Highlights
• Using cyanobacteria extracts at low doses reduces the toxicity risk in cucumber seeds.
• Optimal doses of cytokinins and salicylic acid benefit the early plant growth stages.
• Cyanobacterial siderophores favor plant growth during the seedling phase.
• Strain Nostoc SAB-M612 stood out for their stimulant ability in cucumber seedlings.
Keywords: Cyanobacteria, Bioactive substances, Siderophores, Salicylic acid, Cytokinins
Abstract
This work clarifies some of the substances involved with the biostimulant effect shown by 28 cyanobacteria isolated from different aquatic environments. The production of salicylic acid, cytokinins, siderophores and phosphate solubilization were analyzed in vitro, as well as the phytostimulant/phytotoxic effect on watercress seeds at two different extract concentrations (0.5 and 0.2 mg mL−1). The most prominent plant growth promoting cyanobacteria were verified in vivo at two different doses (0.5 and 0.1 mg mL−1). 21.4 % and 7.1 % of the tested strains produced siderophores or phosphate solubilization, respectively. The production of salicylic acid was stood out for the strains Calothrix SAB-B797, Nostoc SAB-B1300 and Nostoc SAB-M612, while Nostoc SAB-M251 and Trichormus SAB-M304 were noticeable regard to cytokinin production. The highest values of germination occurred when the extracts were applied in low dose (0.5 mg mL−1). Nostoc SAB-M612 provoked the stimulation of aerial and radicular growth in cucumber seedlings.
1. Introduction
At present, due to the increase of the world population and the environmental damages caused by the rapid industrialization, there is a greater demand for food. To meet this requirement, the world needs to significantly improve agricultural productivity in a sustainable and ecological way, since the increase in cultivable land is unfeasible. Therefore, many of the existing agricultural practices must be replaced, as is the case of pesticides and chemical promoters of plant growth. Currently, the best alternative to these harmful crop management has been the use of plant growth promoting bacteria (PGPB). Traditionally, rhizospheric bacteria [1] and symbiotic rhizobia [2] have been highlighted as PGPB agents. However, in recent years, cyanobacteria have attained great importance due to the fact that they have not only been classified as beneficial bio-agents based on their capability to produce biomass for bio-fuels, food supplements (super foods), and bio-fertilizers for safe agriculture, but also for their role in regulating plant productivity [3]. In fact, cyanobacteria are photosynthetic prokaryotes, exceptionally well adapted to a wide array of environments [4]. These organisms have been reported to be beneficial for soil fertility and crop production, because of their ability to fix atmospheric nitrogen, solubilize phosphate and produce plant growth regulators. This type of bacteria releases varied amounts of phytohormones (auxins, gibberellins, cytokinins), polypeptides, amino acids [5], polysaccharides [6] and siderophores [7], for the growth and development of plants, together with ammonia and small nitrogenous polypeptides during the active cell growth, as well as other secondary metabolites after death and decomposition [8]. These types of substances have been described as important factors with stimulating effects in plants [9].
Logically, the approach of a new form of doing agriculture, named as Good Agricultural Practices (GAP), involves the knowledge of the communications between the plants and the microorganisms of their environment (rhizosphere and phyllosphere) [10,11]. For that reason, cyanobacteria are gaining importance as biofertilizers [12], which implies reducing the polluting effects of synthetic fertilizers in favor of an agriculture that is environmentally friendly and economically viable [13]. Last decades, beneficial effects of cyanobacterial inoculation have been reported in different crops, especially for cereals [14] but only few studies have characterized the chemical constituents responsible for plant growth promotion.
The general aim of this work was to study the potential of a group of 28 cyanobacteria, isolated from different aquatic environments, as plant growth promoter clarifying the potential biostimulant substances involved. In order to carry out this study, the following goals were established: (i) to analyse the production of biotic factors related to plant stimulation, such as phosphate solubilization, salicylic acid, cytokinins and siderophores; (ii) to determine the germination index of each strain, which will allow to discard those with phytotoxic effect and (iii) to evaluate the plant growth promoting effect in planta under controlled conditions using cucumber plants. This work could provide relevant information on the role of cyanobacteria as significant biostimulant agents to promote environmentally sustainable agricultural practices, especially in horticultural systems.
2. Materials and methods
2.1. Cyanobacteria collection and preparation of extracts
A total of 28 cyanobacteria strains isolated from freshwater and sea were studied in this work (Table 1). All of them were supplied in lyophilized form from two recognized culture collections: Mosonmagyaróvár Algal culture collection (MACC) and Spanish Bank of Algae (SBA).
Table 1.
