Highlights
-
•
The effect of Co-NPs was evaluated for biochemical enzymatic toxicity.
-
•
Aspartate aminotransferase (ASAT) and Alanine aminotransferase (ALAT) enzymes in serum, liver, and kidney of rats were used.
-
•
The levels of enzymes were increased significantly after being exposed to Co-NPs.
-
•
The elevated level of these enzymes can be useful in the diagnosis of any toxic injury.
Keywords: Biochemical, Enzyme, ASAT, ALAT, Toxicity, Cobalt nanoparticles, Wistar rats, serum, Liver, Kidney
Abstract
Cobalt nanoparticles (Co-NPs) have been extensively used in clinical practices and medical diagnosis. In this study, the potential toxicity effects of Co-NPs with special emphasis over the biochemical enzyme activities, such as aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) in serum, liver, and kidney of Wistar rats were investigated. This toxicity measurement of nanomaterials can support the toxicological data. The biochemical enzymatic variations are powerful tools for the assessment of toxicity. ASAT and ALAT enzymes have been widely used to predict tissue-specific toxicities associated with xenobiotic. The biochemical changes induced by Co-NPs have significance in their toxicological studies because the alterations in biochemical parameters before clinical symptoms indicate either their toxicant safety or detrimental effect. Herein, Co-NPs with particle size <50 nm significantly activated ASAT and ALAT enzymes in the serum, liver, and kidney of rats at concentration-dependent order.
1. Introduction
Nowadays, nanoparticle-based research is an expeditiously developing area with enormous potential in medicine. Cobalt nanoparticles (Co-NPs) are spherical in shape [1]. Co-NPs with magnetic properties shows their potential applications in biotechnology, biomedicine, material sciences, engineering, and environmental areas [2]. Co-NPs can be manufactured by using a laser evaporation process and the contaminants can be removed to produce high purity of Co-NPs [3,4]. Co element is also an indispensable constituent of human nutrients such as vitamin B12 part and is used in drug production especially for cancer treatments [5]. The syntheses of Co-NPs with new shapes are suitable for clinical biomedical practice due to their accumulation in tumor tissue without the need of intratumor injection, high photothermal conversion, excellent optical and photoacoustic imaging performances, and renal excretion [6]. But, the risk may be toxic or cure of cells that can be counted by the death rate of cells [6]. Aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) are the enzymes found mainly in the liver, red blood cells, heart, kidneys, and pancreas cells [7]. The activities of hepatic ASAT, ALAT, γ-GT (gamma-glutamyl transpeptidase), and GPT (serum glutamic pyruvic transaminase) enzymes are commonly used as biomarkers for recent alcohol abuse and in situ toxic compounds [[8], [9], [10], [11]]. The amount of ASAT and ALAT enzymes in the blood is directly related to tissue damages. More or fewer amounts of the enzymes lead to an increase or decrease in tissue damages, respectively [12]. Meanwhile, an increase in the concentration of NPs may cause damage to the cell morphology and structure. The inflammation and necrosis caused by hepatocyte damage can lead to an increase the permeability of the cell membrane. ASAT and ALAT enzymes are released into the body through the cell membrane and hence their concentration in the blood increases [13]. Thus, ASAT and ALAT enzymes are indicators of liver damage [14].
The toxicity of Co-NPs belonging to the cluster of metal and metal oxide NPs may be attributed to either direct uptake of Co-NPs by cells or to the cessation of Co-NPs, leading to the increased level of cationic ions in the tissue with consequent effects on the cells [15]. Until now, there has been limited attention to gaining knowledge related to the potential biochemical enzymatic toxicity of Co-NPs. Co-NPs were found to exert oxidative stress in lung epithelial cells [16], DNA damage in fibroblasts [17], inflammatory response in endothelial cells [18], human mononuclear cells, and neutrophils cytotoxicity [19]. The morphological transformation and genotoxicity induced by Co-NPs were demonstrated in Balb3T3 cells [20]. Furthermore, it was shown that Co-NPs induced a genotoxic effect in human peripheral leukocytes [21]. However, a systematic survey of Co-NPs effects on the viability of non-identical cell types is missing.
