-cyanoacrylate adhesive was the standard dressing used by the senior authors for THAs for several years, based on previously published results [11]. This study sought to compare that standard dressing with the novel mesh dressing for THA. To date, there are no studies comparing the clinical results of the Aquacel Ag and Prineo dressings in THA patients. The primary outcome measure was delayed wound healing. Secondary outcomes evaluated were need for any further intervention on the wound including return to the operating room for wound closure revision or addition of sutures to reinforce the wound in the office.
Material and methods
This retrospective study was approved by the institutional review board. Four hundred and thirty one consecutive THAs performed by 2 surgeons between January 2017 and May 2019 were identified. One hundred and eight were excluded for not using either dressing type. Thus, 323 cases were separated into 2 concurrent cohorts: mesh (n = 186, 58%) and standard dressings (n = 137, 42%) (Fig. 1). These were consecutive cohorts that began at the time the mesh dressing became available for use in our hospital system. Patient demographics were collected, including sex, body mass index, American Society of Anesthesiologists score, diagnosis of diabetes mellitus, smoking status, chronic renal insufficiency, and whether or not patients were on immunosuppressants such as corticosteroids before surgery. The patient cohorts are summarized in Table 1.
Figure 1.
Diagram of patients included in the study.
Table 1.
Patient and procedural information.
| Patient characteristics | Mesh dressing | SD | Standard dressing | SD | P value |
|---|---|---|---|---|---|
| N (%) | 186 (57.6%) | 137 (42.4%) | |||
| Age (years) | 64.64 | 13.42 | 66.74 | 14.92 | .19 |
| Sex (% male) | 51.61% | 61.31% | .08 | ||
| BMI (kg/m2) | 25.99 | 4.47 | 26.95 | 5.47 | .08 |
| ASA score | 1.83 | 0.77 | 1.99 | 0.84 | .08 |
| % DM | 8.06% | 5.11% | .30 | ||
| % Former smokers | 5.38% | 2.92% | .28 | ||
| % Chronic kidney disease | 5.38% | 7.30% | .48 | ||
| % Immunosuppression | 2.69% | 6.57% | .09 | ||
| Surgical time (min) | 97.66 | 35.37 | 104.07 | 22.44 | .06 |
| EBL (mL) | 277.48 | 195.46 | 288.91 | 117.75 | .54 |
SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; DM, diabetes mellitus; EBL, estimated blood loss.
It is important to note that surgical preparation, technique, and closure remained unchanged during the study duration. The skin was prepped with 2% chlorhexidine gluconate in 70% isopropyl alcohol sticks followed by an iodine-impregnated antimicrobial skin adhesive layer. A standard minimally invasive arthroplasty technique was used via either the direct anterior (n = 243, 75.2%) or mini-anterolateral (n = 80, 24.8%) approach per surgeon preference, with specific identification of the superficial and deep fascial layers. On conclusion of the case, the deep and superficial fascia were closed as independent layers with running barbed suture with full backtracking to the starting point. Finally, 2-0 barbed suture was used in the subcuticular layer with backtracking to the center.
After closure, in the standard dressing group, an Aquacel Ag bandage was applied with all edges sealed (Fig. 2). In the mesh group, the polyester mesh was sized and placed on the incision lengthwise followed by 2-octyl cyanoacrylate adhesive and full polymerization (Fig. 3). Both groups were allowed to shower on postoperative day 2 and onward, with instructions not to scrub or rub the dressing too vigorously and blot dry. Aquacel Ag dressings were removed at 1 week and mesh persisted until nonadherent, generally past 3-4 weeks.
Figure 2.
