Abstract
Prosthetic joint infections (PJIs) are typically caused by Staphylococcus aureus and coagulase-negative Staphylococci species. Corynebacterium species are microorganisms of the human skin and mucous membranes that are often considered contaminants when grown in culture. In the past, Corynebacterium species were often classified as diphtheroids based on growing as gram-positive rods in aerobic environments, but with advances in technology, the identification of Corynebacterium species has improved. Corynebacterium can cause infection, but there are few case reports of orthopaedic infection. We present 3 cases of total hip arthroplasty and 3 cases of total knee arthroplasty PJI caused by Corynebacterium species. We found a high failure rate of surgical treatment of Corynebacterium PJI, defined as reoperation for infection. This information adds to the limited literature on these organisms in total joint arthroplasty PJI.
Keywords: Total hip arthroplasty, Total knee arthroplasty, Prosthetic joint infection, Corynebacterium, Reoperation
Introduction
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) reliably relieve pain from osteoarthritis. One of the dreaded complications for both patients and surgeons is prosthetic joint infection (PJI). PJIs have a large economic burden [1], and they are associated with both morbidity and possibly mortality for the patient [2,3]. Treatments for PJI typically include debridement, antibiotics, and implant retention (DAIR); one-stage exchange arthroplasty; or 2-stage exchange arthroplasty. The most commonly identified pathogens causing PJI are gram-positive cocci, specifically Staphylococcus aureus and coagulase-negative Staphylococci species [4].
Corynebacterium species are ubiquitous microorganisms of the human skin and mucous membranes and when isolated from various clinical samples are typically considered contaminants. However, Corynebacterium has increasingly been recognized as the causative pathogen in many clinical scenarios. Historically, distinguishing between Corynebacterium species has been challenging as many biochemical tests are required and many were simply classified as diphtheroids based on growing as gram-positive rods in aerobic environments. However, the increasing presence of matrix-assisted laser desorption ionization-time of flight mass spectrometry has made identification of Corynebacterium species fast, cheap, and accurate [5]. As a result, many cultures that may have previously been identified as diphtheroids are now being identified as a particular Corynebacterium species.
Corynebacterium species are responsible for many types of infections including, but not limited to, skin and soft-tissue infections, pneumonia, postsurgical infections, urinary tract infections, prostatitis, cerebrospinal fluid infections, peritoneal dialysis–related infections, and endocarditis [[6], [7], [8], [9], [10], [11], [12], [13]]. Although there are several case reports of Corynebacterium PJIs, joint infections, or bone infections [7,8,[14], [15], [16]], the data on diagnosing and treating THA and TKA PJI caused by Corynebacterium species are limited. We present 6 THA and TKA PJI cases thought to be caused by Corynebacterium species. The institutional database was retrospectively queried to find these cases. We classified patients by Musculoskeletal Infection Society (MSIS) criteria as well as host and extremity status as defined by McPherson et al. [[17], [18], [19]]. Follow-up was defined as from the operation for Corynebacterium PJI to the most recent clinical follow-up. The operations for Corynebacterium PJI were performed by multiple surgeons at a single institution. Institutional review board approval was obtained for this investigation.
Case histories
Case 1
A 69-year-old woman with a preoperative body mass index (BMI) of 30 kg/m2 and a past medical history of rheumatoid arthritis presented with a painful right THA. She underwent a right THA in January 2017 (Fig. 1). Twenty-two days postoperatively, she developed increasing pain, erythema, swelling, and drainage. On initial presentation, she had an erythrocyte sedimentation rate (ESR) of 100 mm/hr (Ref: 0-15 mm/hr), a C-reactive protein (CRP) of 24 mg/dL (reference: ≤ 0.6 mg/dL), and aspiration showed a synovial white blood cell (WBC) count of 17,650 cells/ mm3 with 54% neutrophils. She underwent DAIR in February 2017, and 5 of 5 cultures grew Corynebacterium striatum. She had persistent drainage after surgery, and 2 weeks later, she underwent resection arthroplasty with placement of an articulating antibiotic spacer (Fig. 2). The patient’s host and extremity status were B2. The patient met MSIS major criteria with 2 positive cultures for C. striatum. She completed antibiotic therapy with intravenous (IV) vancomycin for 6 weeks without additional oral antibiotics. Unfortunately, she had increased pain in her hip in August 2017. Laboratory tests showed her ESR had increased from 9 mm/hr in May to 22 mm/hr in August 2017; her CRP had increased from 4.4 mg/dL in July to 22.7 mg/dL in August 2017; and her aspiration had changed from 4 cells/ mm3 with 60% neutrophils in July to 1446 cells/ mm3 with 60% neutrophils in August 2017. It was decided that she may not have cleared her infection, or she had infection reoccurrence. In September 2017, she underwent a spacer exchange to another articulating spacer. From this surgery, 2 of 2 intraoperative cultures were positive for Enterococcus faecalis. She did well postoperatively, and aspiration before reimplantation showed a WBC count of 55 cells/ mm3 with 8% neutrophils. Reimplantation was performed in May of 2018. She developed recurrent E. faecalis PJI 1 year later and underwent resection and placement of an articulating spacer. The current plan is to retain the articulating spacer if the patient has reasonable pain control and function. Any future infection or failure will likely be treated with a definitive resection arthroplasty. Her most recent clinical follow-up was August 2019, giving 30 months of follow-up.