The detection of ASAT and ALAT enzymes has been widely studied due to their clinical significance in monitoring patients with liver diseases [22,23]. The elevated level of ASAT enzymes may be induced by disorders that affect organs or tissues other than the liver with the most common being striated muscles. The elevated level of ASAT and ALAT up to 300 u/L is considered nonspecific. Normally elevated levels are occurred during the second trimester in asymptomatic normal pregnancy [[24], [25], [26], [27]] whereas elevated levels of ASAT predominate in cirrhosis patients [28]. The toxicity of Co-NPs is one of the major challenges and with a green approach. Co-NPs as a biomarker can reduce the risk of xenobiotics and focus on the most recent emerging developments including synthesis methods, bioimaging, and biosensing applications. Then, it is very important to know the absorbance of ASAT and ALAT in terms of toxicity in different cells and response with the Co-NPs subjected to induced hepatotoxicity and indices of antioxidant status of liver tissues.
2. Materials and methods
2.1. Chemicals
Co-NPs were obtained from Sigma Aldrich, Germany. All other chemicals used in this study were of analytical grade and purchased from Sigma Aldrich (St. Louis. MO, USA). The BSA (bovine serum albumin) (mg/mL): 0.01 g of BSA dissolved in 100 mL of 0.5 M NaOH. Reagent A: the mixture of 2 % Na2CO3, 0.4 % NaOH, 0.16 % Na/K tartarate, and 1 % SDS. Reagent B: 4.0 % CuSO4 (copper reagent). Reagent C: 100 parts of reagent A with 1 part of reagent B. 2 N folins reagent was dissolved in an equal volume of pure water and 1 N folin-phenol reagent was made.
2.2. Rat treatment
Wistar rats were purchased from the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India. They were maintained under controlled conditions in the animal house of the Council of Scientific and Industrial Research, Indian Institute of Chemical Technology, Hyderabad, India, for a week before the experiment. The ethical clearance was obtained from the Animal Ethical Committee (Animal House Facility, Applied Biology Division, Indian Institute of Chemical Technology). The rats were maintained at 22 °C and relative humidity of 30–70 %. Lightening arrangements were 12 h in light and 12 h in dark.
2.3. Biochemical studies
The rats were sacrificed by cervical dislocation. Rat blood was collected without any anticoagulants before being sacrificed. Rat serum was obtained by centrifuging the blood at 1500 rpm for 10 min. The liver and kidney of rats were dissected out and quickly homogenized separately in ice-cold sucrose solutions using Yarco speed homogenizer to make 10 % homogenate (w/v). The pellet was discarded and the supernatant was used as an enzyme source. Serum, kidney, and liver homogenates were used to determine in vitro ASAT and ALAT enzymes in control and nanomaterial-exposed organs.
2.4. Protein, ASAT, and ALAT estimations
The protein was estimated in the liver and kidney of control and exposed cultures with folin-phenol reagent using the standard procedure as described by Lowry and coworkers [29]. ASAT and ALAT activities in rat’s serum, kidney, and liver were estimated by the spectrophotometer method of Yatzidis [30].