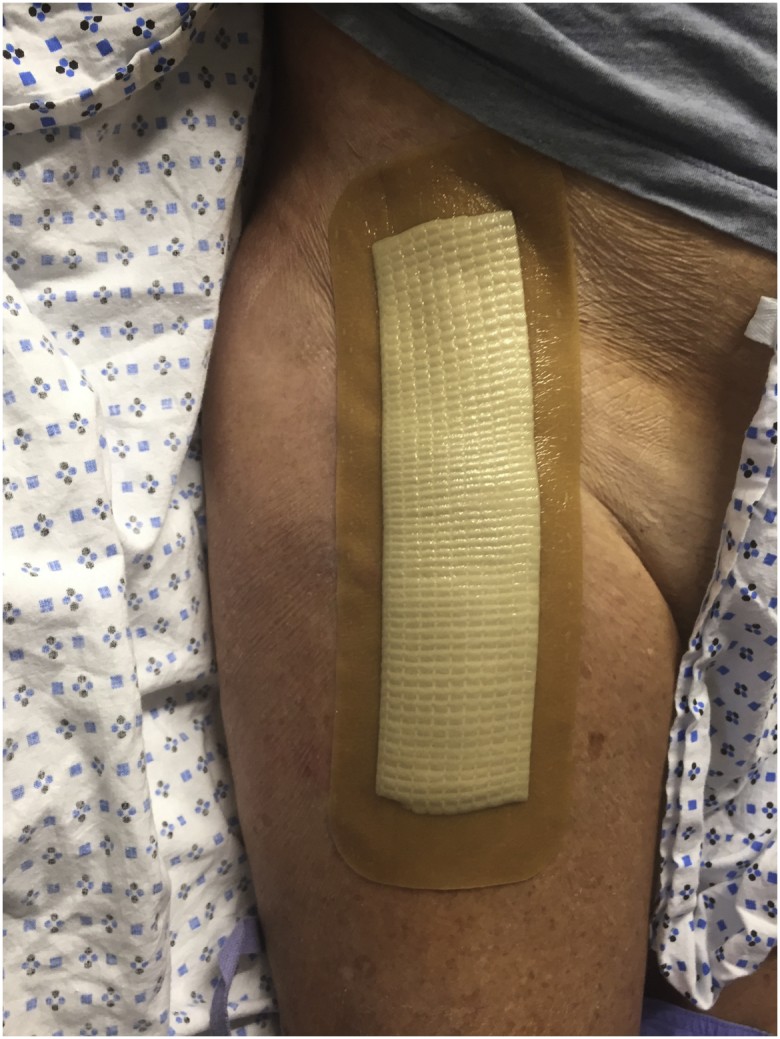
A postoperative patient with the standard dressing.
Figure 3.
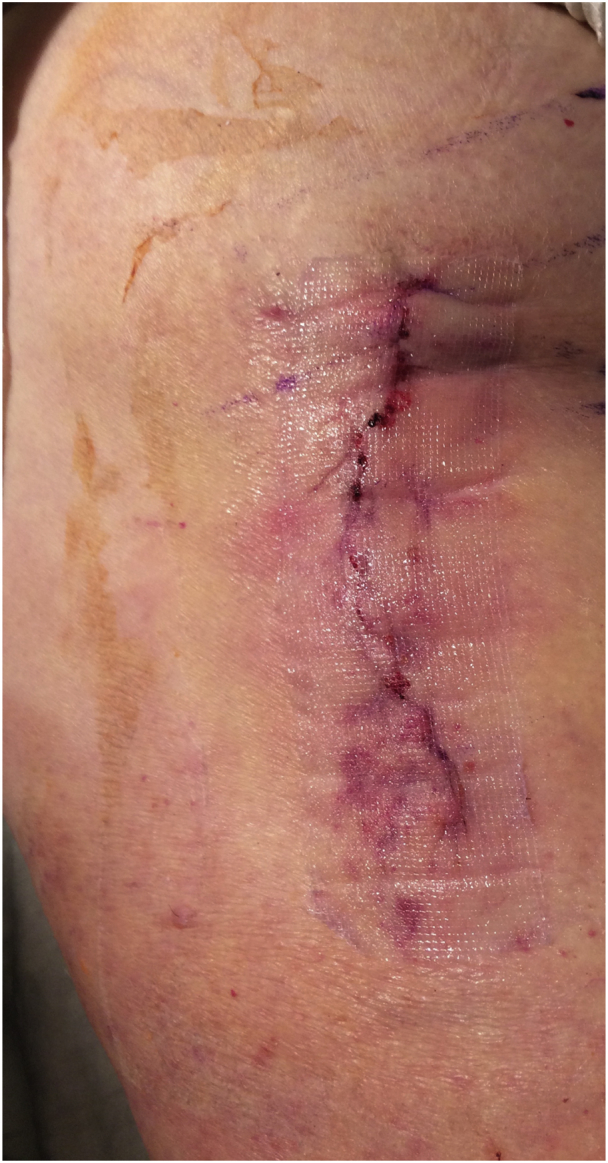
A postoperative patient with the clear mesh dressing.
Tranexamic acid was used for all patients, unless contraindicated by criteria determined by anesthesiology, such as a history of a clotting disorder. Standard 24 hours of antibiotic prophylaxis was used, based on allergy profiles. Aspirin 325 mg twice daily was used for anticoagulation, unless the patient was already taking a stronger anticoagulant for medical reasons, in which case the existing anticoagulant was continued. Postoperative rehabilitation was similar for both groups and consisted of immediate weight-bearing and range of motion exercises under the guidance of hospital physical therapists, with continued physical therapy at home in the postoperative period.
