Abstract
Background and Purpose
In Romania, robust data about the prevalence of obesity and heart failure are lacking, especially in the elderly; therefore, this study aims to analyze the profile of overweight and obese patients aged >65 years admitted to a Romanian hospital for worsening heart failure, and also their risk in the presence of comorbidities.
Patients and Methods
This cross-sectional study was conducted in 126 consecutive elderly patients with overweight and obesity admitted to a Romanian hospital for worsening heart failure. They were divided into three groups: with reduced (<40%) – HFrEF, mid-range (40–49%) – HFmrEF and preserved (≥50%) ejection fraction – HFpEF. Obesity was defined according to the body mass index (BMI) status: obesity, ≥30 kg/m2; overweight, 25–29.9 kg/m2. The Charlson Comorbidity Index (CCI) was calculated to evaluate the severity of comorbidity, with a score ranging from 2 (only heart failure present and age >65 years) to 30 (extensive comorbidity).
Results
NT-proBNP values are negatively correlated with BMI only in patients with HFpEF. Creatinine clearance (p=0.0166), the presence of atrial fibrillation (p=0.0095) and NYHA functional class were independent predictors of increased NT-proBNP values. CCI score is negatively correlated with NT-proBNP values in patients with HFmrEF (r= −0.448, p=0.009) and HFpEF (r= −0.273, p=0.043). The CCI risk was not significantly different between the three groups.
Conclusion
Elderly heart failure patients with overweight or obesity have particular characteristics in terms of NT-proBNP values and presence of comorbidities. In the studied population, NT-proBNP levels were strongly influenced by renal function, NYHA functional class, the presence of atrial fibrillation and left ventricular ejection fraction.
Keywords: obesity, heart failure, comorbidity, elderly patients
Introduction
Heart failure (HF) and obesity represent two major public health issues worldwide, imposing large economic burdens. The prevalence of HF rises to more than 10% among people aged >70 years.1 Despite all therapeutic improvements made in recent decades, the number of hospitalizations, costs, morbidity and mortality remain high in patients with heart failure, especially in those with associated comorbidities.2,3 Also, population aging has clearly changed the epidemiological profile of heart failure patients. The typical elderly patient with comorbidities usually develops HFpEF, while signs and symptoms of heart failure are often non-specific and may not discriminate between heart failure and other medical conditions.1 The diagnosis of heart failure in elderly patients, especially in overweight and obese individuals, remains cumbersome and validated tools are missing.1 Furthermore, managing heart failure in the presence of cardiovascular and non-cardiovascular comorbidities brings particular challenges, and their presence has been identified as a major prognostic indicator for increased morbidity and mortality. Commonly, patients with multiple comorbidities (arterial hypertension, diabetes, lung disease, obesity) develop HFpEF.4 To date, more than 80% of patients diagnosed with HFpEF are overweight or obese.5,6 Unfortunately, no therapy has been found to increase life expectancy in HFpEF patients.7 The Charlson Comorbidity Index (CCI) is an extensively studied and validated predictive tool to assess comorbidity that has been shown to predict mortality.8,9 A comprehensive evaluation of global comorbidity has an important role in decision making and outcome of heart failure patients.
Obesity is an independently acknowledged cardiovascular risk factor, with an important contribution to the development of heart failure.10 Recent reports have shown that up to 49% of heart failure patients are obese and 32–40% are overweight.5 Compared to subjects with a normal BMI, the risk of heart failure is double in subjects with obesity, with a relative risk of 2.12 for women and 1.90 for men.5,6 Moreover, the diagnosis of heart failure in patients with obesity and also in elderly is significantly more difficult because the classical signs and symptoms, such as dyspnea, decreased exercise tolerance, are more difficult to identify, echocardiographic examination is often suboptimal, and the prognostic markers of heart failure, such as NT-proBNP, are decreased.1
Although obesity is an independent risk factor for heart failure, studies show a better prognosis of heart failure in the presence of overweight and obesity, a phenomenon known as the “obesity paradox”.5,6,11 The latest data from the World Health Organization show that in Europe, 21.5% of men and 24.5% of women are overweight or obese.12 However, a recent study including 10 European countries showed that in patients aged ⩾50 years the general prevalence of overweight reaches more than 60%.13 In Romania, robust data about the prevalence of obesity and heart failure is lacking, especially in elderly; therefore, this study aimed to highlight several particularities in terms of heart failure, obesity and comorbidity profile in patients aged > 65 years admitted to a Romanian hospital for worsening heart failure. These patients are frequently underrepresented in large controlled clinical trials, which is why we consider that evidence is required, especially regarding conditions such as obesity and heart failure, both having a climbing prevalence nationwide.