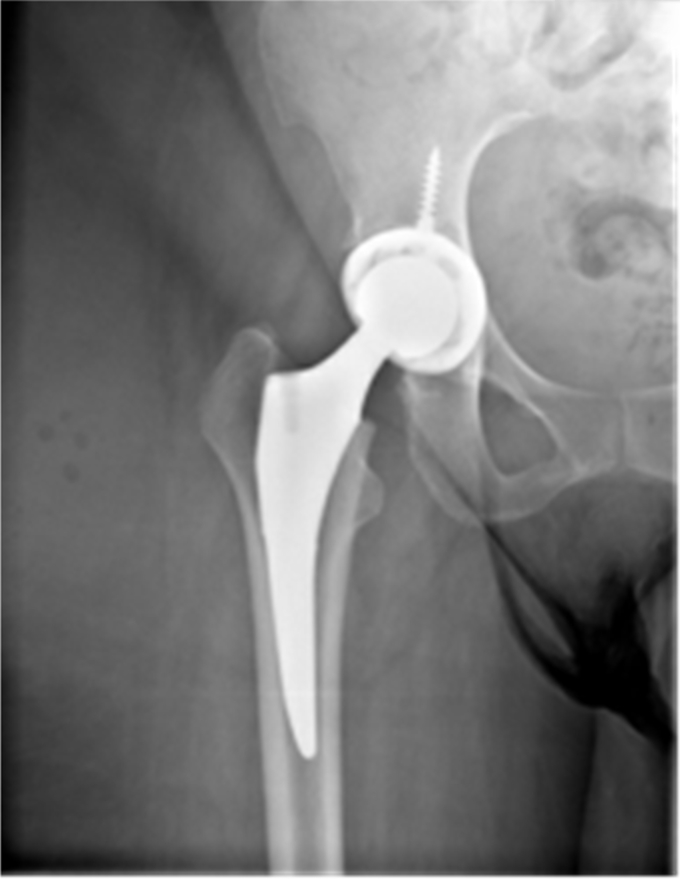
Figure 1.
THA at presentation in case 1. THA, total hip arthroplasty.
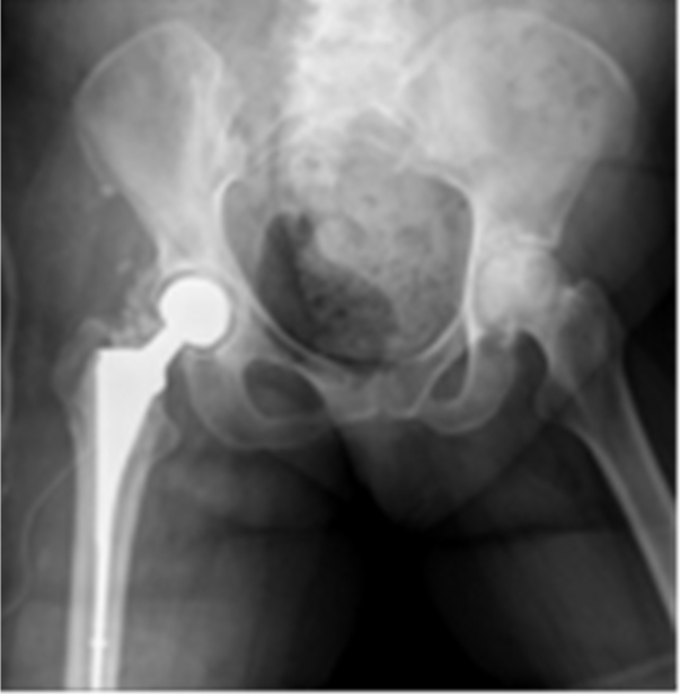
Figure 2.
Articulating spacer in case 1.
Case 2
A 63-year-old man with a preoperative BMI of 40 kg/m2 and a past medical history significant for type 2 diabetes, cerebrovascular accident, and tobacco use underwent right TKA in 2015 for osteoarthritis at an outside institution. He presented to our hospital in June of 2018 because of chronic right knee pain with a 15-degree flexion contracture. On presentation, his hemoglobin A1C was 8%, ESR was 46 mm/hr, CRP was 1.91 mg/dL, and aspiration showed an elevated synovial WBC count of 112,650 cells/ mm3 and 92% neutrophils. His presenting radiographs are shown in Figure 3a and b. The patient had multiple interventions regarding smoking cessation without success. He underwent resection and placement of a static spacer in August of 2018 (Fig. 4). C. striatum grew from 3 of 6 operative cultures. The patient’s host status and extremity status were B2. He met major MSIS criteria with 2 positive cultures of C. striatum. He was treated with 6 weeks of IV vancomycin. He underwent reimplantation in January of 2019. At his latest follow-up, his range of motion was 4 degrees shy of full extension with flexion to 100 degrees. His most recent clinical follow-up was in September 2019, giving 13 months of follow-up.