Prospection of cyanobacteria producing siderophores, salicylic acid, cytokinins, phosphate solubilization and watercress germination promotion. Different letters indicate values significantly different in a Fisher’s Least Significant Difference test (LSD) at P < 0.05.
| Code | Genera | Source1 | Bioactivity2 | Salicylic acid (μg mL−1) | Cytokinins (ng mL−1) | GI3 (0.5 mg mL−1) | GI (2.0 mg mL−1) |
|---|---|---|---|---|---|---|---|
| SAB-B0070 | Gloeocapsa | FW | – | 5.94 bc | 2.63 ab | 92.95 a | 77.03 ab |
| SAB-B797 | Calothrix | FW | – | 8.40 d | 3.05 abc | 95.80 a | 103.03 bc |
| SAB-B866 | Dolichospermum | FW | – | 5.80 bc | 3.41 abc | 94.44 a | 75.22 ab |
| SAB-B912 | Anabaena | FW | Sid+ | 5.50 bc | 2.18 a | 102.75 ab | 120.81 c |
| SAB-B974 | Trichormus | FW | – | 5.65 bc | 2.13 a | 99.45 a | 95.98 ab |
| SAB-B1211 | Leptolyngbya | FW | – | 5.83 c | 2.71 a | 100.76 ab | 111.97 c |
| SAB-B1267 | Leptolyngbya | FW | – | 6.87 c | 2.49 a | 92.91 a | 79.72 ab |
| SAB-B1269 | Cyanobacteria | FW | Sid+ | 5.76 bc | 2.06 a | 101.53 ab | 83.02 ab |
| SAB-B1300 | Nostoc | FW | – | 8.23 d | 3.54 abc | 94.70 a | 90.21 ab |
| SAB-B1328 | Lyngbya | FW | Sid+ | 5.55 bc | 3.52 abc | 103.42 ab | 101.80 bc |
| SAB-B1356 | Nodularia | FW | Sid+ | 4.71 b | 4.19 abc | 97.46 a | 102.04 bc |
| SAB-B1572 | Anabaena | FW | – | 5.19 bc | 3.57 a | 101.84 ab | 72.61 ab |
| SAB-B1579 | Synechococcus | FW | Sid+ | 1.93 a | 2.64 ab | 67.85 a | 24.90 a |
| SAB-M128 | Trichormus | SW | – | 4.54 b | 5.54 cd | 80.82 a | 48.84 a |
| SAB-M132 | Nostoc | SW | – | 4.81 b | 3.83 abc | 71.94 a | 59.64 a |
| SAB-M134 | Trichormus | SW | – | 4.84 b | 5.27 cd | 94.26 a | 52.66 a |
| SAB-M150 | Nostoc | SW | – | 4.88 b | 5.53 cd | 97.02 a | 70.65 ab |
| SAB-M189 | Nostoc | SW | – | 4.99 b | 4.65 abc | 92.24 a | 74.26 ab |
| SAB-M205 | Tolypothrix | SW | – | 5.14 bc | 4.31 abc | 90.83 a | 80.49 ab |
| SAB-M221 | Trichormus | SW | Sid+ | 5.07 b | 5.76 cd | 83.87 a | 65.77 ab |
| SAB-M251 | Nostoc | SW | – | 5.21 b | 6.39 d | 87.95 a | 66.98 ab |
| SAB-M304 | Trichormus | SW | – | 4.76 b | 6.46 d | 74.43 a | 19.08 a |
| SAB-M307 | Anabaena | SW | – | 4.68 b | 5.93 cd | 88.67 a | 85.31 ab |
| SAB-M405 | Calothryx | SW | – | 5.27 bc | 3.15 abc | 93.99 a | 78.14 ab |
| SAB-M465 | Tolypothrix | SW | – | 4.95 bc | 5.52 bcd | 94.32 a | 84.60 ab |
| SAB-M612 | Nostoc | SW | – | 8.76 d | 4.94 abc | 108.60 b | 83.37 ab |
| SAB-M661 | Nostoc | SW | P-sol+ | 5.40 b | 4.28 abc | 99.63 a | 87.39 ab |
| SAB-M683 | Nostoc | SW | P-sol+ | 5.38 b | 3.24 abc | 102.59 ab | 106.55 bc |
Source: Freshwater (FW); Seawater (SW).
Bioactivity: production of siderophores (Sid) or phosphate solubilization (P-sol).
GI: Germination Index.
To carry out all the in vitro and in vivo tests, lyophilized biomass was subjected to a quick sonication process (Branson Sonicator 150, Amplitude 40 %, 30 s), in order to obtain aqueous extracts at different concentrations (see later sections). In order to eliminate particles in suspension, extracts were precipitated after sonication at 1000 rpm during 5 min.
2.2. In vitro Production of bioactive substances
The protocol used for the detection of siderophores (Sid) was a modified method of Schwyn and Neilands [15]. The sonicated extracts of cyanobacteria, at concentrations of 10 mg mL−1 (DM: dry matter), were inoculated in culture medium prepared with the following components (composition per liter): Chrome Azurol S, 60.5 mg; Hexadecyltrimethylammonium (HDTMA), 72.9 mg; Pipes, 30.24 mg; Agar, 15 g. The medium was adjusted to pH 7.2 before the addition of 10 mL of 1 mM FeCl3.6H2O solution. After 14 days of incubation at 30 °C the presence of siderophores was detected by a change of color from blue to brown-orange.
Phosphate solubilization (P-sol) activity was demonstrated by growing the strains in culture medium containing 2.5 % tricalcium phosphate. A zone of clearance of the medium was detected in the case of phosphate solubilizing colonies after 14 days of incubation at 30 °C [16].