Patients and Methods
Study Design and Population
This is a cross-sectional study which consecutively enrolled 126 overweight and obese patients aged 65 years and over who were admitted for worsening heart failure to the Cardiology Department of the Clinical Rehabilitation Hospital in Cluj-Napoca, Romania. The estimated glomerular filtration rate (eGFR) was calculated using the Cockroft–Gault equation. We compared the baseline clinical characteristics, laboratory data, echocardiographic parameters, in-hospital therapies and adverse outcomes following admission between the three groups. Also, the Charlson Comorbidity Index (CCI) was calculated in order to evaluate the severity of comorbidity, with a score ranging from 2 (only heart failure present and age >65 years) to 30 (extensive comorbidity). For the purpose of the study, we used the basic CCI score, not the age-adjusted one. The assigned value for heart failure - 1 point was not incorporated in the final CCI score because it was one of the inclusion criteria. The comorbidity risk was evaluated as low if CCI = 2, moderate if CCI = 3–4 and high if CCI ≥ 5.8
Diagnosis of Heart Failure and Overweight/Obesity
Heart failure was defined according to the 2016 European Society of Cardiology guidelines for the diagnosis and management of acute and chronic heart failure criteria.1 Thus, all the included patients had NT-proBNP values above 125 pg/mL. Using the current terminology for describing heart failure, the patients were divided into three groups according to the left ventricular ejection fraction: with reduced (<40%) – HFrEF, mid-range (40–49%) – HFmrEF and preserved (≥50%) ejection fraction – HFpEF. NT-proBNP assays were performed upon initial assessment. NT-proBNP levels were determined on the day of admission and their measurement was carried out using the chemiluminescence method.
Based on anthropometric measurements, the body mass index (BMI) was calculated. Patients with a body mass index (BMI) ≥25 kg/m2 were included. Patients with a BMI between 25 and 30 were classified as overweight patients. Obesity was subdivided into 3 categories: class 1 -BMI 30 to < 35 kg/m2, class 2 - BMI 35 to < 40 kg/m2, class 3 - BMI 40 kg/m2 or higher.
Sociodemographic and Clinical Variables
Patients’ baseline demographic and clinical characteristics such as diabetes mellitus, arterial hypertension, smoking status, dyslipidemia are delineated in Table 1, stratified by left ventricular ejection fraction (LVEF). The mean age of the analyzed study population was 70.44±9.09 years, and 69 (54.8%) were men. Patients with HFpEF represented 45.2% of the studied population.
Table 1.
Patients Demographic and Clinical Baseline Characteristics by Tertile Left Ventricular Ejection Fraction
| All Patients | LVEF < 40% | LVEF 40–49% | LVEF ≥ 50% | p value a | ||
|---|---|---|---|---|---|---|
| Heart failure patients | 126 | 32(25.4) | 37(29.4%) | 57(45.2) | ||
| Age | Mean ± SD | 70. 44± 9. 09 | 67.18 ±9.35 | 71.29±8.7 | 71.70±8.8 | 0.06 |
| Sex | Women | 57(45.2%) | 15 (46.8) | 14 (37.83) | 28 (49.12) | NS |
| Men | 69 (54.8%) | 17 (53.12) | 23 (62.16) | 29 (50.87) | ||
| NYHA class | II | 44 (34.9) | 10 (31.25) | 17 (45.94) | 17 (29.82) | NS |
| III | 67(53.2) | 17 (53.12) | 18 (48.64) | 32 (56.14) | ||
| IV | 15 (11.9) | 5 (15.62) | 2 (5.4) | 8 (14.03) | ||
| NT-proBNP (pg/mL) | Mean ±SD (median) | 2728.22±3686.30 (1371.5) | 4363.75±6019 (1915) | 2047.58±2111 (1362) | 2231.19±2240 (1286) | NS |
| Creatinine clearance (%) | 30–59 | 25 (19.8) | 11 (34.37) | 5 (13.51) | 9 (15.78) | 0.058 |
| 60–89 | 40 (31.7) | 5 (15.6) | 16 (43.24) | 19 (33.33) | ||
| ≥90 | 61 (48.4) | 16 (50) | 16 (43.24) | 29 (50.87) | ||
| Diabetes mellitus (%) | Yes | 43 (34.1) | 9 (28.1) | 11 (29.72) | 23 (40.35) | NS |
| No | 83 (65.87) | 23 (71.87) | 26 (70.27) | 34 (59.64) | ||
| Hypertension (%) | Yes | 89 (70.6) | 17 (53.12) | 26 (70.27) | 46 (80.70) | 0.023 |
| No | 37 (29.4) | 15 (46.87) | 11 (29.72) | 11 (19.29) | ||