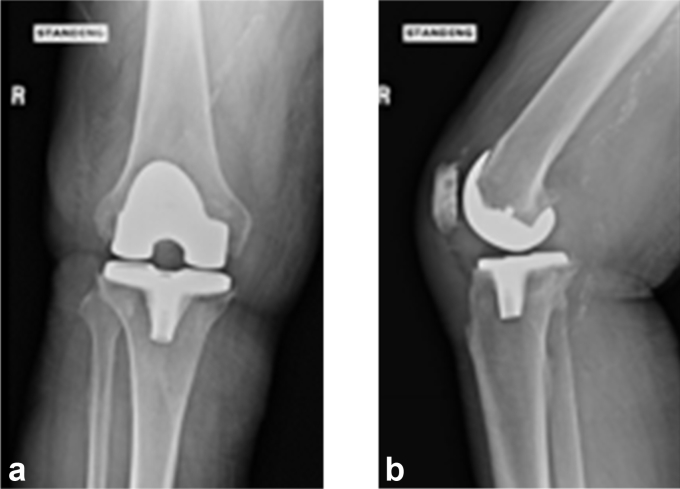
Figure 3.
(a) and (b). AP and lateral radiograph of TKA at presentation in case 2. TKA, total knee arthroplasty.
Figure 4.
Static spacer in case 2.
Case 3
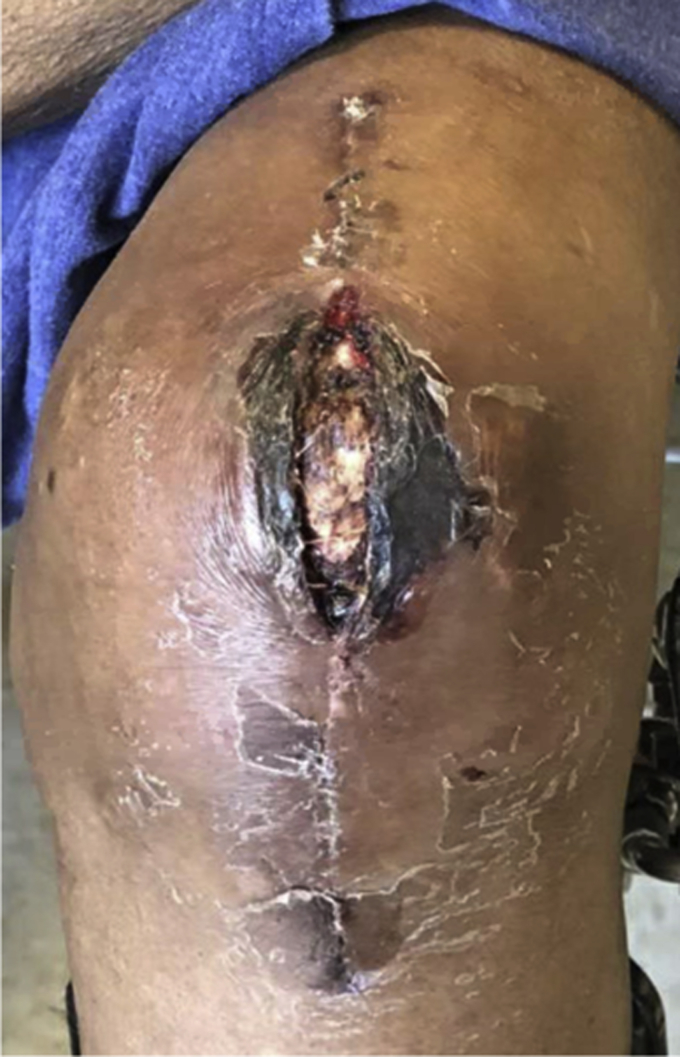
A 70-year-old woman with a BMI of 28.8 kg/m2 and a past medical history significant for rheumatoid arthritis, type 2 diabetes mellitus, and hypoalbuminemia underwent a left TKA for osteoarthritis in May 2018 at our institution. Her hemoglobin A1C was 6.3% before her index TKA. Seven weeks after index TKA, she was admitted for wound dehiscence, purulent drainage, and fever (Fig. 5). Aspiration on admission revealed synovial WBC count of 172,060 cells/ mm3 with 84% neutrophils, and blood tests revealed an ESR of 116 mm/hr and CRP of 35 mg/dL. She underwent DAIR and was started on IV vancomycin and piperacillin-tazobactam postoperatively. Four of 4 intraoperative cultures were positive for C. striatum, and 3 of the 4 were also positive for Peptoniphilus asaccharolyticus and Prevotella bivia. She met major MSIS criteria for PJI with 2 positive cultures, and her host and extremity grade were C2. Two weeks later while still in the hospital, purulent drainage was noted from the incision site and she underwent a resection with placement of a static spacer. She continued to have signs of infection, leading to repeat irrigation and debridement (I&D). Vascular surgery was consulted for acutely increased left lower extremity pain and darkening of her toes. A computed tomography angiogram revealed severe chronic multilevel vascular disease in her left leg, and she ultimately underwent above-knee amputation (AKA) in July 2018 after a multidisciplinary team concluded that amputation provided the best chance to eradicate her severe infection in the setting of poor blood supply. She was discharged in August 2018 after 6 revisions of her AKA. She subsequently required repeated hospitalizations for multiple infections and complications of peripheral vascular disease in the contralateral extremity, and she ultimately expired from sepsis 11 months after her index TKA in April 2019.