Quantification of salicylic acid (Sal) and cytokinins (Cyt) was performed from sonicated extracts of cyanobacteria prepared at the concentration of 100 mg mL−1 and 25 mg mL−1, respectively. The quantification of both phytohormones was carried out by immunodiagnostic tests (Plant Cytokinin CYT ELISA Kit, MyBiosource MBS269996; Plant Salicylic acid SA ELISA Kit, MyBiosource MBS9314138). The absorbance of the samples was finally carried out at a wavelength of 450 nm, in a Thermo Scientific Multiskan FC spectrophotometer.
2.3. Germination index bioassays
The phytotoxic effect of the extracts of cyanobacteria was verified in 100 watercress seeds at concentrations of 2 and 0.5 mg mL-1 (DM: dry matter), by the technique described by Zucconi [17]. The germination index calculation was made taking into account the percentage of germination of the seeds and the lengthening of the radicles, based on the following formula:
| GI= (GSs% * REs) / (GSdw% * REdw) |
Where:
GI: Germination Index
GSs%: percentage of germinated seed in the presence of the sonicated sample
GSdw%: percentage of germinated seed in the presence of distilled water
REs: mean of radicle elongation (mm) in the presence of the sonicated sample
REdw: mean of radicle elongation (mm) in the presence of distilled water
2.4. In vivo evaluation of phytostimulant activity
The best strains of cyanobacteria selected from the previous experiments were analysed in vivo. The objective in this case was to determine its potential as growth promoting agents in cucumber seedlings. A randomized experimental design consisting of 2 treatments at different concentrations of extract, 0.5 and 0.1 mg mL−1 (DM: dry matter), was established. 1 L of each extract subjected to ultrasound was prepared to irrigate 30 cucumber seedlings placed in pots with a standard mixture prepared from organic substrate and vermiculite (ratio 3: 1 v / v). A set of cucumber seedlings was used as a control experiment (non-inoculated plants). Plants were kept in a greenhouse at a controlled temperature of 25 ± 1 °C and a photoperiod of 12 h. After 40 days, the effect of the phytostimulants was evaluated, as described previously [18]. Parameters measured to evaluate the promoter effect in vivo were stem and root length, stem diameter, ratio root / stem, leaf number and fresh weight.
2.5. Statistical analyses
The data obtained was subjected to statistical analysis using the Statgraphics Centurion XVII program. The selection of the best phytostimulant strains was carried out by applying a multivariate analysis of variance (ANOVA) and a Fisher multiple comparison test (least significant difference test) at P < 0.05. A linkage between groups was used as a grouping method for the variables analysed in vitro. The interval measured in this case was the Euclidean distance squared. In addition, principal components were analysed to identify similarities or differences between the best strains, as well as groups of closely related variables. Finally, both in vitro and in vivo variables were correlated by calculating the Pearson correlation coefficient.
3. Results and discussion
3.1. In vitro bioprospection of plant growth promoting cyanobacteria
3.1.1. Production of siderophores, phosphate solubilization, salicylic acid and cytokinins
All the cyanobacteria were tested to detect the presence of different metabolites or capacities of agronomic interest. By one hand, production of siderophores and phosphate solubilization were investigated by qualitative protocols. Table 1 shows that 21.4 % and 7.1 % of the strains were capable to produce siderophores or solubilizate phosphate, respectively. The diversity of the cyanobacteria capable of producing siderophores was noticeable while only 2 strains of Nostoc spp. (SAB-M661 and SAB-M683) were described as phosphate solubilizing. However, other Nostoc strains did not show this capability (SAB-B1300, SAB-M132, SAB-M150, SAB-M189, SAB-M251 and SAB-M612), therefore phosphate solubilizing is independent from the genera and it could be probably a subespecific character.
Siderophores are organic compounds produced by microorganisms which help to chelate ferric iron under iron deficient conditions, and make them available to the microbes and plants [19]. It has been described that cyanobacterial species such as Anabaena flos-aquae and Anabaena cylindrica have the ability to produce siderophores for chelating micronutrients such as Fe or Cu [20,21]. In this work, several strains of Anabaena spp. were capable of producing siderophores, though other genera were also involved in this phenomena such as Cyanobacteria, Lyngbya, Nodularia, Synechococcus and Trichormus (Table 1).
It has been traditionally considered that cyanobacteria could remove available phosphorus in soil by incorporating it into cell constituents or by absorbing it in excessive amounts, and then gradually releasing it to plants by exudation, autolysis or microbial decomposition of dead cells [21]. Some authors have described that cyanobacteria are able to store polyphosphate bodies to overcome short periods of P-starvation. Polyphosphate granules are used as an intracellular P-reserve and are generally present in exponentially growing cells under P-rich conditions. They can accumulate at high levels in N2-fixing cells and degrade in P-deficient cells [22]. More recently, it has also been suggested that cyanobacteria can improve the bioavailability of phosphorus to the plants by solubilizing and mobilizing the insoluble organic phosphates present in the soil with the help of phosphatase enzymes [23].
Regarding the production of phytohormone like-bioactive substances, several strains highlighted due to the production of salicylic acid and cytokinins (Table 1). Strains named Calothrix SAB-B797, Nostoc SAB-B1300 and Nostoc SAB-M612 were noticeable in relation to the production of salicylic acid, while Nostoc SAB-M251 and Trichormus SAB-M304 produced the most quantities of cytokinins.