2.5. Statistical analysis
The data represents the mean and standard deviation of three biological replicates from each animal group. The data was analyzed by the Dunnett test to illustrate the significant concentration differences between the control and treated-culture groups. Statistical significance was considered at P < 0.05. Statistical analysis for Co-NPs concentrations in organs and biochemical examinations were calculated by:
Where X stands for pyruvic acid formed in μg
stands for volume of serum in ml and
stand for molecular weight of pyruvic acid (for converting μg into μmoles)
3. Results
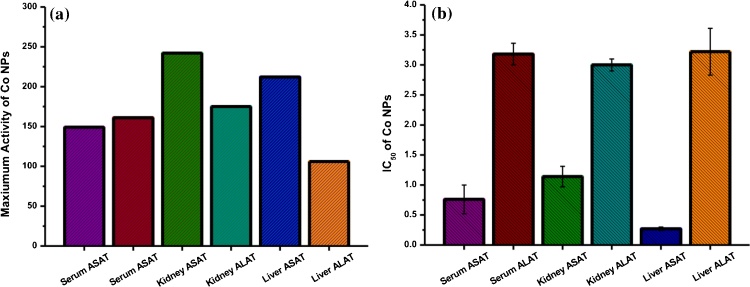
In the present systematic study, Aspartate aminotransferase (ASAT) and Alanine aminotransferase (ALAT) activities in serum, liver, and kidney reacted with Co-NPs at different concentrations were evaluated. Co-NPs with particle size < 50 nm significantly activated Aspartate aminotransferase and Alanine aminotransferase in serum, liver, and kidney. Similarly, the ASAT and ALAT, significant changes and activation were observed with each concentration of Co-NPs. The significant activation of ASAT in serum was observed at the concentration of 1.0, 2.5, and 5.0 mM of Co-NPs (Table S1). The maximum activation was about 149 % at 5.0 mM concentration of Co-NPs (Fig. 1a) and the IC50 was observed <1.00 mM (Fig. 1b). Similarly, the ALAT significant activation in serum was observed at 1.0, 5.0, and 10.0 mM concentrations of Co-NPs (Table S2) and the IC50 was observed at 3.18 ± 0.18 mM of Co-NPs (Fig. 1b). The maximum activation was observed at 161 % at 10 mM Co-NPs (Fig. 1a).
Fig. 1.
(a) The maximum activation of Co-NPs (5 mM) for ASAT and ALAT in different samples. (b) IC50 of Co-NPs for ASAT and ALAT in different samples.
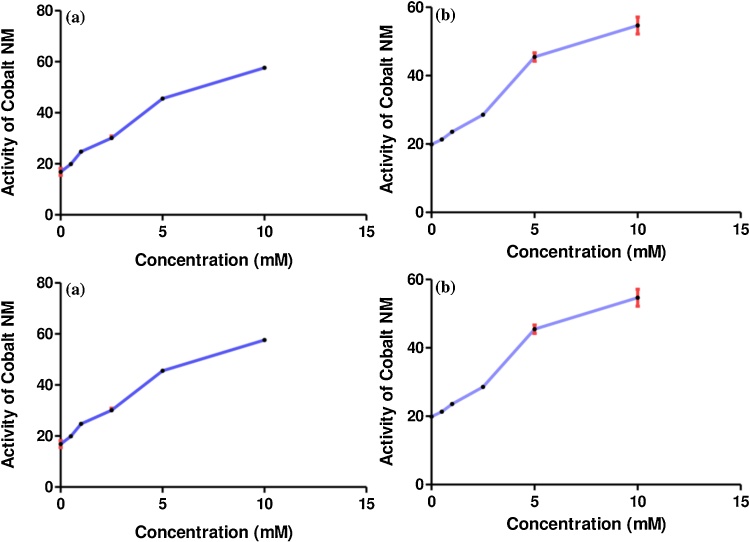
Furthermore, ASAT significant changes in kidney were observed in the Co-NPs concentration of 0.5, 1.0, 2.5, 5.0, and 10.0 mM (Table S3). The IC50 was observed to be 1.14 ± 0.17 mM Co-NPs (Fig. 1b). The maximum activation was 242 % at 10.00 mM of Co-NPs concentration (Fig. 1a) with the activity of 57.61 ± 0.61 (Fig. 2a). Meanwhile, the ALAT significant changes in kidney were observed at 1.0, 2.5, 5.0, and 10.0 mM Co-NPs and the observed IC50 was 3.00 ± 0.10 mM (Table S4). A maximum activation was 175 % at 10.0 mM concentration of Co-NPs (Table S4 and Fig. 1). The activity of Co-NPs was increased from 0.5 mM to 10.0 mM compared to the control (Fig. 2b).
Fig. 2.
The activity of Co-NPs with different concentrations on the ASAT (a) and ALAT (b) in the kidney of rats.
