Eligibility criteria for a biologic treatment for severe asthma include poor disease control despite a full medication plan according to Global Initiative for Asthma steps 4–5 [1]. Adherence to inhaled therapy should be verified as part of that prescription requirement [2]. In fact, it has been demonstrated that poor adherence is a major cause of uncontrolled asthma, regardless of its severity [3]. Furthermore, biologics do not exert a disease-modifying effect [4]; in contrast to allergen immunotherapy, which is able to permanently modulate the way the immune system reacts to allergens beyond the immunotherapy treatment course [5], biologic therapy withdrawal usually leads to asthma relapse [4]. Thus, a low adherence rate to inhaled treatment in patients undergoing biologic therapy raises some issues related to sustainability.
Short abstract
Less than half of severe asthmatic patients show a >80% adherence rate to inhaled treatment just before and during biologic therapy. This has implications in biologic treatment sustainability and disease prevalence estimation. http://bit.ly/3cRTJB0
To the Editor:
Eligibility criteria for a biologic treatment for severe asthma include poor disease control despite a full medication plan according to Global Initiative for Asthma steps 4–5 [1]. Adherence to inhaled therapy should be verified as part of that prescription requirement [2]. In fact, it has been demonstrated that poor adherence is a major cause of uncontrolled asthma, regardless of its severity [3]. Furthermore, biologics do not exert a disease-modifying effect [4]; in contrast to allergen immunotherapy, which is able to permanently modulate the way the immune system reacts to allergens beyond the immunotherapy treatment course [5], biologic therapy withdrawal usually leads to asthma relapse [4]. Thus, a low adherence rate to inhaled treatment in patients undergoing biologic therapy raises some issues related to sustainability.
According to the available evidence, suboptimal adherence is particularly prevalent among patients affected by difficult-to-treat asthma, who are reported to take only ∼50% of their prescribed drugs [6, 7]. However, so far, only a few studies, most of them including small populations, have specifically investigated adherence to inhaled medications in patients receiving monoclonal antibodies as add-on therapy before and during the biologic treatment, and none of them has compared subpopulations undergoing different biologic treatments [8–10].
The present study aimed to investigate the adherence rate to inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients affected by severe asthma prior to and during treatment with omalizumab or mepolizumab.
The electronic database of the Veneto region (in the northeast of Italy) Drug Regulatory Agency was interrogated in order to track the use of asthma medications of each subject living in the area, from medical prescription to drug supply at the pharmacy. Patients undergoing omalizumab or mepolizumab treatment for ≥6 months were selected. Adherence was estimated as the amount of drugs bought by the patients out of the prescribed inhaled treatment within a 6-month period before and after the start of biologic treatment. Five adherence-rate classes were identified: 0%, 1–19%, 20–39%, 40–79% and >80%. The Veneto Drug Regulatory Agency Ethical Committee approved the analysis.
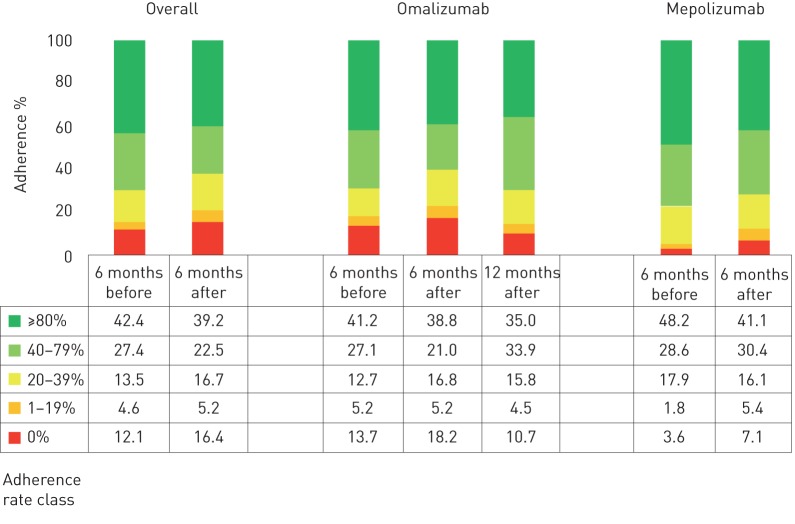
Overall, 347 patients were included (males 48.9%, mean age 43.8 years). 291 of them were treated with omalizumab whilst 56 patients had been prescribed mepolizumab. Within the omalizumab subgroup, all patients were atopic and 40.2% of them had nasal polyps; mean±sd baseline forced expiratory volume in 1 s (FEV1) was 69.8±17.8% of predicted and on average, they experienced 6.5±2.3 exacerbations per year. In the mepolizumab subgroup, 34.7% were atopic and 61.3% suffered from nasal polyposis; baseline FEV1 was 70.2±16.9% of predicted and the average exacerbation rate was 5.1±3.2 per year. The distribution of the patients according to the adherence rate class to ICS/LABA 6 months before biologic treatment start was: 42.4% with >80% adherence; 27.4% between 79% and 40% adherence; 13.5% between 39% and 20% adherence; 4.6% between 19% and 1%; and 12.1% with 0% adherence. No significant differences between omalizumab and mepolizumab subgroups could be observed in any of the adherence rate classes (figure 1). The same analysis was performed 6 months after biologic treatment start. The distribution of the patients in the five adherence-rate classes previously identified was: 39.2% with >80% adherence; 22.5% between 79% and 40% adherence; 16.7% between 39% and 20% adherence; 5.2% between 19% and 1% adherence; and 16.4% with 0% adherence. Again, a very similar trend was registered in both the omalizumab and mepolizumab subgroups (figure 1). Furthermore, when comparing the baseline and the 6-month follow-up, no significant differences could be detected in any of the adherence classes (figure 1). Regarding the subgroup of patients treated with omalizumab, a 12-month follow-up was available. The adherence rate class analysis revealed the following distribution: 35.0% with >80% adherence; 33.9% between 79% and 40% adherence; 15.8% between 39% and 20% adherence; 4.5% between 19% and 1% adherence; and 10.7% with 0% adherence.