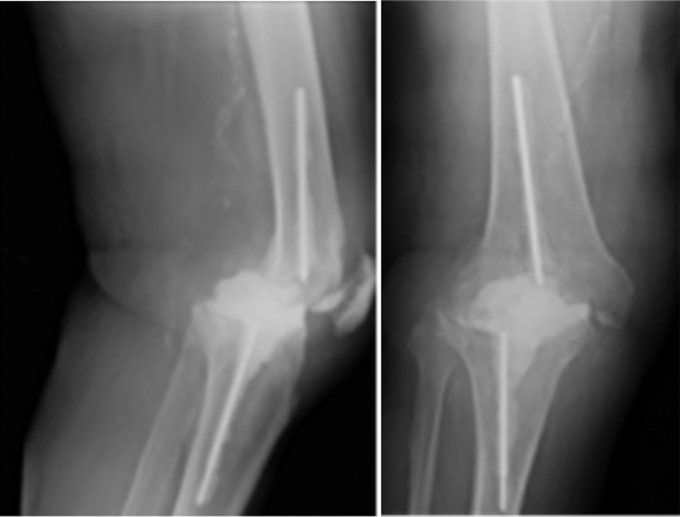
Figure 5.
Wound dehiscence in case 3.
Case 4
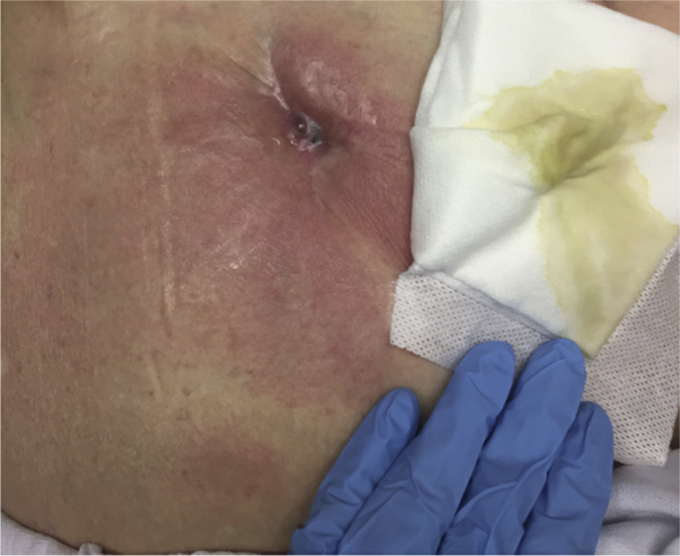
A 77-year-old woman with a BMI of 37 kg/m2 and past medical history significant for atrial fibrillation, bilateral TKA, and right THA presented to us with chronic THA PJI. She underwent index right THA in 2011 and had a 2-stage exchange in 2013 for PJI. She presented to our institution in January 2019 with continued hip pain, erythema, and a draining sinus tract while taking oral ampicillin and doxycycline for suppression (Fig. 6). Radiographs showed lucency around her cup, and the preoperative ESR and CRP were 90 mm/hr and 12.71 mg/dL, respectively. She underwent a resection arthroplasty in May of 2019 with placement of a static spacer. Five of 6 intraoperative cultures grew C. striatum, meeting major MSIS criteria for PJI. Her host and extremity status were A2. She received 8 weeks of IV vancomycin and ertapenem postoperatively. She was placed on ertapenem in addition to vancomycin because one of 6 intraoperative cultures grew Mycobacterium avium, and at presentation to our institution, she had a chronic sinus while on ampicillin and doxycycline. In August of 2019, she showed no signs or symptoms of infection, ESR and CRP remained within normal limits, and an aspiration of the hip had no microbial growth. She underwent reimplantation in November 2019. Seven weeks after her reimplantation, she suffered a dislocation that could not be closed reduced. She therefore underwent open reduction and her femoral head was exchanged from a 36- to 40-mm head. Ten weeks after her reimplantation, she presented to the emergency department with fevers and chills as well as new erythema and drainage at her incision site. The ESR was 129 mm/hr, CRP was 22.9 mg/dL, and joint aspirate was positive for E. faecium. She subsequently underwent DAIR in January of 2020. Four of 4 intraoperative cultures were positive for E. faecium, and 2 of 4 cultures were positive for Staphylococcus epidermidis. She was placed on a 6-week course of IV vancomycin. Her most recent follow-up was in February 2020, giving 9 months of follow-up.