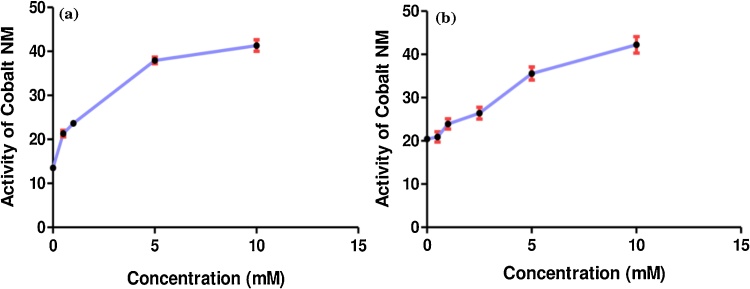
The ASAT in the liver was significantly activated at 0.5, 1.0, 5.0, and 10.0 mM concentrations of Co-NPs and the observed IC50 was 0.27 ± 0.03 mM (Table S5). Further, 212 % activation was observed at 10.0 mM concentration of Co-NPs with increasing their activity (Fig. 3a). Similarly, the ALAT activation in the liver was significant for all concentrations including 0.5, 1.0, 2.5, 5.0, and 10.0 mM concentrations of Co-NPs (Table S6). The IC50 was observed at 3.22 ± 0.39 mM Co-NPs. The maximum increase of their activity was observed with the maximum concentration of Co-NPs at 10.0 mM (Fig. 3b).
Fig. 3.
The activity of Co-NPs with different concentrations on the ASAT (a) and ALAT (b) in the liver of rats.
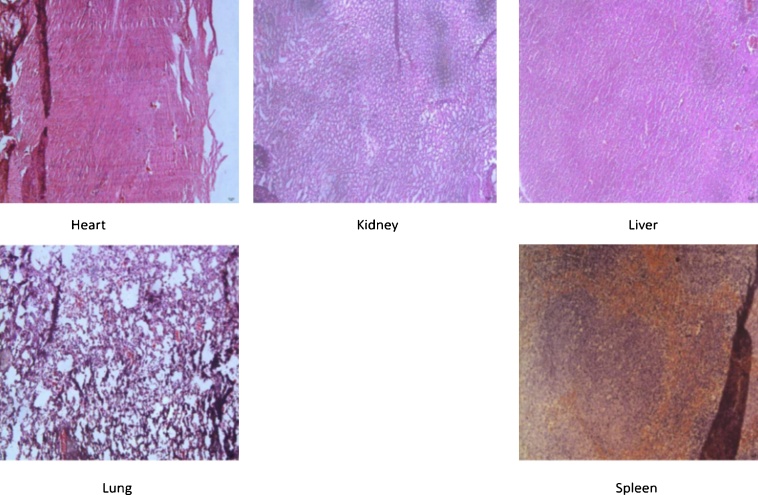
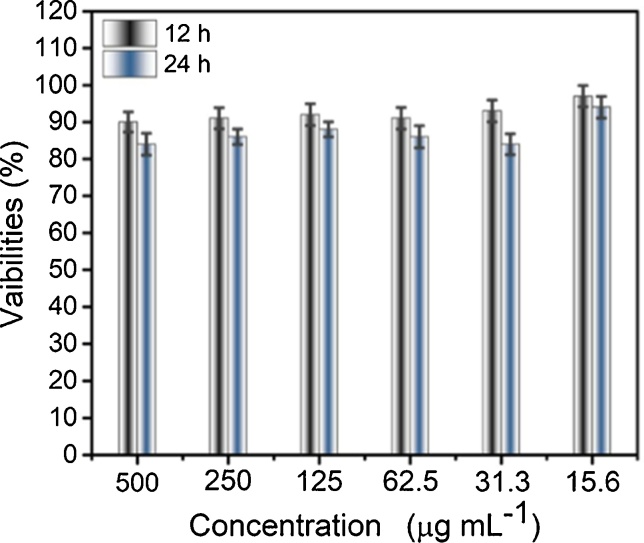
A patho-morphology analysis on heart, liver, spleen, lung, and kidney in different groups was conducted to estimate the toxicity of the Co-NPs. The spleen, heart, and lung were optional data for hemotoxylin and eosin (H&E) stain. The H&E stained images of the samples are given in Fig. 4. In addition, the cell viability report of the samples at different concentrations of Co-NPs is shown in Fig. 5. The results showed that the Co-NPs were predominantly accumulated with ASAT and ALAT, suggesting the transferring of Co-NPs to the samples.