The attending surgeon assessed the patient for delayed wound healing at the initial 2-week office visit or sooner if contacted by the patient, visiting nurse, or post–acute care facility. Detailed information regarding first and final follow-up for each group is displayed in Table 2. Delayed wound healing was diagnosed by visual and manual inspection for drainage, or wound edge separation. If the drainage was enough to wet a sterile piece of gauze on palpation, or if the wound was separated enough to show the deep dermal tissue or deeper, a diagnosis of delayed wound healing was recorded. If the mesh was still in place at the time of the office visit, then the wound was examined through the clear mesh dressing without removing it.
Table 2.
Mean days until first and last follow-up for each cohort.
| Follow-up | Mesh dressing | SD | Range | Standard dressing | SD | Range |
|---|---|---|---|---|---|---|
| First follow-up (days) | 16.01 | 2.12 | 3-25 | 17.00 | 1.98 | 3-28 |
| Last follow-up (days) | 159.02 | 137.98 | 25-780 | 126.89 | 138.31 | 28-740 |
SD, standard deviation.
Categorical variables were reported as percentages. Categorical variables between the groups were evaluated using the chi-square test, and continuous variables were evaluated with a paired Student’s t-test. A P value of <.05 indicated statistically significant differences. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL).
Results
When comparing the mesh and standard dressing groups, no significant differences in demographics (Table 1) were observed. In addition, there were no significant differences in risk factors for wound healing complications including American Society of Anesthesiologists score, body mass index, diabetes mellitus, history of smoking, chronic kidney disease, or the number of patients taking immunosuppressant medication in either cohort. The total length of surgery was comparable between groups.
We observed 22 cases (6.8%) of delayed wound healing in this patient cohort (Table 3). Seven cases of delayed wound healing occurred in the mesh group and 15 in the standard dressing group (3.8% vs 10.9%; P = .01). There was no significant difference in reoperation rate due to wound complications in the mesh group compared with the standard dressing group (2 [1.1%] vs 2 [1.5%], P = .76). The rate of deep infections was also not significantly different in the mesh group compared with the standard dressing group (2 [1.1%] vs 1 [0.7%], P = .75).
Table 3.
Wound complications between groups.
| Complications | Mesh dressing | Standard dressing | P value |
|---|---|---|---|
| % Wound complications | 3.76% | 10.95% | .01 |
| % Reoperation | 1.08% | 1.46% | .76 |
| % Deep infection | 1.08% | 0.73% | .75 |
Bolded P values indicate statistical significance (P < .05).
In both the mesh and standard dressings, no documented adverse events related to the dressing (eg, allergy, blistering, rash, etc.) occurred. The mesh dressing persisted in place at the 2-week visit in all patients. The silver dressing was removed at 7 days in all patients, with none persisting at the 2-week visit.
Discussion
In this study, mesh dressings were associated with significantly fewer episodes of delayed wound healing than standard dressings in a consecutive series of 323 THAs (Table 3). The fewer cases of delayed wound healing with mesh dressings may be due to the tension-sharing properties and longer duration of occlusive protection. In several studies, mesh dressings have demonstrated strength equivalent to a 3-0 suture evenly distributed across the width of the mesh [7,18]. The additional mesh layer adds further reinforcement to the wound closure, increasing the tensile strength during early ambulation in physical therapy. The mesh also creates a physical barrier over the incision that persists for 3-4 weeks instead of the occlusive dressing that persists for only 1 week. The mesh dressing has been reported to prevent the entry of 99% of pathogens into the wound [19].
An important factor in dressing selection is cost. The standard dressing costs approximately $40 as compared to the mesh dressing costing approximately $80 in our institution. Although this initial price is higher, wound complications can require more frequent office visits to evaluate the wound and other interventions such as suture reinforcement and reoperations that create an additional financial burden. Although a formal cost analysis is beyond the scope of this study, we hypothesize that the reduction in delayed wound healing using the mesh dressing is an economically responsible decision. This has been corroborated by other studies, showing that the mesh dressing, when used as a substitute for staples or superficial sutures, resulted in a $56-$80 saved per patient in a 90-day economic model [20]. In our study, the mesh was an additional layer of strength and protection on top of a thorough subcuticular closure, rather than a substitute. In addition, in total knee replacements, this mesh closure compared with staples was found to have a significantly shorter hospital length of stay, lower rate of discharge to skilled nursing facility vs home, and a lower rate of 30-, 60-, and 90-day readmissions, with no significant difference in hospital costs [21].