Cyanobacteria play a significant role in providing phytohormones that promote plant growth. Cytokinins are involved in the activation of the functions of cell division, organogenesis and late senescence of plants, meanwhile, salicylic acid is directly related to the activation of the defensive plant response. Some authors described the production of cytokinins by the genera Arthronema and Calothrix or auxins by Oscillatoria and Chlorogloea [24]. Their structural–functional plasticity confers great versatility and enables them to adapt and inhabit a wide range of environments and niches. In addition, they establish symbioses with different members of the plant kingdom [25,26] and are capable of colonizing roots and eliciting growth promotion and defence responses in plants [27,28].
3.1.2. Screening of plant growth promoter strains based on germination index assay
In order to narrow search, phytostimulation test was carried out. Those strains which presented or exceeded 100 % of Germination Index (GI) were considered as phytostimulant at 0.5 and / or 2.0 mg mL−1. Thus, 7 strains reached GI values equal to or greater than 100 % when they were tested at 0.5 mg mL−1 while 6 of them were higher than 100 % when the extracts were applied at 2 mg mL−1 (Table 1). On the one hand, strain Nostoc SAB-M612 was the best growth promoter in watercress seeds after applying at low dose (GI = 108.6 %); Anabaena SAB-B912 and Leptolyngbya SAB-B1211 were the most phytostimulant strains at 2.0 mg mL−1 of extract (120.81 % and 111.97 % respectively). On the other hand, four of them were the most phytotoxic strains, Synechococcus SAB-M1579 and Trichormus SAB-M304 (Table 1).
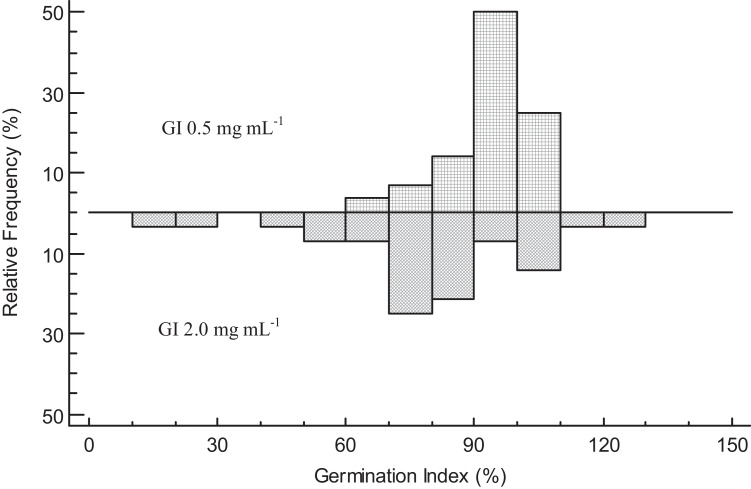
The diagram of relative frequencies revealed that the results were most homogeneous when the extracts were applied at low dose, since around 70 % of the strains showed GI values around 90–110 % (Fig. 1). Contrary to detected at low dose, the most extreme results in terms of phytotoxicity and phytostimulation were detected when those were applied at higher dose (2.0 mg mL−1).
Fig. 1.
Diagram of relative frequencies concerning the variable Germination Index (GI) at 0.5 and 2.0 mg mL−1.
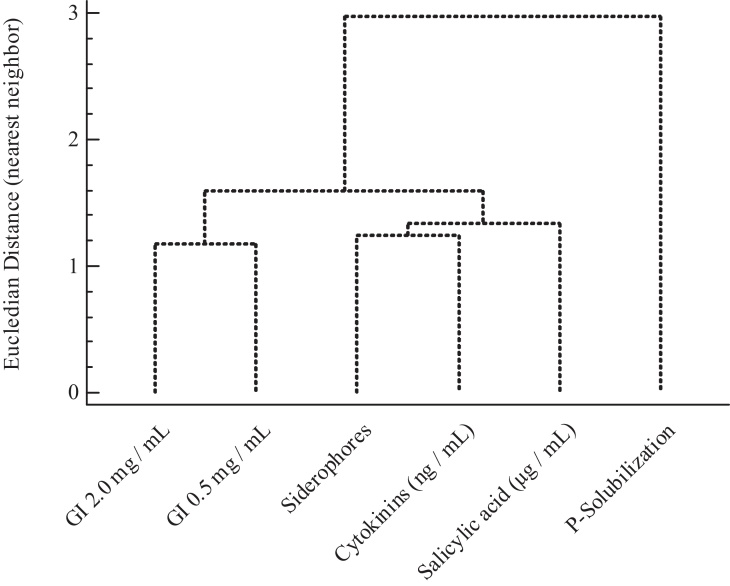
As seen in Fig. 2, there is an important link between germination data and salicylic acid production by cyanobacteria. On the other hand, the production of cytokinins and siderophores could also be decisive during the germination and root elongation phase, although less important than salicylic acid. Both facts could reveal that, although the effect of salicylic acid, cytokinins and siderophores could have more weight in the early stages of plant development, the same does not occur with the ability solubilize phosphate (Fig. 2), which character is founded farthest in the dendrogram and could be more important during advanced phases of plant growth.
Fig. 2.
Dendrogram of the euclidean distance by the nearest neighbor method: clustering in vitro variables (GI: Germination Index).