Figure 6.
Draining sinus tract at presentation in case 4.
Case 5
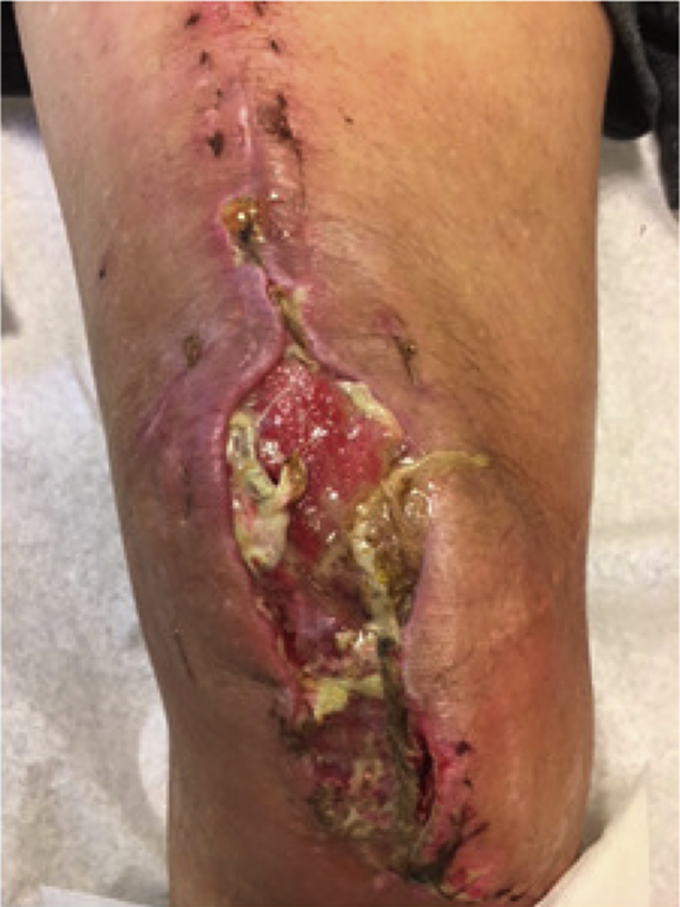
A 66-year-old man with a BMI of 24.13 kg/m2 and with a past medical history significant for rheumatoid arthritis and left olecranon septic bursitis presented with a chronic, right TKA PJI. His index TKA was performed in 2015. The patient’s infected olecranon bursitis first occurred after a rheumatoid nodule was removed in 2014 and his elbow was noted to have drainage; he underwent multiple I&Ds of the elbow. These were culture positive for Pseudomonas aeruginosa, Staphylococcus warneri, and S. epidermidis. The patient presented to our institution in February of 2018 with a sinus tract about his TKA. He continued to have medical care at an outside institution, and later in February, he underwent a DAIR at an outside hospital. He presented back to our institution in April with a nonhealing wound. Blood work from April 2018 showed an ESR of 65 mm/hr, CRP 43.9 mg/dL, and a knee aspirate showed a WBC count of 2853 cells/ mm3 with 46% neutrophils. In April 2018, the patient underwent resection arthroplasty with placement of an articulating spacer and a right knee gastrocnemius flap to cover a soft-tissue defect. Seven intraoperative cultures were taken, 3 of which were positive for C. striatum. The patient met major MSIS criteria with 2 positive cultures. The patient’s host status and extremity status were C3. The patient completed 6 weeks of IV vancomycin. Three weeks after resection the patient began having subjective fevers and increased purulent and foul-smelling drainage (Fig. 7). The decision was made to perform an AKA in May 2018. This was further complicated by a surgical site abscess, and the patient underwent 5 additional debridements. At his most recent follow-up he has had no other issues with his leg. His most recent clinical follow-up was in May 2019, giving 12 months of follow-up.
Figure 7.
Poor wound healing with drainage after resection and before AKA in case 5. AKA, above-knee amputation.