| Overweight (%) | Yes | 16 (12.7) | 3 (9.3) | 6 (16.21) | 7 (12.28) | NS |
| Obesity grade I (%) | Yes | 61 (48.4) | 15 (46.87) | 15 (40.54) | 31 (54.38) | |
| Obesity grade II (%) | Yes | 32 (25.4) | 9 (28.12) | 10 (27.02) | 13 (22.8) | |
| Obesity grade III (%) | Yes | 17 (13.5) | 5 (15.62) | 6 (16.21) | 6 (10.52) | |
| Smoking (%) | Yes | 47 (37.3) | 11 (34.37) | 15 (40.54) | 21 (36.84) | NS |
| No | 79(62.7) | 21 (65.62) | 22 (59.45) | 36 (63.15) | ||
| Total-cholesterol (mg/dl) | Mean ± SD | 167.19±46.45 | 152.71±46.54 | 168.59±42.93 | 174.42±47.51 | NS |
| LDL – cholesterol (mg/dl) | Mean ± SD | 103.03±42.34 | 87.90±30.75 | 107.05±51.41 | 108.91±40.02 | 0.06 |
| HDL cholesterol (mg/dl) | Mean ± SD | 39.80±12.13 | 39.65±15.49 | 39.02±10.36 | 40.40±11.2 | NS |
| Triglycerides (mg/dl) | Mean ± SD | 137.10±73.63 | 132.03±79.01 | 139.91±85.74 | 138.12±62.43 | NS |
| Glycemia (mg/dl) | Mean ± SD | 112.56±34.52 | 113.18±39.58 | 106.44±23.30 | 116.08±37.35 | NS |
| Uric acid (mg/dl) | Mean ± SD | 7.86±2.34 | 8.47±2.59 | 7.69±2.00 | 7.66±2.41 | NS |
| Creatinine (mg/dl) | Mean ± SD | 1.11±0.39 | 1.29±0.5 | 1.07±0.37 | 1.02±0.28 | 0.02 (Significant difference EF<40 vs 40–49 and EF <40 vs ≥50) |
| Ischaemic heart disease (%) | Yes | 59 (46.8) | 15 (46.87) | 17 (45.94) | 27 (47.36) | NS |
| No | 67 (53.2) | 17 (53.120) | 20 (54.05) | 30 (52.63) | ||
| Left atrium >40 mm | DA | 97 (77) | 25 (78.12) | 30 (81.08) | 42 (73.68) | NS |
| NU | 29(23) | 7 (21.87) | 7 (19.91) | 15 (26.31) | ||
| Diastolic dysfunction | Without | 21 (16.7) | 8 (25) | 7 (18.91) | 6 (10.52) | NS |
| Relaxation abnormality | 34 (27) | 6 (18.75) | 8 (21.62) | 20 (35.08) | ||
| Pseudonormal | 31 (24.6) | 6 (18.75) | 13 (35.13) | 12 (21.05) | ||
| Restrictive | 40 (31.7) | 12 (37.5) | 9 (24.32) | 19 (33.33) | ||
| Pulmonary artery systolic pressure | 49.57 ± 16.27 | 55.68±14.16 | 51.68±12.67 | 44.12±18.05 | 0.028 Significant difference between EF<40% vs ≥50% |
Note: aTwo-sided p < 0.05 was statistically significant.
Statistical Analysis
Statistical analysis was carried out using MedCalc (v 10.3.0.0, MedCalc Software, Ostend, Belgium) and SPSS Statistics for Windows (v 16.0, IBM Corporation, Armonk, NY, USA) software programs. Normal distribution was assessed using the Kolmogorov–Smirnov test. The results regarding categorical variables are presented as numbers and percentages. For numerical data, mean, standard deviation and median values were calculated (depending on distribution normality). In order to perform group comparisons, we used ANOVA and Kruskal–Wallis tests (for numerical data) or Chi square test (for non-numerical data). Pearson and Spearman coefficients were used to describe correlations between data. In order to perform multivariate analysis, we applied logarithm to NT-proBNP values. Multivariate analysis (for assessing independent predictive factors for log NT-proBNP) was performed taking into consideration age, gender, diabetes, the presence of arterial hypertension, left ventricular ejection fraction, atrial fibrillation, creatinine clearance, body mass index, CCI. A two-sided P < 0.05 was determined to be statistically significant.
Results
Profile of Heart Failure
The study population comprised 110 participants with obesity and 16 with overweight. No statistically significant differences were found in terms of left ventricular ejection fraction between patients with obesity class I, II and III. The most frequent precipitating factor for hospital admission was atrial fibrillation with rapid ventricular response. The main etiology of HF was ischemic (50%), followed by rheumatic heart disease.
Among the patients, 25.4% had reduced ejection fraction (HFrEF) with a mean NT-proBNP value of 4363.76 ± 6017 pg/mL, 29.4% had mid-range ejection fraction (HFmrEF) with a mean NT-proBNP value of 2047.58 ±2111 pg/mL, and 45.2% had preserved ejection fraction (HFpEF) with a mean NT-proBNP value of 2231.19 ± 2240 pg/mL.