Fig. 4.
H&E stained images of the liver, kidney, heart, lung, and spleen.
Fig. 5.
Cell viability at different concentrations of Co-NPs.
4. Discussion
The liver is the primary organ where exogenous synthetic substances are utilized and in the long run, discharged; as a result, liver cells are presented significantly with these chemical substances, which lead the liver brokenness, cell damage, and even organ failure [31]. Cobalt metals are known to be genotoxic in vitro, whereas Co2+ ions are known to be a health hazard to rodents [32]. Co-NPs induced greater cytotoxicity and genotoxicity in liver cells, cell membrane damage, oxidative stress, immune inflammation, and DNA damage, which may play an important role in the effects of Co-NPs on the liver cells [33]. It is proven that Co-NPs induce time and concentration-dependent cytotoxicity. While, it was unknown that whether the Co2+ ions release from the prostheses or by corrosion of wear particles, the mechanism of Co cytotoxicity is encompassed in that oxidative pressure assumes a vital role [[34], [35], [36]]. A recent study demonstrated that NPs can cross cytomembranes with no evident damage to cell integrity [37]. Young-min Kwon et al. reported the potential toxicity of Co-NPs in macrophages, although Co2+ in the culture medium resulting from the corrosion of Co-NPs cannot have significant effects [38]. The latest study suggested that the cytotoxicity of Co-NPs was probably mediated by Co-NPs rather than metal ions dissolved by nanoparticles in the extracellular culture medium. Although the toxicity of Co2+ and Co-NPs has been confirmed, the specific mechanism has not been explained in the study [39]. This study by Akhtar et al. shows that PEG-coated CoFe2O4 synthesized by hydrothermal technology is an excellent model of drug carrier and can use curcumin, which is a natural chemical and has no side effects and no other diseases reported [40]. The accumulation of Co in the liver causes alterations of hepatic function [41]. Degeneration, inflammation, and necrosis prevented by hepatocyte damage can increase the permeability of the cell membrane. Subsequently, ASAT and ALAT as clinical biomarkers are released into the body through the cell membrane and hence their concentration in the blood increases. ASAT and ALAT are indicators of liver damage [42]. Recent findings in vitro studies suggest that Co-NPs can penetrate cell membranes and other biological dams [43]. The ASAT and ALAT catalyze the following reaction:
R−CHNH2.COOH + RC(COOH) ↔ H3C.CO.COOH + R.CH.NH2.COOH
α-Oxaglutarate + l-aspartate ↔ l-glutamate + oxaloacetate
α-Oxaloglutarate + l-alanine ↔ l-glutamate + Pyruvate.
The oxaloacetate in the presence of aniline citrate is converted into pyruvate, in both the cases of pyruvate reacts with 2,4-DNPH and form a red-colored complex dinitrophenylhydrazine which was be measured on a spectrophotometer.
In this study, the activities of ASAT and ALAT enzymes increased significantly their activation in the serum, liver, and kidney, when compared with the control. Thus, these results were further compared with the results that show consistency with the previous studies. The current study shows the significance of Co-NPs to increase the activity of ALAT and ASAT enzymes in the serum of exposed tissues in a concentration-dependent manner suggesting possible injuries to the liver tissue. ALAT enzyme is one of the main groups of the family of phosphatases [44]. The dysfunction of the heart and liver is associated with the elevation of ALAT and ASAT in the serum [45,46].
The present study shows the results of the liver, kidney, and serum of exposed tissues with 0.5, 1.0, 2.5, 5.0, and 10.0 mM concentrations of Co-NPs. Moreover, the data reveals that Co-NPs induced a concentration-dependent increase. A significant concentration-dependent of Co-NPs increase in pro-inflammatory was also found in the previous study [47]. Several reports are available on light microscopy studies of rat liver subjected to Co-NPs [48]. Co-NPs were at concentration-dependent order to activate both ASAT and ALAT activities in serum, liver, and kidney. These findings suggest that Co-NPs could induce concentration-dependent and the maximum increase of 106 % was observed at 10.0 mM concentration of Co-NPs for ALAT in the liver. The relative toxicity based on IC50 showed that liver ASAT was more potent in causing the toxicity followed by serum ASAT, kidney ASAT, kidney ALAT, serum ALAT, and liver ALAT (Table S7). The probable mechanisms of toxicity could be Co-NPs induce adverse biological effects through the activation of the immune response.