Case 6
A 72-year-old female with a preoperative BMI of 36.13 kg/m2 and past medical history significant for rheumatoid arthritis presented with a chronic, right THA PJI. She had her index surgery at our institution in 2012. In 2013, she underwent revision for aseptic loosening of her acetabular component. In 2015, the patient had a custom triflange acetabular component for pelvic discontinuity. In March 2015, 2 weeks after her revision, the patient was noted to have persistent drainage at her first post-operative visit, concerning for PJI. From March to December of 2015, she was treated with multiple DAIRs, with intraoperative cultures over this time period coming back positive for a variety of organisms including Escherichia coli, vancomycin-resistant E. faecium, Proteus vulgaris, and Serratia marcescens. The patient eventually underwent resection arthroplasty in December of 2015. The patient was stable on suppressive cephalexin until May 2018 when she presented with a sinus tract about the hip. Blood work showed an ESR of 108 mm/hr and a CRP of 17.9 mg/dL. Aspiration of the hip showed an elevated synovial WBC count of 22,448 cells/ mm3 with 92% neutrophils. The patient underwent an I&D in July 2018 with 4 of 5 intraoperative cultures positive for C. striatum and 3 of 5 cultures positive for vancomycin sensitive E. faecium. The patient met major MSIS criteria as she had a sinus tract. The patient’s host status and extremity status were C2. The patient was treated with tedizolid for 8 weeks, as she developed bullous dermatitis previously on vancomycin and had a history of vancomycin resistant organisms. The patient was noted to have persistent drainage following the I&D. An aspiration in September 2018 showed a synovial WBC count of 18,310 cells/ mm3 with 93% neutrophils. She underwent an I&D in November 2018. Three out of 3 intraoperative cultures grew C. striatum, and one of the 3 cultures was positive for vancomycin-sensitive E. faecium. At the most recent follow-up, she was noted to have a nonhealing wound over her hip, and she is currently being treated with local wound care. Her most recent clinical follow-up was in September 2019, giving her 14 months of follow-up. Given the persistent nature of her infection and poor host and extremity status, there is currently no plan for reimplantation.
Discussion
Corynebacterium is a rare but challenging pathogen that causes PJIs. In the current case series, we found a high failure rate of surgical and medical treatment of Corynebacterium PJI, defined as reoperation for infection (Table 1). Most patients were treated with IV antibiotics as these organisms are typically resistant to oral antibiotics. This information adds to the limited literature on these organisms and can aid surgeons, infectious disease physicians, and the patient regarding the difficult management of Corynebacterium PJIs.
Table 1.
Details of Corynebacterium prosthetic joint infections.
| Patient case number | TKA or THA | Type of surgery for Corynebacterium-related PJI | Preoperative culture from aspirate | Intraoperative cultures | Type of antibiotic(s) | Duration of antibiotic(s) (wk) | Infection recurrence | Additional surgical treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | THA | DAIR | NG | Corynebacterium striatum (5/5) | IV vancomycin | 6 | Yes | Resection and articulating spacer, spacer exchange, reimplantation, resection and articulating spacer |
| 2 | TKA | Resection arthroplasty with static spacer | Corynebacterium striatum | Corynebacterium striatum (3/6) | IV vancomycin | 6 | No | Reimplantation |
| 3 | TKA | DAIR | Corynebacterium striatum | Corynebacterium striatum (4/4), Prevotella bivia (3/4), Peptoniphilus asaccharolyticus (3/4) | IV vancomycin and piperacillin/tazobactam | 12 | Yes | Resection with static spacer, I&D, AKA with multiple debridements |
| 4 | THA | Resection arthroplasty with static spacer | No aspirate | Corynebacterium striatum (5/6), Mycobacterium avium (1/6) | IV vancomycin and ertapenem | 8 | Yes | Reimplantation, revision, DAIR |
| 5 | TKA | Resection arthroplasty with articulating spacer | Corynebacterium striatum | Corynebacterium striatum (3/7) | IV vancomycin | 6 | Yes | AKA with multiple debridements |
| 6 | THA | I&D | Corynebacterium striatum | Corynebacterium striatum (4/5), Enterococcus faecium (3/5) | PO tedizolid | 8 | Yes | I&D |
THA, total hip arthroplasty; TKA, total knee arthroplasty; PJI, prosthetic joint infection; DAIR, debridement, antibiotics, and implant retention; NG, no growth; IV, intravenous; PO, oral; AKA, above-knee amputation; I&D, irrigation and debridement.
There are limited reports of Corynebacterium orthopaedic infections. von Graevenitz et al. [16] found 73 Corynebacterium isolates from open fracture and PJIs in 60 patients over a 5-year time period. In this study, some of the most frequent species were Corynebacterium amycolatum, C. striatum, and Corynebacterium diphtheria. Scholle [15] discussed the case of a spontaneous joint infection in a left knee with C. striatum. They noted that this isolate was resistant to most antibiotics. Achermann et al. [7] describes the case of a Corynebacterium bovis shoulder PJI. This patient was treated with a 2-stage exchange protocol and 3 months of antibiotics. Sonication of the prosthesis helped to identify the pathogen. These studies highlight that Corynebacterium can be difficult to detect and can cause true orthopaedic infection.