NT-proBNP Values
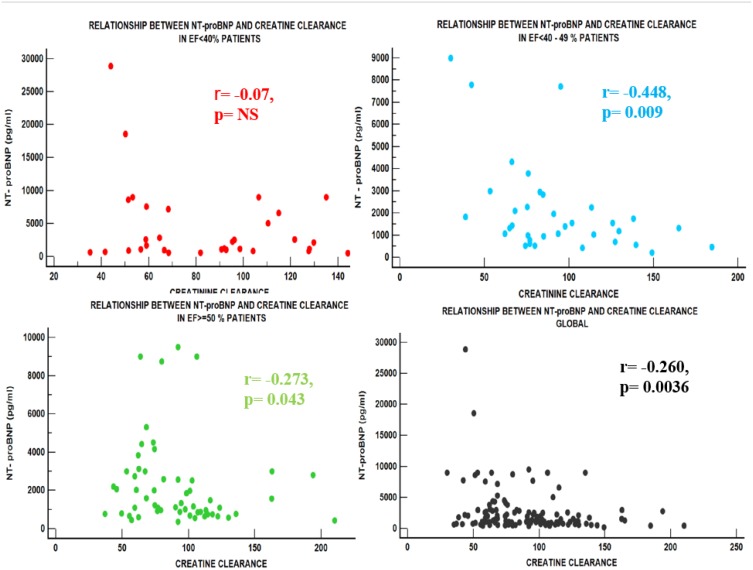
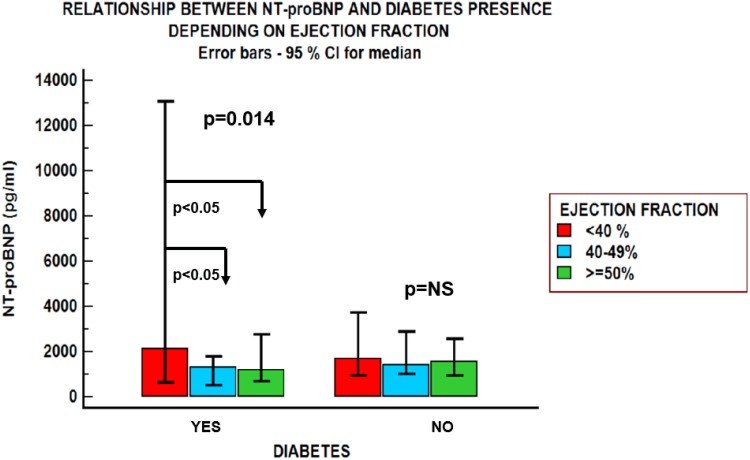
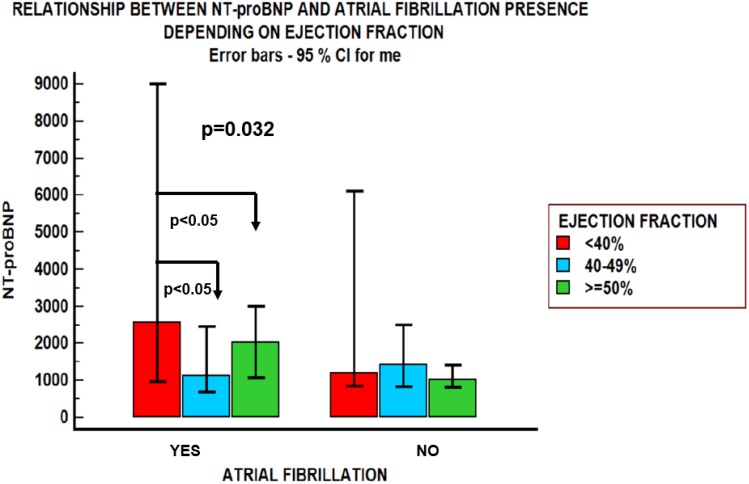
NT-proBNP values negatively correlated with BMI only in patients with HFpEF (r=−0.243, p=0.07); in the other groups, NT-proBNP was not significantly influenced by BMI. An inverse relationship was found between NT-proBNP values and creatinine clearance estimated by the Cockcroft–Gault equation in patients with HFmrEF: r=−0.448, p=0.009 and HFpEF: r= −0.273, p=0.043, but not in the HFrEF group (Figure 1). NT-proBNP levels were influenced by the presence of atrial fibrillation and diabetes mellitus (Figures 2 and 3), but were not influenced by age in any of the three groups, regardless of sex (Table 2). There was a significant difference in NT-proBNP values between patients with HFrEF, who had more elevated values, and those with HFmrEF and HFpEF (5226.33±5969 vs 1139.5±604.93 pg/mL and 5226.33±5969 vs 1953.3±2009 pg/mL). The same relationship was found in the presence of atrial fibrillation (5878±8077 vs 1851.83±1815 pg/mL and 5878±8077 vs 2906.87 ±2710 pg/mL).
Figure 1.
The relationship between NT-proBNP and creatinine clearance (Cockroft–Gault formula). NT-proBNP levels negatively correlated with the creatinine clearance estimated by Cockcroft–Gault equation in the group of patients with HFmrEF: r=−0.448, p=0.009 and HFpEF: r= −0.273, p=0.043, but not in the HFrEF group.
Figure 2.
NT-proBNP values in patients with obesity and heart failure depending on the left ventricular ejection fraction and the presence of diabetes mellitus. NT-proBNP levels were influenced by the presence of diabetes mellitus. Patients with diabetes mellitus and heart failure had significantly different levels of natriuretic peptides (NT-proBNP), depending on left ventricular ejection fraction. NT-proBNP values were not significantly different between the three groups of patients with heart failure without diabetes mellitus.
Figure 3.