One downside is that mesh dressings have been reported to cause adverse reactions including allergic dermatitis in several studies [[4], [5], [6],22,23]. The product packaging warns the provider to be cautious using mesh in patients sensitive to cyanoacrylate, formaldehyde, benzalkonium chloride, or any pressure-sensitive adhesive [24,25]. In our study, there were no allergic reactions to either the mesh or standard dressing. Surgeons should be cautious in specific patients with allergies to determine the best dressing individually.
This study has several limitations. First, this being a retrospective study, there are inherent limitations and biases that a larger, prospective study would have avoided, including selection bias. Second, it was not possible to blind the surgeon to the dressing type used in this study at the 2-week evaluation. Third, as this is a retrospective study, the dressing type used was not randomized, but assigned because of surgeon preference. One concern is if the primary surgeon selected higher risk patients to the mesh dressing cohort. However, no significant difference in wound-healing risk factors was identified between groups. Other elements of variation were also controlled for including the identical wound closure steps and postoperative physical therapy protocol. Finally, because of the prolonged adherence of the mesh dressing to the wound, patients treated with mesh had their wounds evaluated through the mesh. Although the mesh is clear (see Fig. 3), small areas may have been obscured. Future studies should be performed prospectively in a blinded fashion or over a longer period of time to monitor for SSI and PJI. A decrease in delayed wound healing and other wound complications could lead to potentially fewer SSI and PJI in THA patients, but with the numbers available for this study, we did not note any significant differences in reoperation rate or SSI. A larger study specifically powered to detect this would be required.
Conclusions
In this cohort of patients, mesh dressings appear to be a safe and reliable dressing option for THA and are associated with a decrease in early wound healing complications when compared to standard, silver-impregnated occlusive dressings at 2 weeks. Further research is needed to determine whether or not this early decrease in wound complications confers reduced risk for SSI or PJI.
Conflict of interest
Dr. H. John Cooper is a paid speaker and consultant as well as received research support from KCI Inc., KCI USA, and KCI Medical Canada.
Appendix A. Supplementary data
References
- 1.Blomfeldt R., Kasina P., Ottosson C., Enocson A., Lapidus L.J. Prosthetic joint infection following hip fracture and degenerative hip disorder: a cohort study of three thousand, eight hundred and seven consecutive hip arthroplasties with a minimum follow-up of five years. Int Orthop. 2015;39:2091. doi: 10.1007/s00264-015-2989-y. [DOI] [PubMed] [Google Scholar]
- 2.Triantafyllopoulos G.K., Soranoglou V.G., Memtsoudis S.G., Sculco T.P., Poultsides L.A. Rate and risk factors for periprosthetic joint infection among 36,494 primary total hip arthroplasties. J Arthroplasty. 2018;33:1166. doi: 10.1016/j.arth.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Pulido L., Ghanem E., Joshi A., Purtill J.J., Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper H.J., Bas M.A. Closed-incision negative-pressure therapy versus antimicrobial dressings after revision hip and knee surgery: a comparative study. J Arthroplasty. 2016;31:1047. doi: 10.1016/j.arth.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Sharma G., Lee S.W., Atanacio O., Parvizi J., Kim T.K. In search of the optimal wound dressing material following total hip and knee arthroplasty: a systematic review and meta-analysis. Int Orthop. 2017;41:1295. doi: 10.1007/s00264-017-3484-4. [DOI] [PubMed] [Google Scholar]
- 7.Smith E.L., DiSegna S.T., Shukla P.Y., Matzkin E.G. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29:283. doi: 10.1016/j.arth.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Agodi A., Auxilia F., Barchitta M. Risk of surgical site infections following hip and knee arthroplasty: results of the ISChIA-GISIO study. Ann Ig. 2017;29:422. doi: 10.7416/ai.2017.2174. [DOI] [PubMed] [Google Scholar]
- 9.Miller A.G., Swank M.L. Dermabond efficacy in total joint arthroplasty wounds. Am J Orthop (Belle Mead NJ) 2010;39:476. [PubMed] [Google Scholar]