In our series, many patients had compromised host and extremity status, had undergone prior operations for PJI, or had polymicrobial infection, which could have been factors associated with the high rate of infection recurrence [[20], [21], [22]]. Corynebacterium is an opportunistic pathogen that has been reported in those with prolonged hospitalizations, immunocompromised states, or prosthetic devices [9]. It is challenging to tell if it is the Corynebacterium or the complex nature of the infections in this study that led to a high rate of infection recurrence, but it is likely that both play a role.
Rates of infection eradication vary depending on the surgical treatment and the organism. Two-stage exchange protocol in TJA has led to infection eradication in 73%-78% of patients [23,24], while DAIR has been less promising with success rates ranging from 37% to 66% [[25], [26], [27]]. In the present study, all 3 patients treated with DAIR or I&D required reoperation for infection. Only one patient with resection arthroplasty who went on to reimplantation has remained infection free at latest follow-up (Table 1). Neither DAIR nor 2-stage exchange was associated with good infection eradication, albeit in a very small series.
It is important to note that Corynebacterium species are resistant to many antibiotics. Isolates can be resistant to penicillin, levofloxacin, sparfloxacin, cephalosporins, clindamycin, erythromycin, minocycline, linezolid, and imipenem [15,[28], [29], [30], [31]]. Because many Corynebacterium species are resistant to oral antibiotics, a course of IV antibiotics is typically necessary. This makes long-term suppression with oral antibiotics unlikely to be successful in these patients.
Summary
In conclusion, our case series of Corynebacterium PJIs demonstrates that this organism can be the cause of THA and TKA PJI. C. striatum was an organism in all our cases and was resistant to many oral antibiotics, eliminating the option for oral suppression. These infections were often present in patients who were medically compromised hosts and were associated with a high rate of recurrence. Therefore, these patients should be followed up closely by both orthopaedic surgery and infectious disease physicians for signs of infection recurrence. In addition, an effort should be made to medically optimize these patients before surgery.
Conflict of interest
Michael P. Bolognesi reports royalties from Zimmer Biomet and TJO; paid consultancy for Total Joint Orthopaedics and Zimmer Biomet; stock or stock options in TJO and Amedica; research support from or supplier as a principal investigator of DePuy, Synthes, Exactech, and PCORI; other financial or material support from Zimmer Biomet, DePuy, Stryker, TJO, Smith & Nephew, DJO, and Exactech; medical/orthopaedic publications editorial/governing board of Arthroplasty Today; and a board member/committee appointments for EOA, AAHKS, AAOS, JOA, and JSOA. William A. Jiranek reports royalties from DePuy; speakers bureau/paid presentations for DePuy; and a board member/committee appointments for Hip Society. The other authors declare no potential conflicts of interest.
Appendix A. Supplementary data
References
- 1.Kurtz S.M., Lau E., Watson H., Schmier J.K., Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Lum Z.C., Natsuhara K.M., Shelton T.J., Giordani M., Pereira G.C., Meehan J.P. Mortality during total knee periprosthetic joint infection. J Arthroplasty. 2018;33(12):3783. doi: 10.1016/j.arth.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Natsuhara K.M., Shelton T.J., Meehan J.P., Lum Z.C. Mortality during total hip periprosthetic joint infection. J Arthroplasty. 2019;34(7S):S337. doi: 10.1016/j.arth.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Tande A.J., Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konrad R., Berger A., Huber I. Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheria-associated bacteria. Euro Surveill. 2010;15(43) doi: 10.2807/ese.15.43.19699-en. [DOI] [PubMed] [Google Scholar]
- 6.Chandran R., Puthukkichal D.R., Suman E., Mangalore S.K. Diphtheroids-important nosocomial pathogens. J Clin Diagn Res. 2016;10(12):DC28. doi: 10.7860/JCDR/2016/19098.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achermann Y., Trampuz A., Moro F., Wust J., Vogt M. Corynebacterium bovis shoulder prosthetic joint infection: the first reported case. Diagn Microbiol Infect Dis. 2009;64(2):213. doi: 10.1016/j.diagmicrobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Cazanave C., Greenwood-Quaintance K.E., Hanssen A.D., Patel R. Corynebacterium prosthetic joint infection. J Clin Microbiol. 2012;50(5):1518. doi: 10.1128/JCM.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funke G., von Graevenitz A., Clarridge J.E., 3rd, Bernard K.A. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10(1):125. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong H.L., Koh H.I., Lee A.J. Native Valve endocarditis due to Corynebacterium striatum confirmed by 16S ribosomal RNA sequencing: a case report and literature review. Infect Chemother. 2016;48(3):239. doi: 10.3947/ic.2016.48.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mottola C., Mendes J.J., Cristino J.M., Cavaco-Silva P., Tavares L., Oliveira M. Polymicrobial biofilms by diabetic foot clinical isolates. Folia Microbiol (Praha) 2016;61(1):35. doi: 10.1007/s12223-015-0401-3. [DOI] [PubMed] [Google Scholar]