5. Conclusion
The biochemical enzymes of ASAT and ALAT increased significantly in serum, liver and kidney tissues exposed to Co-NPs. The elevated levels of ASAT and ALAT enzymes were reported in serum, kidney, and liver with toxic chemicals. These enzymes increased in serum and simultaneously increased in tissues like liver and kidney due to increased permeability of the plasma membrane. This is also suggestive of increased synthesis of these enzymes as an adaptive mechanism due to the chemical stress. The relative toxicity based on IC50 showed liver ASAT was more potent in causing toxicity than serum ASAT, kidney ASAT, kidney ALAT, serum ALAT, and liver ALAT. These results suggest that the in vitro exposure of Co-NPs caused alteration in the serum and in cellular activities of vital organs like liver and kidney, inducing the changes in the physiological and metabolic activities of the individual.
CRediT authorship contribution statement
Akhtar Rasool: Conceptualization, Methodology, Investigation, Validation, Writing - original draft. Muhammad Zulfajri: Visualization, Validation, Writing - review & editing. Arif Gulzar: Validation, Data curation. Marlia Mohd Hanafiah: Software, Writing - review & editing. Syeda Azeem Unnisa: Formal analysis, Project administration. Mohammed Mahboob: Supervision, Methodology, Validation.
Declaration of Competing Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank the CSIR-Indian Institute of Chemical Technology, Hyderabad, India, for providing the lab facilities to conduct this work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00453.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Biao L., Jian-guo G., Qi W., Qing-jie Z. Preparation of nanometer cobalt particles by polyol reduction process and mechanism research. Mater. Trans. 2005;46:1865–1867. [Google Scholar]
- 2.Ha Y., Ko S., Kim I., Huang Y., Mohanty K., Huh C., Maynard J.A. Recent advances incorporating superparamagnetic nanoparticles into immunoassays. ACS Appl. Nano Mater. 2018;1:512–521. doi: 10.1021/acsanm.7b00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faraji M., Yamini Y., Rezaee M. Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010;7:1–37. [Google Scholar]
- 4.Tartaj P., Morales Mdel P., Veintemillas-Verdaguer S., Gonzalez-Carreno T., Serna C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003;36:182–197. [Google Scholar]
- 5.Czarnek K., Terpilowska S., Siwicki A.K. Selected aspects of the action of cobalt ions in the human body. Cent. J. Immunol. 2015;40:236–242. doi: 10.5114/ceji.2015.52837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubins H.B., Robins S.J., Collins D., Fye C.L., Anderson J.W., Elam M.B., Faas F.H., Linares E., Schaefer E.J., Schectman G., Wilt T.J., Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N. Engl. J. Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 7.Huang X.J., Choi Y.K., Im H.S., Yarimaga O., Yoon E., Kim H.S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors. 2006;6:756–782. [Google Scholar]
- 8.Lieber C.S., Weiss D.G., Groszmann R., II Veterans affairs cooperative study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol. Clin. Environ. Res. 2003;27:1765–1772. doi: 10.1097/01.ALC.0000093743.03049.80. [DOI] [PubMed] [Google Scholar]
- 9.Lieber C.S. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J. Hepatol. 2000;32:113–128. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 10.Mowe M., Bohmer T. Increased levels of alcohol markers (γGT, MCV, ASAT, ALAT) in older patients are not related to high alcohol intake. J. Am. Geriastrics Soc. 1996;44:1136–1137. doi: 10.1111/j.1532-5415.1996.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 11.Liappas I.A., Nicolaou C., Chatzipanagiotou S., Tzavellas E.O., Piperi C., Papageorgiou C., Boufidou F., Bagos P., Soldatos C.R. Vitamin B12 and hepatic enzyme serum levels correlate with interleukin-6 in alcohol-dependent individuals without liver disease. Clin. Biochem. 2007;40:781–786. doi: 10.1016/j.clinbiochem.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Gowda S., Desai P.B., Hull V.V., Math Aa K., Vernekar S.N., Kulkarni S.S. A review on laboratory liver function tests. Pan Afr. Med. J. 2009;3:1–11. entral.nih.gov/articlerender.fcgi?artid=PMC2984286 [PMC free article] [PubMed] [Google Scholar]