3.2. Application of the best selected cyanobacteria as growth promoters in cucumber seedlings
One of the main criteria taken into account for the selection of the best plant growth promoting strains was the Germination Index. In this way, the possible phytotoxic effect that cyanobacterial extracts could have on bioassays performed on cucumber seedlings was ruled out. Thus, based on the results shown in Table 1, the selected strains were SAB-B912, SAB-B1211, SAB-M612 and SAB-M683. Bioactivity (phosphate solubilization and production of siderophores) and the ability to produce phytohormones from these strains were important aspects to consider, but they were not considered for this preliminary selection.
In order to objectively evaluate the results derived from the bioassay performed on cucumber seedlings, the plant growth promotion was expressed as the percentage of increase / decrease of all the variables analysed, based on the data observed in the control plant set (plants not inoculated with extracts of cyanobacteria).
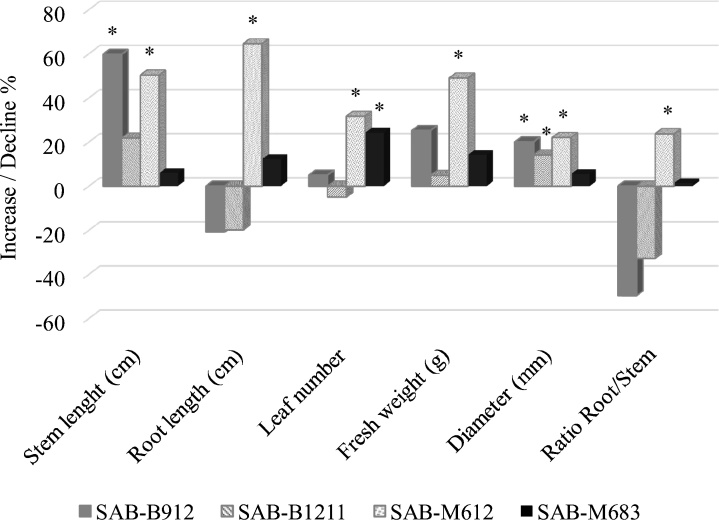
As indicated above (see the Materials and Methods section), bioassays in cucumber seedlings were carried out from two different extract concentrations (0.5 and 0.1 mg mL−1). Taking into account that the statistical analyses did not show significant differences between both treatments (data not shown), Fig. 3 represents the mean values for each variable analysed, regardless of the treatment applied at 0.1 mg mL−1. In this way, the results obtained revealed a clear growth promoting effect after the application of the sonicated extracts of the strains Anabaena SAB-B912 and Nostoc SAB-M612. However, only the strain named Nostoc SAB-M612 was capable of increasing the Root / Stem ratio in relation to what was observed in the set of control plants. This fact was due to the remarkable increase in the length of the root of the treated plants (more than 60 % increase in root growth). In addition, extracts of SAB-M612 increased the stem length (50 %), leaf production (30 %), fresh weight (close to 50 %) and thickness of the treated plants (more than 20 %) in relation to untreated plants (Fig. 3). A more discrete promoting effect was detected after the application of the strains SAB-B912 and SAB-M683. On the other hand, strains SAB-B912 and SAB-B1211 slightly promoted the aerial plant growth but not the radicular development.
Fig. 3.
Measurement of plant growth parameters after application of selected cyanobacteria extracts in cucumber seedlings. Data are represented as the percentage of increase or decrease based on the control plants not treated with cyanobacteria. Mean of 30 replicates was used in each case (*: asterisk indicates values significantly different in a Fisher’s Least Significant Difference test at P < 0.05).
* significantly higher values at P < 0.05.
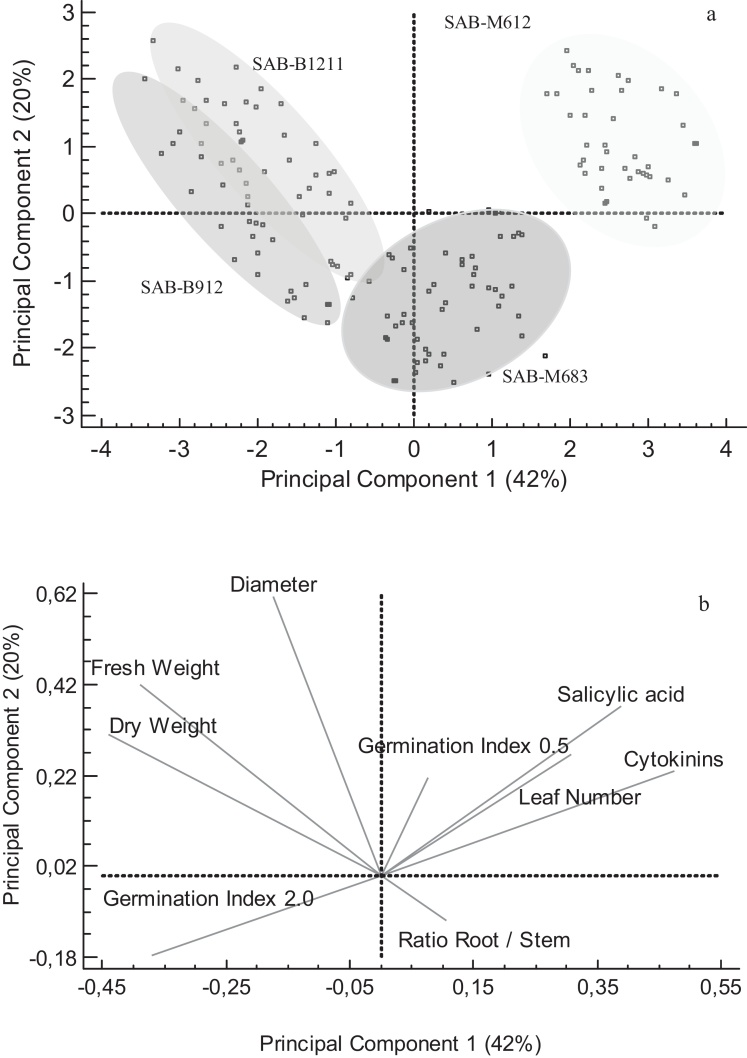