FIGURE 1.
Adherence rate in the study population.
Our findings highlight an astonishingly low adherence to inhaled treatment in severely asthmatic patients before and during biologic therapy, regardless the type of prescribed biologic drug and the biologic treatment duration. Adherence rate assessment still represents a challenge, as currently, none of the available strategies is free from potential bias [11]. However, tracking the journey of asthma medications from the prescribers to the pharmacy where the patients buy the drug represents a quite accurate proxy of adherence rate.
No information is available on the correct use of the inhaler device; it represents a potential limitation of our study, as inhalation technique evaluation is part of the global adherence assessment. In fact, although when talking about severe asthma patients, one could expect that the correct approach to the device is an acquired skill, it has been demonstrated that errors in the inhalation technique in severe asthmatics persist despite educational interventions [12].
Generally speaking, the low adherence within the severe or difficult-to-treat asthma population has been previously highlighted [6, 8–10]. However, the issue becomes even more relevant when talking about biologic therapy. In fact, adherence to traditional treatment, despite being extremely difficult to assess with proper accuracy, plays a pivotal role in identifying the truly uncontrolled patients among the severely asthmatic, which is relevant to establish eligibility for biologic treatment [1, 2]. The requirements indicated by Italian Drug Regulatory Agency for biologic drug prescription do not explicitly include the adherence evaluation, even if it is fully recommended by local, national and international guidelines on severe asthma [1, 13]. In Italy, only referral centres with recognised expertise in the field of severe asthma management are authorised to prescribe biologic treatments but if and how adherence is assessed is not standardised, and every single centre applies its own methodology. Our findings suggest that even in specialised centres, adherence assessment and implementation should be considered an unmet need and a standardised approach to the issue should be considered. Notably, according to our results, the adherence rate before and during the biologic therapy does not significantly differ. The observation suggests that the adherence rate is not affected by the frequency of follow-up visits, which increases due to the biologic treatment schedule. It has been demonstrated that reminder strategies and more intensive follow-up could have a positive impact on the adherence rate [14]; however, according to our findings and to other published reports, it does not seem to be the case for severe asthmatics undergoing biologic treatment.
Recently, a study conducted in Australia explored the adherence to inhaled therapy through an electronic monitoring device in 69 patients affected by severe asthma and eligible for biologic treatment or thermoplasty [8]. The authors reported that 55.6% of patients had a documented adherence >75%. Despite the differences in terms of adherence evaluation methodology, the aforementioned data are quite consistent with our results and with previous studies [6, 9, 10]. In contrast to our report, no information was available concerning the adherence rate during the biologic treatment or after thermoplasty.
When the biologic treatment is ongoing, poor adherence to traditional medications could be positively interpreted as a marker of biologic treatment efficacy, even if in that case, a progressive decrease in adherence rate could be expected over time from the beginning of biologic treatment. On the contrary, our results show a similar adherence rate before and during the biologic therapy course, until 1 year after its introduction (in the case of omalizumab).
However, as a disease-modifying effect of biologic drugs has not been demonstrated [4], the sustainability of the latter in poorly adherent patients may raise some concerns. In fact, since no difference in terms of adherence to inhaled drugs was observed before and during the biologic treatment, one could wonder whether better asthma control could have been achieved by improving adherence instead of including a biologic drug in the treatment plan. The literature provides evidence that good adherence is not enough to achieve satisfactory asthma control in a not negligible asthma subpopulation [2]. In those cases, it can be discussed whether biologic drugs may replace an ineffective ICS/LABA treatment instead of acting as an add-on therapy, as suggested by the guidelines [1]. However, the relevance of adherence optimisation in the management of asthma should be always verified.
Furthermore, the treatment that is needed to obtain asthma control, or that is not enough to maintain control despite its maximal regimen, is part of the severe asthma definition, so nonadherence could severely impact severe asthma prevalence estimation [15] and consequently, health planning and resources allocation.