- 13.Giannini E.G., Testa R., Savarino V. Liver enzyme alteration: a guide for clinicians. Can. Med. Assoc. J. 2005;172:367–379. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall P., Cash J. What is the real function of the liver “function” tests? Ulster Med. J. 2012;81:30–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Horev-Azaria L., Kirkpatrick C.J., Korenstein R., Marche P.N., Maimon O., Ponti J., Romano R., Rossi F., Golla-Schindler U., Sommer D., Uboldi C., Unger R.E., Villiers C. Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data. Toxicol. Sci. 2011;122:489–501. doi: 10.1093/toxsci/kfr124. [DOI] [PubMed] [Google Scholar]
- 16.Monteiller C., Tran L., MacNee W., Faux S., Jones A., Miller B., Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup. Environ. Med. 2007;64:609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papageorgiou I., Brown C., Schins R., Singh S., Newson R., Davis S., Fisher J., Ingham E., Case C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28:2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Peters K., Unger R.E., Kirkpatrick C.J., Gatti A.M., Monari E. Effects of nano-scaled particles on endothelial cell function in vitro Studies on viability, proliferation and inflammation. J. Mater. Sci. Mater. Med. 2004;15:321–325. doi: 10.1023/b:jmsm.0000021095.36878.1b. [DOI] [PubMed] [Google Scholar]
- 19.Papis E., Gornati R., Prati M., Ponti J., Sabbioni E., Bernardini G. Gene expression in nanotoxicology research: analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxicol. Lett. 2007;170:185–192. doi: 10.1016/j.toxlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Colognato R., Bonelli A., Ponti J., Farina M., Bergamaschi E., Sabbioni E., Migliore L. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23:377–382. doi: 10.1093/mutage/gen024. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg D.M., Remtulla M.A., Lustig V. The Diagnostic accuracy of three recommended methods for serum aspartate aminotransferase assays in patients suspected of myocardial infarction and hepatobiliary diseases. Clin. Biochem. 1988;21:323–328. doi: 10.1016/s0009-9120(88)80090-0. [DOI] [PubMed] [Google Scholar]
- 22.Gella F.J., Orivella T., Pastor M.C., Arenas J., Moreno R., Durban R., Gomez J.A. A simple procedure for the routine determination of aspartate aminotransferase and alanine aminotransferase with pyridoxal phosphate. Clin. Chim. Acta. 1985;153:241–247. doi: 10.1016/0009-8981(85)90358-4. [DOI] [PubMed] [Google Scholar]
- 23.Lustig V., Papanastasiou-Diamandis A., Goldberg D.M. Evaluation of commercially formulated aspartate aminotransferase and alanine aminotransferase activity determinations by the scandinavian committee on enzymes and IFCC methods as modified for use with automated enzyme analysers. Clin. Biochem. 1988;21:283–290. doi: 10.1016/s0009-9120(88)80082-1. [DOI] [PubMed] [Google Scholar]
- 24.Vagvala S.H., O’Connor S.D. Imaging of abnormal liver function tests. Clin. Liver Dis. 2018;11:128–134. doi: 10.1002/cld.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green R.M., Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 26.Jung K., Grützmann K.D. Comparative determinations of aminotransferase activities in serum with so-called “optimised” methods. Clin. Chim. Acta. 1977;81:299–304. doi: 10.1016/0009-8981(77)90064-x. [DOI] [PubMed] [Google Scholar]
- 27.Bacq Y., Zarka O., Bréchot J.F., Mariotte N., Vol S., Tichet J., Weill J. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996;23:1030–1034. doi: 10.1002/hep.510230514. [DOI] [PubMed] [Google Scholar]
- 28.Kamath P.S. Clinical approach to the patient with abnormal liver test results. Mayo Clin. Proc. 1996;71:1089–1095. doi: 10.4065/71.11.1089. [DOI] [PubMed] [Google Scholar]