The Principal Component Analyses revealed the presence of two main components (PC1 and PC2) that explained more than 60 % of the data variability (Fig. 4). This analysis was performed bearing in mind both in vivo growth parameters (root and stem length, leaf number, fresh weight and stem diameter) and in vitro phytohormone production. In view of the results shown in the dispersion diagram (Fig. 4a), clear significant differences were established between SAB-M612 and the rest of the tested strains. With respect to the Principal Component graphic, PC1 proved to have a greater influence on the production of phytohormones and the growth parameters concerning the plant weight (Fig. 4b). While the production of cytokinins and salicylic acid was positively related with the Germination Index at 0.5 mg mL−1 and the Root / Stem Ratio, other parameters concerning the plant size and weight were linked to Germination Index at 2.0 mg mL−1 (Fig. 4b).
Fig. 4.
Principal Component Analyses including in vitro and in vivo variables: Dispersion Diagram to stablish global differences or similarities among the cyanobacteria tested (a); the influence of the Principal Components on the variability of the parameters analyzed (b).
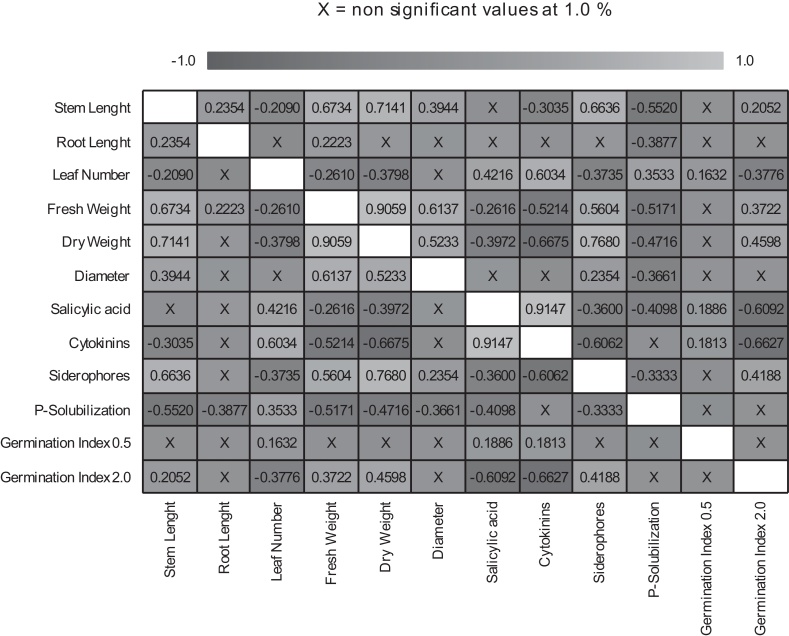
The relationship between the in vitro and in vivo variables analysed is shown by a Pearson correlation analysis (Fig. 5). Taking into account that Pearson's R value oscillated between -1 and +1, negative relationships between variables are represented in dark grey, while positive relationships are indicated in light grey.
Fig. 5.
Correlation Pearson test including in vitro and in vivo variables analyzed (P < 0.01). Pearson's R value oscillated between -1 (dark grey) and +1 (light grey).
In view of the results shown in Fig. 5, it should be noted the positive relationship between the variable Fresh weight and other parameters related to the plant size (Root and stem length, as well as stem diameter). It is also worth noting the direct relationship between the leaf number and the capacity of the strains selected to produce salicylic acid and cytokinins (R2 = 0.4216 and 0.6034, respectively).
The strong positive relationship between the production of salicylic acid and cytokinins was especially surprising (R2 = 0.9147). Nonetheless, the excess of salicylic acid and cytokinins in the germination phase could be phytotoxic, as was detected in the germination tests on cress seeds (Table 1, Fig. 1, Fig. 5). This effect was specifically detected when some of the sonicated extracts of cyanobacteria were applied at 2 mg / mL−1 and tended to decrease when the applied concentration of the sonicated extracts was lower.
The production of both phytohormones correlated negatively with the plant weight (Fig. 5) and the production of siderophores. Surprisingly, the production of salicylic acid was inversely related to the ability to solubilize phosphates (R2 = -0.4098), while the production of both cytokinins was negatively correlated with the stem length.