The relationship between NT-proBNP and ejection fraction in heart failure patients with obesity and atrial fibrillation vs their counterparts without atrial fibrillation. Patients with heart failure and atrial fibrillation presented different levels of NT-proBNP levels, depending on left ventricular ejection fraction. Patients with HFrEF and atrial fibrillation presented higher natriuretic peptide levels in comparison with those with HFmrEF and HFpEF. In the absence of atrial fibrillation these differences were attenuated. The presence of atrial fibrillation (p=0.0095) was an independent predictor of increased NT-proBNP values.
Table 2.
Change in NT-proBNP Values by Comorbidities and Echocardiographic Parameters in the Three Arms
| NT-proBNP Value (pg/mL) | All Patients | LVEF < 40% | LVEF 40–49% | LVEF ≥ 50% | p value | |
|---|---|---|---|---|---|---|
| Creatinine clearance | 30–59 | 4647.76±6618 (2073) | 7304.27±9062 (2572) | 4404.4 ± 3786 (3000) | 1536.1 ±973 (1100) | NS |
| 60–89 | 2575.31±2184 (2019.5) | 2434. ±2839 (982) | 1767.06±1223 (1314) | 3288.11±2477 (2793) | NS | |
| ≥90 | 2036.78±2314 (1184) | 2945.18±2871 (1669) | 1574.06±1735 (1254) | 1790. 89 ±2189 (1021) | NS | |
| Atrial fibrillation | Mean ± SD (median) | 3306.50±4598 (1912) | 5878±8077 (2572) | 1851.83±1815 (1125) | 2906.87 ±2710 (2039) | 0.032 Significant differences between group 1 vs group 2 and group 1 vs group 3 |
| Diabetes mellitus | Yes | 2460.90±3383.68 (1317) | 5226.33± 5969 (2138) | 1139.5±604.93 (1317) | 1953.3±2009 (1176) | 0.014 Significant differences between group 1 vs group 2 and group 1 vs group 3 |
| No | 2817.58±3831.87 (1505) | 4026.21±6137 (1692) | 2396.84±2377.66 (1421) | 2424.87±2399.9 (1572) | NS | |
| Arterial hypertension | Yes | 2550.91±3552 (1556) | 4266.47±6879 (1692) | 1994.76±2031 (1320) | 2211.8±1944 (1590) | 0.08 |
| No | 3145.13±4002 (1188) | 4474±5109 (2296) | 2167.63±2383 (1404) | 2310.54±3313 (893) | NS | |
| Overweight | 3072.66±2781 (2000) | 6103.33±4713 (8645) | 1645.2±1521 (1067) | 2793.42±1651 (2527) | 0.07 | |
| 1st degree obesity | 2873.42±4472 (1339) | 5102.40±8101 (1184) | 2106±1831.4 (1833) | 2166.19±2127 (1233) | NS | |
| 2nd degree obesity | 2759.37±2957 (1525.5) | 3847.44±3536 (2612) | 2815.1±3068 (1653.5) | 1963.23±2371 (1108) | NS | |
| 3rd degree obesity | 1789.43±2225 (1119) | 2033.4±1802 (1692) | 957.5±489.75 (936) | 2543.8±3632 (924) | NS | |
| Smoking | Yes | 2814±2884 (1320) | 3535.45±3563.7 (1184) | 2509.33±2304 (1757) | 2653.71±2946 (1115) | NS |
| No | 2675.87±4117 (1404) | 4797.61±7016 (2296) | 1717.76±1952 (1067) | 1977.68±1685 (1501) | NS | |
| Left atrium dilated (>40 mm) | Yes | 3026.56±4037 (1724.5) | 4829.36±6594 (2296) | 2069.4±2211 (1379) | 2627.65±2487 (2000) | NS |
| No | 1705.35±1776 (1095) | 2700.85±3015 (1120) | 1938.5±1681 (1235) | 1147.53±572.85 (1021) | NS |
Note: p < 0.05 was statistically significant.
Creatinine clearance (p=0.0166), the presence of atrial fibrillation (p=0.0095) and NYHA functional class were independent predictors of increased NT-proBNP values.
NT-proBNP levels were not significantly influenced by the presence of echocardiographic left ventricular hypertrophy or atrial dilatation in any of the three groups (Table 2).
Profile of Comorbidities
The prevalence of comorbidities ranged from 15% for chronic pulmonary diseases to 34% for diabetes without chronic complications and 36% for peptic ulcer disease. A high CCI risk score was identified in 50.8% of patients and only 5.6% had a CCI = 2. The CCI risk was not significantly different between patients with heart failure with reduced (<40%) – HFrEF, mid-range (40–49%) – HFmrEF and preserved (≥50%) – HFpEF ejection fraction (EF) (p=0.068). Heart failure patients with low CCI scores were younger compared to those with moderate or high CCI scores (p<0.001). CCI score negatively correlated with NT-proBNP values in patients with HFmrEF (r= −0.448, p=0.009) and HFpEF (r= −0.273, p=0.043).
Medical Treatment
Table 3 synthesizes the treatment of patients divided into the three groups, stratified by LVEF. The most frequently used antiarrhythmic drug was amiodarone, administered to 28% of patients, especially those with atrial fibrillation. The in-hospital and discharge pharmacological treatment included the standard heart failure therapy recommended by current guidelines.