In conclusion, regardless the ability or not of inhaled treatment to significantly improve the asthma control, our findings highlight the need for a focus on adherence particularly in patients who are potentially eligible for biologic drugs. In fact, a systematic and careful evaluation of adherence rate before confirming the diagnosis of severe and/or difficult-to-control asthma supports proper treatment selection for patients, by decreasing the risk of overdiagnosis and overtreatment with expensive biologic drugs. Notably, verifying the regular and appropriate use of inhaled therapies appears to be mandatory even during biologic treatment and at each follow-up visit, in order to properly evaluate how to tailor the different treatment options for achieving asthma control in the best way.
Although challenging and probably time-consuming to all physicians, adherence assessment in severe asthma, especially before and during a biologic treatment, should be considered as part of a personalised medicine approach.
Footnotes
Conflict of interest: M. Caminati has nothing to disclose.
Conflict of interest: A. Vianello has nothing to disclose.
Conflict of interest: M. Andretta has nothing to disclose.
Conflict of interest: A.M. Menti has nothing to disclose.
Conflict of interest: S. Tognella has nothing to disclose.
Conflict of interest: L. Degli Esposti has nothing to disclose.
Conflict of interest: C. Micheletto has nothing to disclose.
Conflict of interest: C. Bovo has nothing to disclose.
Conflict of interest: G. Senna has nothing to disclose.
References
- 1.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019 Available from: www.ginasthma.org. Date last accessed: November 2019.
- 2.Corren J, Panettieri RA. How important is adherence to inhaled medications before starting a biologic therapy for asthma? J Allergy Clin Immunol Pract 2018; 6: 1578–1579. doi: 10.1016/j.jaip.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 3.Blake KV. Improving adherence to asthma medications: current knowledge and future perspectives. Curr Opin Pulm Med 2017; 23: 62–70. doi: 10.1097/MCP.0000000000000334 [DOI] [PubMed] [Google Scholar]
- 4.Caminati M, Polk B, Rosenwasser LJ. What have recent advances in therapy taught us about severe asthma disease mechanisms? Expert Rev Clin Immunol 2019; 15: 1145–1153. doi: 10.1080/1744666X.2020.1672536 [DOI] [PubMed] [Google Scholar]
- 5.Komlósi ZI, Kovács N, Sokolowska M, et al. Mechanisms of subcutaneous and sublingual aeroallergen immunotherapy: what is new? Immunol Allergy Clin North Am 2020; 40: 1–14. doi: 10.1016/j.iac.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Gamble J, Stevenson M, McClean E, et al. The prevalence of nonadherence in difficult asthma. Am J Respir Crit Care Med 2009; 180: 817–822. doi: 10.1164/rccm.200902-0166OC [DOI] [PubMed] [Google Scholar]
- 7.Boulet L-P, Vervloet D, Magar Y, et al. Adherence: the goal to control asthma. Clin Chest Med 2012; 33: 405–417. doi: 10.1016/j.ccm.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Tay TR, Radhakrishna N, et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J 2018; 51: 1701836. doi: 10.1183/13993003.01836-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen DJ, Holmes LJ, Hince KA, et al. Nonadherence with inhaled preventer therapy in severe asthmatic patients on long-term omalizumab. Eur Respir J 2018; 52: 1801025. doi: 10.1183/13993003.01025-2018 [DOI] [PubMed] [Google Scholar]
- 10.Jeffery MM, Shah ND, Karaca-Mandic PJ, et al. Trends in omalizumab utilization for asthma: evidence of suboptimal patient selection. J Allergy Clin Immunol Pract 2018; 6: 1568–1577. doi: 10.1016/j.jaip.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 11.Senna G, Caminati M, Lockey RF. Allergen immunotherapy adherence in the real world: how bad is it and how can it be improved? Curr Treat Options Allergy 2015; 2: 39–53. doi: 10.1007/s40521-014-0037-6 [DOI] [Google Scholar]
- 12.Sulaiman I, Greene G, MacHale E, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J 2018; 51: 1701126. doi: 10.1183/13993003.01126-2017 [DOI] [PubMed] [Google Scholar]
- 13.Regione del Veneto Veneto Region Drug Regulatory Agency guidelines on the management of severe asthma. 2019 www.regione.veneto.it/web/sanita/linee-di-indirizzo-regionale1. Date last accessed: November 2019.
- 14.McBrien CN, Menzies-Gow A. Less is more: the impact of maintenance treatment adherence in severe asthma clinical trials. Eur Respir J 2019; 53: 1900599. doi: 10.1183/13993003.00599-2019 [DOI] [PubMed] [Google Scholar]
- 15.Vianello A, Caminati M, Andretta M, et al. Prevalence of severe asthma according to the drug regulatory agency perspective: an Italian experience. World Allergy Organ J 2019; 12: 100032. doi: 10.1016/j.waojou.2019.100032 [DOI] [PMC free article] [PubMed] [Google Scholar]