It was noticeable the link between the production of siderophores and the parameters concerning to plant size and weight. However, contrary to expectations, P-solubilization negatively correlated with the rest of plant growth parameters (Fig. 5); this effect could be related with phenological state of the cucumber seedling.
In this work, it was evident that these cyanobacterial strains release varied quantities of phytohormones for plant growth and development together with other secondary bioactive metabolites. In view of the results obtained in this work, it is worth highlighting the influence of the production of both phytohormones in the germination phase. However, this effect was beneficial mainly at the lowest dose used since it was phytotoxic when higher extract doses were applied.
Recent studies also showed that cyanobacterial inoculation leads to modulation of the rhizosphere microbiome, leading to alterations in the structure and abundance of microbial communities involved in the mineralization and solubilization of nutrients [[29], [30], [31]]. Concretely, some studies have shown that the inoculation of cyanobacterium Calothrix elenkinii significantly brought about beneficial changes in the plant and rhizosphere microbiome [[30], [31], [32]]. Therefore, the effect provoked by the cyanobacteria results from the collaborative action among them and other microbial groups or species inhabiting in the soil rhizosphere.
4. Conclusions
Cyanobacteria are a group of photosynthetic organisms that can easily survive with the minimum requirement of light, carbon dioxide and water. They are phototrophic and appear naturally in a multitude of agroecosystems distributed throughout the world. They fix nitrogen and produce some bioactive compounds, which improve the nutritional status of the soil, promote the growth of crops and protect them from plant pathogens. Due to their natural diversity, the capacity of cyanobacteria to grow in a variety of locations could be exploited for their commercial application as plant growth promoters.
However, the beneficial effects of cyanobacteria by treating with crude extracts on some vegetables, herbaceous and crops plants have already been well established, only few studies have characterized the chemical constituents responsible for plant growth promotion. In this work, it was surprising that the parameters related to the size and weight of the plant (with the exception of the number of leaves) correlated negatively with the production of salicylic acid and cytokinins, as well as with phosphate solubilization, contrary to what was detected in the case of siderophores production. This fact leads us to think that during the initial stages of germination and elongation of the root it is essential to establish the balance between salicylic and cytokinins, while in the later stages of seedling, the presence of siderophore-like chelating agents is decisive for the optimal development of the plant.
Funding
This research has received funding from the European Union’s Horizon 2020 Research and Innovation program under the Grant Agreement No. 727874 (SABANA Project).
CRediT authorship contribution statement
A.J. Toribio: Investigation, Data curation, Writing - original draft. F. Suárez-Estrella: Investigation, Conceptualization, Methodology, Visualization, Writing - original draft, Writing - review & editing. M.M. Jurado: Investigation, Methodology, Writing - original draft. M.J. López: Investigation, Methodology, Writing - original draft. J.A. López-González: Investigation, Methodology, Writing - original draft. J. Moreno: Conceptualization, Methodology, Supervision, Writing - review & editing.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interests associated with this publication and that there has been no significant financial support for this work that may have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other people who meet the authorship criteria but are not listed. We further confirm that the order of the authors listed in the manuscript has been approved by all of us.
We confirm that we have taken into account the protection of intellectual property associated with this work and that there are no impediments to publication with respect to intellectual property. In doing so, we confirm that we have followed the regulations of our institutions regarding intellectual property.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00449.
Appendix B. Supplementary data
The following are Supplementary data to this article:
References
- 1.Castro-Sowinski S., Herschkovitz Y., Okon Y., Jurkevitch E. Effects of inoculation with plant growth-promoting rhizobacteria on resident rhizosphere microorganisms. FEMS Microbiol. Lett. 2009;276:1–11. doi: 10.1111/j.1574-6968.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Yang S., Tang F., Zhu H. Symbiosis specificity in the legume - rhizobial mutualism. Cell. Microbiol. 2012;14:334–342. doi: 10.1111/j.1462-5822.2011.01736.x. [DOI] [PubMed] [Google Scholar]
- 3.Singh J.S., Kumar A., Rai A.N., Singh D.P. Cyanobacteria: a precious bioresource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016;7:1–19. doi: 10.3389/fmicb.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh J.S. Cyanobacteria: a vital bio-agent in eco-restoration of degraded lands and sustainable agriculture. Climate Change Environ. Sustain. 2014;2:133–137. [Google Scholar]
- 5.Karthikeyan N., Prasanna R., Sood A., Jaiswal P., Nayak S., Kaushik B.D. Physiological characterization and electron microscopic investigations of cyanobacteria associated with wheat rhizosphere. Folia Microbiol. 2009;54:43–51. doi: 10.1007/s12223-009-0007-8. [DOI] [PubMed] [Google Scholar]