Table 3.
Medical Treatment of Patients Stratified by LVEF
| Total | All Patients | LVEF < 40% | LVEF 40–49% | LVEF ≥ 50% | p value |
|---|---|---|---|---|---|
| 126 | 32 | 37 | 57 | ||
| Angiotensin converting enzyme inhibitors (ACEIs) – No (%) | 75 (59.5%) | 15(46.8%) | 19(51.3%) | 41(71.9%) | 0.0340 |
| Angiotensin AT1 receptor blockers (ARBs) – No (%) | 49 (38.8%) | 22(68.7%) | 9(24.3%) | 18(31.5%) | 0.0054 |
| Sacubitril/valsartan – No (%) | 12 (9.5%) | 8(25%) | 1 (2.7%) | 0(0) | 0.0018 |
| Beta-blockers – No (%) | 121(96%) | 30(93.7%) | 35(94.5%) | 56 (98.2%) | NS |
| Ivabradine – No (%) | 10 (7.9%) | 4 (12.5%) | 4 (10.8%) | 2(3.5%) | NS |
| Long-lasting nitrates – No (%) | 28(22.2%) | 2(6.2%) | 23(62.1%) | 3 (5.2%) | <0.0001 |
| Calcium channel blockers – No (%) | 6 (4.7%) | 3 (9.3%) | 1(2.7%) | 2(3.5%) | NS |
| Antiarrhythmics – No (%) | 39 (30.9%) | 8 (25%) | 11(29.7%) | 20(35%) | NS |
| Anti-aldosterone agents (MRAs) – No (%) | 69 (54.7%) | 30 (93%) | 28 (75%) | 11 (19%) | NS |
| Anticoagulants – No (%) | 72 (57.1%) | 20(62.5%) | 19(51.3%) | 33(57.8%) | NS |
Note: p < 0.05 was statistically significant.
Discussion
Evaluation of biomarkers such as NT-proBNP upon hospital admission is needed in patients with heart failure because it can facilitate in-hospital treatment and early discharge. However, existing data shows that patients with obesity have lower values of NT-proBNP,1,5,14 altering its prognostic and risk stratification value. The exact pathophysiological mechanism is not fully understood. A possible explanation could be that C-type (NPR-C) natriuretic peptide receptors are well represented in adipose tissue, lipolysis being partially induced by these natriuretic peptides, so their serum concentration is lower in obese patients. Also, reduced cardiac distensibility in obese patients lowers the synthesis of natriuretic peptides.14,15 On the other hand, advancing age is known to be a potential cause of elevated natriuretic peptide levels.16 Furthermore, the association of the single marker-based risk stratification approach and a comprehensive comorbidity risk evaluation helps the clinician in optimizing prognostic estimation, especially in elderly patients with heart failure and multiple comorbidities.
Commonly, elderly patients with obesity do not have a significant impairment of left ventricular systolic function, which is often preserved or only slightly impaired.5,6 Current guidelines highlight the fact that one in six patients with heart failure remains misdiagnosed.1 Misdiagnosis comes from the fact that obesity by itself can cause dyspnea, exercise intolerance and ankle swelling, poor quality echocardiographic images and low NT-proBNP levels.1
In the current study, the majority of elderly patients with obesity had HFpEF. However, there were no significant differences between the three groups in terms of cardiovascular risk factors or various comorbidities, which were evaluated individually and as “a whole” using the Charlson Comorbidity Index score (CCI), excepting renal impairment, which was more often seen in the HFrEF group. Also, we registered significantly higher pulmonary artery systolic pressures in HFrEF patients than in those with HFpEF.
Patients with HFpEF and HFmrEF commonly have various associated cardiovascular comorbidities.1,17 In the current study, the patients presented a wide spectrum of comorbidities and half of them had a high CCI risk score suggesting a worse prognosis and a greater likelihood of rehospitalization. Charlson Comorbidity Index score is the most widely used tool for the assessment of comorbidities and there is strong evidence regarding its ability to predict non-sudden death in heart failure.18 We found no differences in patients’ CCI scores regarding ventricular function (reduced, mid-range or preserved). However, patients with obesity and HFpEF or HFmrEF with high CCI scores had lower NT-proBNP values than those with low CCI scores. So, the CCI score may serve as a simple, inexpensive tool for risk prediction and stratification in elderly heart failure patients with various comorbidities, along with specific biomarkers for heart failure, and also it may pave the way to a personalized treatment strategy.
There are studies which suggest increasing values of NT-proBNP with age.19 In the current study, NT-proBNP levels did not significantly increase with age, but the CCI score was significantly higher in patients with advanced age. So, the presence of obesity in elderly patients may falsely normalize NT-proBNP levels; a multimarker diagnostic and predictive strategy, including various biomarkers which reflect different pathophysiological mechanisms in heart failure, may be extremely useful in these patients.