- 10.Cai J., Karam J.A., Parvizi J., Smith E.B., Sharkey P.F. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case-control study. J Arthroplasty. 2014;29:1098. doi: 10.1016/j.arth.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Grosso M.J., Berg A., LaRussa S., Murtaugh T., Trofa D.P., Geller J.A. Silver-impregnated occlusive dressing reduces rates of acute periprosthetic joint infection after total joint arthroplasty. J Arthroplasty. 2017;32:929. doi: 10.1016/j.arth.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Kuo F.-C., Chen B., Lee M.S., Yen S.-H., Wang J.-W. AQUACEL® Ag surgical dressing reduces surgical site infection and improves patient satisfaction in minimally invasive total knee arthroplasty: a prospective, randomized, controlled study. Biomed Res Int. 2017;2017:1262108. doi: 10.1155/2017/1262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ethicon US LLC DERMABONDTM PRINEOTM skin closure system (22 cm) instructions for use. 2016. http://hostedvl106.quosavl.com/cgi-isapi/server. dll?8080?IFUs?.cmt1bWFyMTJAaXRzLmpuai5jb20=?GetOneDocPur eFullTxt?p2frdflcj7n4t01muj4u25m7nc?8
- 14.Ethicon US LLC Su 06TR071 Study report for in vitro evaluation of microbial barrier properties of DERMABOND ProTape. Data on file. https://www.jnjmedicaldevices.com/sites/default/files/user_uploaded_assets/pdf_assets/2019-08/DERMABOND-Portfolio-Value-Summary-072113-170501.pdf
- 15.Siddiqui M., Bidaye A., Baird E. Wound dressing following primary total hip arthroplasty: a prospective randomised controlled trial. J Wound Care. 2016;25(40):42. doi: 10.12968/jowc.2016.25.1.40. [DOI] [PubMed] [Google Scholar]
- 16.Parvizi D., Friedl H., Schintler M.V. Use of 2-octyl cyanoacrylate together with a self-adhering mesh (DermabondTM PrineoTM) for skin closure following abdominoplasty: an open, prospective, controlled, randomized, clinical study. Aesthetic Plast Surg. 2013;37:529. doi: 10.1007/s00266-013-0123-3. [DOI] [PubMed] [Google Scholar]
- 17.Blondeel P.N., Richter D., Stoff A., Exner K., Jernbeck J., Ramakrishnan V. Evaluation of a new skin closure device in surgical incisions associated with breast procedures. Ann Plast Surg. 2014;73:631. doi: 10.1097/SAP.0b013e3182858781. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Xue D., Yin H. Barbed versus traditional sutures for wound closure in knee arthroplasty: a systematic review and meta-analysis. Sci Rep. 2016;6:19764. doi: 10.1038/srep19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merollini K.M.D., Zheng H., Graves N. Most relevant strategies for preventing surgical site infection after total hip arthroplasty: guideline recommendations and expert opinion. Am J Infect Control. 2013;41:221. doi: 10.1016/j.ajic.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Sadik K., Flener J., Gargiulo J. A US hospital budget impact analysis of a skin closure system compared with standard of care in hip and knee arthroplasty. Clin Outcomes Res. 2019;11:1. doi: 10.2147/CEOR.S181630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton N., Schmitz N.-D., Johnston S.S. Economic and clinical comparison of 2-octyl cyanoacrylate/polymer mesh tape with skin staples in total knee replacement. J Wound Care. 2018;27:S12. doi: 10.12968/jowc.2018.27.Sup4.S12. [DOI] [PubMed] [Google Scholar]
- 22.Holte A.J., Tofte J.N., Dahlberg G.J., Noiseux N. Use of 2-octyl cyanoacrylate adhesive and polyester mesh for wound closure in primary knee arthroplasty. Orthopedics. 2017;40:e784. doi: 10.3928/01477447-20170531-03. [DOI] [PubMed] [Google Scholar]
- 23.Chawla H., van der List J.P., Fein N.B., Henry M.W., Pearle A.D. Barbed suture is associated with increased risk of wound infection after unicompartmental knee arthroplasty. J Arthroplasty. 2016;31:1561. doi: 10.1016/j.arth.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Davis M.D.P., Stuart M.J. Severe allergic contact dermatitis to dermabond Prineo, a topical skin adhesive of 2-octyl cyanoacrylate increasingly used in surgeries to close wounds. Dermatitis. 2016;27:75. doi: 10.1097/DER.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 25.Dunst K.M., Auboeck J., Zahel B., Raffier B., Huemer G.M. Extensive allergic reaction to a new wound closure device (Prineo) Allergy. 2010;65:798. doi: 10.1111/j.1398-9995.2009.02243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.