- 6.Maqubela M.P., Mnkeni P.N.S., Malam Issa O., Pardo M.T., D’Acqui L.P. Nostoc cyanobacterial inoculation in South African soils enhances soil structure, fertility, and maize growth. Plant Soil. 2009;315:79–92. [Google Scholar]
- 7.Řezanka T., Palyzová A., Sigler K. Isolation and identification of siderophores produced by cyanobacteria. Folia Microbiol. 2018;63:569–579. doi: 10.1007/s12223-018-0626-z. [DOI] [PubMed] [Google Scholar]
- 8.Vaishampayan A., Sinha R.P., Häder D.P., Dey T., Gupta A.K., Bhan U., Rao A.L. Cyanobacterial biofertilizers in rice agriculture. Bot. Rev. 2001;67:453–516. [Google Scholar]
- 9.Mazhar S., Hasnain S. Screening of native plant growth promoting cyanobacteria and their impact on Triticum aestivum var. Uqab 2000 growth. Afr. J. Agric. Res. 2011;6:3988–3993. [Google Scholar]
- 10.Ortiz-Castro R., Contreras-Cornejo H.A., Macías-Rodríguez L., López-Bucio J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009;4:1–12. doi: 10.4161/psb.4.8.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thajuddin N., Subramanian G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 2005;89:50–57. [Google Scholar]
- 12.Renuka N., Guldhe A., Prasanna R., Singh P., Bux F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol. Adv. 2018;36:1255–1273. doi: 10.1016/j.biotechadv.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Singh J.S., Pandey V.C., Singh D.P. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011;140:339–353. [Google Scholar]
- 14.Shariatmadari Z., Riahi H., Seyed Hashtroudi M., Ghassempour A., Aghashariatmadary Z. Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci. Plant Nutr. 2013;59:535–547. [Google Scholar]
- 15.Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 16.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 17.Zucconi F., Pera A., Forte M., de Bertoldi M. Evaluating toxicity in immature compost. Biocycle. 1981;22:54–57. [Google Scholar]
- 18.Santoro M.V., Zygadlo J., Giordano W., Banchio E. Volatile organic compounds from rhizobacteria increase biosynthesis of essential oils and growth parameters in peppermint (Mentha piperita) Plant Physiol. Biochem. 2011;49:177–1082. doi: 10.1016/j.plaphy.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed E., Holmström S.J. Siderophores in environmental research: roles and applications. Microb. Biotechnol. 2014;7:196–208. doi: 10.1111/1751-7915.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman S., Lammers P., Berman M., Sanders-Loehr J. Siderophore-mediated iron uptake in different strains of Anabaena sp. J. Bacteriol. 1983;156:1144–1150. doi: 10.1128/jb.156.3.1144-1150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller W.H., Roger R.N. Utilisation of the phosphorus of algal cells as measured by the Neubauer technique. Soil Sci. 1952;74:417–429. [Google Scholar]
- 22.McKnight D.M., Morel F.M. Copper complexation by siderophores from filamentous blue-green algae. Limnol. Oceanogr. 1980;25:62–71. [Google Scholar]
- 23.Tang E.P.Y., Vincent W.F., Proulx D., Lessard P., de la Noüe J. Polar cyanobacteria versus green algae for tertiary waste-water treatment in cool climates. J. Appl. Phycol. 1997;9:371–381. [Google Scholar]
- 24.Parveen S., Pandey V.D. Alkaline phosphatase activity in freshwater cyanobacteria. Plant Arch. 2011;11:827–830. [Google Scholar]
- 25.Tarakhovskaya E.R., Maslov Y.I., Shishova M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007;54:163–170. [Google Scholar]
- 26.Prasanna R., Jaiswal P., Nayak S., Sood A., Kaushik B.D. Cyanobacterial diversity in the rhizosphere of rice and its ecological significance. Indian J. Microbiol. 2009;49:89–97. doi: 10.1007/s12088-009-0009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasanna R., Nain L., Ancha R., Shrikrishna J., Joshi M., Kaushik B.D. Rhizosphere dynamics of inoculated cyanobacteria and their growth promoting role in rice crop. Egypt. J. Biol. 2009;11:26–36. [Google Scholar]
- 28.Prasanna R., Babu S., Rana A., Kabi S.R., Chaudhary V., Gupta V., Kumar A., Shivay Y.S., Nain L., Pal R.K. Evaluating the establishment and agronomic proficiency of cyanobacterial consortia as organic options in wheat-rice cropping sequence. Exp. Agric. 2013;49:416–434. [Google Scholar]
- 29.Prasanna R., Babu S., Bidyarani N., Kumar A., Triveni S., Monga D., Mukherjee A.K., Kranthi S., Gokte-Narkhedhar N., Adak A., Yadav K., Nain L., Saxena A.K. Prospecting cyanobacteria fortified composts as plant growth promoting and biocontrol agents in cotton. Exp. Agric. 2015;51:42–65. [Google Scholar]
- 30.Manjunath M., Kanchan A., Ranjan K., Venkatachalam S., Prasanna R., Ramakrishnan B., Hossain F., Nain L., Shivay Y.S., Rai A.B., Singh B. Beneficial cyanobacteria and eubacteria synergistically enhance bioavailability of soil nutrients and yield of okra. Heliyon. 2016;2:e00066. doi: 10.1016/j.heliyon.2016.e00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priya H., Prasanna R., Ramakrishnan B., Bidyarani N., Babu S., Thapa S., Renuka N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015;171:78–89. doi: 10.1016/j.micres.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Ranjan K., Priya H., Ramakrishnan B., Prasanna R., Venkatachalam S., Thapa S., Tiwari R., Nain L., Singh R., Shivay Y.S. Cyanobacterial inoculation modifies the rhizosphere microbiome of rice planted to a tropical alluvial soil. Appl. Soil Ecol. 2016;108:195–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.