The presence of renal dysfunction in heart failure patients is frequently seen in clinical practice, being referred to as the cardiorenal syndrome. High NT-proBNP levels are a marker of poor prognosis in these patients.20 Furthermore, extrapolated from a large body of literature, BNP and NT-proBNP levels are assumed to be higher in patients with decreasing renal function (progressive kidney disease).1,21 In the present study, involving elderly participants with obesity, an inverse relationship between creatinine clearance and NT-proBNP was demonstrated in the HFmrEF and HFpEF groups.
Diabetes mellitus is a well-known independent cardiovascular risk factor, frequently seen in elderly patients with obesity, also leading to HFpEF development.1,22 However, in diabetic patients, heart failure biomarkers such as elevated NT-proBNP levels remain extremely accurate in predicting cardiovascular events.23 Hyperglycemia levels may accelerate ventricular secretion of BNP by inducing a hypertonic state, cell dehydration, hypervolemia, with ventricular wall tension.24 There is strong evidence regarding NT-proBNP as an important tool in predicting the risk of cardiovascular events in diabetic patients, which seems to be even superior to albuminuria.25 In our study, NT-proBNP levels were influenced by the presence of diabetes mellitus; patients with obesity and a LVEF higher than 40% presented lower NT-proBNP values than those with low LVEF. This suggests a better prognosis in obese patients with diabetes mellitus and HFpEF in comparison with their counterparts with HFrEF.
Also, NT-proBNP levels were influenced by the presence of atrial fibrillation (AF). Obesity is an independent risk factor for atrial fibrillation development.26,27 One unit increase in BMI brings an additional 4% risk of AF development.28 Obesity-related cardiomyopathy with left atrial dilatation and dysfunction may be, at least in part, responsible for the development, recurrence or progression of atrial fibrillation. However, studies show that obese heart failure patients with AF have a significantly lower risk of all-cause mortality and rehospitalization for worsening heart failure compared to normal weight patients.29 In our study, AF was an independent predictor of log NT-proBNP. In patients with heart failure and AF, BNP is mainly produced in the atrium, although there are fewer myocardial cells.30 NT-proBNP values are elevated in AF, being a potential prognostic tool, also with possible utility in therapeutic decision. However, NT-proBNP values decrease in patients with atrial fibrillation and obesity.31
The so-called “obesity paradox” is a well-documented phenomenon; recent studies have focused on characterizing specific subgroups.1,5 Significant increases of the BMI attenuate this paradox.32 Also, the “obesity paradox” is described in AF patients, lower rates of all-cause mortality, stroke, systemic embolism, and myocardial infarction being registered in patients efficiently anticoagulated.33
Conclusion
Elderly individuals with obesity and heart failure have particular characteristics in terms of left ventricular ejection fraction, NT-proBNP values, renal function and comorbidities. The majority of elderly patients with obesity had HFpEF. They presented a wide spectrum of comorbidities and half of them had a high CCI risk score. CCI score negatively correlated with NT-proBNP values in patients with HFmrEF and HFpEF. NT-proBNP levels were strongly influenced by renal function, NYHA functional class, the presence of atrial fibrillation and left ventricular ejection fraction. So, a composite risk stratification strategy based on a combination of heart failure-specific biomarkers such as NT-proBNP and comorbidity prognostic risk such as CCI may greatly enhance accuracy in diagnosing heart failure in overweight and obese elderly patients with various comorbidities, especially those with HFpEF.
Ethics and Consent Statement
The selected patients were informed about the study protocol and gave their signed informed consent. The study was carried out in agreement with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. This study was approved by the institutional Ethics Committee of “Iuliu Hatieganu” University of Medicine and Pharmacy Cluj-Napoca, Romania (permission code: 119/13.03.2017).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ponikowski P1, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;27:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2.Filippatos G, Khan SS, Ambrosy AP, et al. International REgistry to assess medical practice with longitudinal observation for Treatment of Heart Failure (REPORT-HF): rationale for and design of a global registry. Eur J Heart Fail. 2015;17(5):527–533. doi: 10.1002/ejhf.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jhund PS. Obesity and heart failure: when ‘epidemics’ collide. Eur Heart J. 2017;38(24):1934–1936. doi: 10.1093/eurheartj/ehw357 [DOI] [PubMed] [Google Scholar]
- 4.Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17(7):665–671. doi: 10.1002/ejhf.304 [DOI] [PubMed] [Google Scholar]