- 29.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Yatzidis H. New method for direct determination of “True” creatinine. Clin. Chem. 1974;20:1131–1134. [PubMed] [Google Scholar]
- 31.Adams D.H., Ju C., Ramaiah S.K., Uetrecht J., Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Boeck M., Kirsch-volders M., Lison D. Cobalt and antimony: genotoxicity and carcinogenicity. Mutat. Res. 2003;533:135–152. doi: 10.1016/j.mrfmmm.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y.K., Deng X.X., Yang H.L. Cytotoxicity and genotoxicity in liver cells induced by cobalt nanoparticles and ions. Bone Jt. Res. 2016;5:461–469. doi: 10.1302/2046-3758.510.BJR-2016-0016.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado C., Appelbe A., Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Hear. Lung Circ. 2012;21:759–760. doi: 10.1016/j.hlc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Oldenburg M., Wegner R., Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplasty. 2009;24:825. doi: 10.1016/j.arth.2008.07.017. e15-825.e20. [DOI] [PubMed] [Google Scholar]
- 36.Pelclova D., Sklensky M., Janicek P., Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin. Toxicol. 2012;50:262–265. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 37.Bossi E. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 2016;6:22254. doi: 10.1038/srep22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon Y.M., Xia Z., Glyn-Jones S. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed. Mater. 2009;4 doi: 10.1088/1748-6041/4/2/025018. [DOI] [PubMed] [Google Scholar]
- 39.Liu Ya-ke. Toxicity and bioactivity of cobalt nanoparticles on the monocytes. Orthop. Surg. 2015;7.2:168–173. doi: 10.1111/os.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhtar Shahnaz. Toxicity of PEG-Coated CoFe 2 O 4 nanoparticles with treatment effect of curcumin. Nanoscale Res. Lett. 2018;13.1:52. doi: 10.1186/s11671-018-2468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolov I.G., Joki N., Vicca S., Patey N., Auchère D., Benchitrit J., Flinois J.P., Ziol M., Beaune P., Drüeke T.B., Lacour B. Tissue accumulation of lanthanum as compared to aluminum in rats with chronic renal failure - Possible harmful effects after long-term exposure. Nephron - Exp. Nephrol. 2010;115:e112–e121. doi: 10.1159/000313492. [DOI] [PubMed] [Google Scholar]
- 42.Kumari S.A., Madhusudhanachary P., Patlolla A.K., Tchounwou P.B. Hepatotoxicity and ultra structural changes in wistar rats treated with Al2O3 nanomaterials. Trends Cells Mol. Biol. 2016;11:77–88. [PMC free article] [PubMed] [Google Scholar]
- 43.Schrand A.M., Rahman M.F., Hussain S.M., Schlager J.J., Smith D.A., Syed A.F. Metal-based nanoparticles and their toxicity assessment. WIREs Nanomedicine and Nanobiotechnology. 2010;2:544–568. doi: 10.1002/wnan.103. [DOI] [PubMed] [Google Scholar]
- 44.Harrison S., Page C.P., Spina D. Airway nerves and protein phosphatases. Gen. Pharmacol. 1999;32:287–298. doi: 10.1016/s0306-3623(98)00204-3. [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen S.S., Danscher G., Stoltenberg M., Larsen A., Bruun J.M., Mygind T., Kemp K., Soballe K. Cobalt-chromium-molybdenum alloy causes metal accumulation and metallothionein up-regulation in rat liver and kidney. Basic Clin. Pharmacol. Toxicol. 2007;101:441–446. doi: 10.1111/j.1742-7843.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 46.Day C.P. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 47.David S., Passirani C., Carmoy N., Morille M., Mevel M., Chatin B., Benoit J.P., Montier T., Pitard B. DNA nanocarriers for systemic administration: characterization and in vivo bioimaging in healthy mice. Mol. Ther. - Nucleic Acids. 2013;2:e64. doi: 10.1038/mtna.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding W., Manley S., Ni H. The emerging role of autophagy in alcoholic liver disease. Exp. Biol. Med. 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.