- 12.Reece R.M., Cunha C.B., Rich J.D. Corynebacterium minutissimum vascular graft infection: case report and review of 281 cases of prosthetic device-related Corynebacterium infection. Scand J Infect Dis. 2014;46(9):609. doi: 10.3109/00365548.2014.918650. [DOI] [PubMed] [Google Scholar]
- 13.van Asten S.A., La Fontaine J., Peters E.J., Bhavan K., Kim P.J., Lavery L.A. The microbiome of diabetic foot osteomyelitis. Eur J Clin Microbiol Infect Dis. 2016;35(2):293. doi: 10.1007/s10096-015-2544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux V., Drancourt M., Stein A., Riegel P., Raoult D., La Scola B. Corynebacterium species isolated from bone and joint infections identified by 16S rRNA gene sequence analysis. J Clin Microbiol. 2004;42(5):2231. doi: 10.1128/JCM.42.5.2231-2233.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholle D. A spontaneous joint infection with Corynebacterium striatum. J Clin Microbiol. 2007;45(2):656. doi: 10.1128/JCM.00827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Graevenitz A., Frommelt L., Punter-Streit V., Funke G. Diversity of coryneforms found in infections following prosthetic joint insertion and open fractures. Infection. 1998;26(1):36. doi: 10.1007/BF02768750. [DOI] [PubMed] [Google Scholar]
- 17.McPherson E.J., Tontz W., Jr., Patzakis M. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop (Belle Mead NJ) 1999;28(3):161. [PubMed] [Google Scholar]
- 18.McPherson E.J., Woodson C., Holtom P., Roidis N., Shufelt C., Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8. [PubMed] [Google Scholar]
- 19.Parvizi J., Gehrke T. International consensus group on periprosthetic joint I. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. doi: 10.1016/j.arth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Bozic K.J., Lau E., Kurtz S. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794. doi: 10.2106/JBJS.K.00072. [DOI] [PubMed] [Google Scholar]
- 21.Ji B., Zhang X., Xu B. The fate of immunocompromised patients in the treatment of chronic periprosthetic joint infection: a single-centre experience. Int Orthop. 2018;42(3):487. doi: 10.1007/s00264-018-3763-8. [DOI] [PubMed] [Google Scholar]
- 22.Tan T.L., Kheir M.M., Tan D.D., Parvizi J. Polymicrobial periprosthetic joint infections: outcome of treatment and identification of risk factors. J Bone Joint Surg Am. 2016;98(24):2082. doi: 10.2106/JBJS.15.01450. [DOI] [PubMed] [Google Scholar]
- 23.Ford A.N., Holzmeister A.M., Rees H.W., Belich P.D. Characterization of outcomes of 2-stage exchange arthroplasty in the treatment of prosthetic joint infections. J Arthroplasty. 2018;33(7S):S224. doi: 10.1016/j.arth.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud T., Lyons M.C., Naudie D.D., Macdonald S.J., McCalden R.W. Assessing the gold standard: a review of 253 two-stage revisions for infected TKA. Clin Orthop Relat Res. 2012;470(10):2730. doi: 10.1007/s11999-012-2358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzam K.A., Seeley M., Ghanem E., Austin M.S., Purtill J.J., Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25(7):1022. doi: 10.1016/j.arth.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 26.Parvizi J., Azzam K., Ghanem E., Austin M.S., Rothman R.H. Periprosthetic infection due to resistant staphylococci: serious problems on the horizon. Clin Orthop Relat Res. 2009;467(7):1732. doi: 10.1007/s11999-009-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weston J.T., Watts C.D., Mabry T.M., Hanssen A.D., Berry D.J., Abdel M.P. Irrigation and debridement with chronic antibiotic suppression for the management of infected total knee arthroplasty: a contemporary Analysis. Bone Joint J. 2018;100-B(11):1471. doi: 10.1302/0301-620X.100B11.BJJ-2018-0515.R1. [DOI] [PubMed] [Google Scholar]
- 28.Kocazeybek B., Ozder A., Kucukoglu S., Kucukates E., Yuksel H., Olga R. Report of a case with polymicrobial endocarditis related to multiresistant strains. Chemotherapy. 2002;48(6):316. doi: 10.1159/000069710. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka Y., Ohkusu K., Kawamura Y., Baba S., Ezaki T., Kimura S. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn Microbiol Infect Dis. 2006;54(2):109. doi: 10.1016/j.diagmicrobio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Sierra J.M., Martinez-Martinez L., Vazquez F., Giralt E., Vila J. Relationship between mutations in the gyrA gene and quinolone resistance in clinical isolates of Corynebacterium striatum and Corynebacterium amycolatum. Antimicrob Agents Chemother. 2005;49(5):1714. doi: 10.1128/AAC.49.5.1714-1719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarr P.E., Stock F., Cooke R.H., Fedorko D.P., Lucey D.R. Multidrug-resistant Corynebacterium striatum pneumonia in a heart transplant recipient. Transpl Infect Dis. 2003;5(1):53. doi: 10.1034/j.1399-3062.2003.00002.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.