- 5.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox în heart failure. Prog Cardiovasc Dis. 2018;61(2):151–156. doi: 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92(2):266–279. doi: 10.1016/j.mayocp.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Montero D, Flammer AJ. Exercise intolerance in heart failure with preserved ejection fraction: time to scrutinize diuretic therapy? Eur J Heart Fail. 2017;19(8):971–973. doi: 10.1002/ejhf.811 [DOI] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373e83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 9.Formiga F, Moreno-Gonzalez R, Chivite D, et al. High comorbidity, measured by the charlson comorbidity index, associates with higher 1-year mortality risks in elderly patients experiencing a first acute heart failure hospitalization. Aging Clin Exp Res. 2017;30(8):927–933. doi: 10.1007/s40520-017-0853-1 [DOI] [PubMed] [Google Scholar]
- 10.Badimon L, Bugiardini R, Cenko E, et al. Position paper of the European Society of Cardiology-working group of coronary pathophysiology and microcirculation: obesity and heart disease. Eur Heart J. 2017;38(25):1951–1958. doi: 10.1093/eurheartj/ehx181 [DOI] [PubMed] [Google Scholar]
- 11.Padwal R, McAlister F, McMurray JJ, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes. 2014;38(8):1110–1114. doi: 10.1038/ijo.2013.203 [DOI] [PubMed] [Google Scholar]
- 12.Yumuk V1, Tsigos C, Fried M, et al. Obesity management task force of the european association for the study of obesity. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. doi: 10.1159/000442721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peralta M, Ramos M, Lipert A, Martins J, Marques A. Prevalence and trends of overweight and obesity in older adults from 10 European countries from 2005 to 2013. Scand J Public Health. 2018;46(5):522–529. doi: 10.1177/1403494818764810 [DOI] [PubMed] [Google Scholar]
- 14.Madamanchi C, Alhosaini H, Sumida A, et al. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176(3):611–617. doi: 10.1016/j.ijcard.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care. 2014;37(11):2899–2908. doi: 10.2337/dc14-0669 [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Card Fail. 2017;23(8):628–651. doi: 10.1016/j.cardfail.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 17.Lourenço AP, Leite-Moreira AF, Balligand JL, et al. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the working group on myocardial function of the European society of cardiology. Eur J Heart Fail. 2018;20(2):216–227. doi: 10.1002/ejhf.1059 [DOI] [PubMed] [Google Scholar]
- 18.Clarke B, Howlett J, Sapp J, et al. The effect of comorbidity on the competing risk of sudden and nonsudden death in an ambulatory heart failure population. Can J Cardiol. 2011;27(2):254e61. doi: 10.1016/j.cjca.2010.12.053 [DOI] [PubMed] [Google Scholar]
- 19.Richards AM. The relationship of plasma NT-proBNP to age and outcomes in heart failure. JACC Heart Fail. 2016;4(9):746–748. doi: 10.1016/j.jchf.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 20.Srisawasdi P, Vanavanan S, Charoenpanichkit C, et al. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol. 2010;133(1):14–23. doi: 10.1309/AJCP60HTPGIGFCNK [DOI] [PubMed] [Google Scholar]
- 21.Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest. 2014;44(3):303–308. doi: 10.1111/eci.12234 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert RE, Krum H. Heart failure in diabetes: effects of antihyperglycaemic drug therapy. Lancet. 2015;385(9982):2107–2117. doi: 10.1016/S0140-6736(14)61402-1 [DOI] [PubMed] [Google Scholar]
- 23.Saeed A, Ballantyne CM. Assessing cardiovascular risk and testing in type 2 diabetes. Curr Cardiol Rep. 2017;3(3):19. doi: 10.1007/s11886-017-0831-4 [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Hu W, Su H, et al. Levels of B-type natriuretic peptide in chronic heart failure patients with and without diabetes mellitus. Exp Ther Med. 2013;5(1):229–232. doi: 10.3892/etm.2012.760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol. 2012;19(5):944–951. doi: 10.1177/1741826711420015 [DOI] [PubMed] [Google Scholar]
- 26.Wang HJ, Si QJ, Shan ZL, et al. Effects of body mass index on risks for ischemic stroke, thromboembolism, and mortality in Chinese atrial fibrillation patients: a single-center experience. PLoS One. 2015;10(4):e0123516. doi: 10.1371/journal.pone.0123516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. [DOI] [PubMed] [Google Scholar]
- 28.Lavie CJ, De Schutter A, Parto P, et al. Obesity and prevalence of cardiovascular diseases and prognosis-the obesity paradox updated. Prog Cardiovasc Dis. 2016;58(5):537–547. doi: 10.1016/j.pcad.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 29.Yagawa M1, Nagatomo Y, Izumi Y, et al. Effect of obesity on the prognostic impact of atrial fibrillation in heart failure with preserved ejection fraction. Circ J. 2017;81(7):966–973. doi: 10.1253/circj.CJ-16-1130 [DOI] [PubMed] [Google Scholar]
- 30.Knudsen CW, Omland T, Clopton P, et al. A. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol. 2005;46(5):838–844. doi: 10.1016/j.jacc.2005.05.057 [DOI] [PubMed] [Google Scholar]
- 31.Zheng LH, Wu LM, Yao Y, et al. Impact of body mass index on plasma N-terminal ProB-type natriuretic peptides in Chinese atrial fibrillation patients without heart failure. PLoS One. 2014;9(8):e105249. doi: 10.1371/journal.pone.0105249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López J, Cortés-Bergoderi M. Update: systemic diseases and the cardiovascular system (i): obesity and the heart. Rev Esp Cardiol. 2011;64(2):140–149. doi: 10.1016/j.recesp.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Sandhu RK, Ezekowitz J, Andersson U, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation) trial. Eur Heart J. 2016;37(38):2869–2878. doi: 10.1093/eurheartj/ehw124 [DOI] [PubMed] [Google Scholar]