Abstract
Rasmussen encephalitis (RE) is a disorder characterized by drug-resistant seizures and progressive unihemispheric atrophy, hemiparesis, and varying degrees of cognitive decline. The pathophysiology of RE remains elusive, with hypotheses suggesting underlying autoimmune- and T cell-mediated processes. In this case report, we describe a single patient's clinical course from the first day of presentation until definitive treatment for atypical Rasmussen encephalitis at a tertiary care pediatric center. The patient exhibited several atypical features of Rasmussen encephalitis, including a posterior predominance of initial seizure onset with the development of severe choreoathetosis and ipsilateral cerebellar atrophy. He subsequently developed coexistent autoimmune disorders in the form of psoriasis and uveitis, and underwent multiple forms of immunotherapy with limited benefit.
This patient shows an association of RE with other autoimmune conditions supporting an autoimmune mechanism of disease while exhibiting several atypical features of RE. Rarely, occipital lobe seizures have been documented as the presenting semiology of this syndrome. This case highlights the need to be mindful of atypical features that may delay hemispherectomy, which remains the definitive treatment. It also suggests that children may be predisposed to the development of autoimmune disorders in later stages of the disease.
Keywords: Rasmussen encephalitis, atypical Rasmussen encephalitis, Bien criteria, Drug resistant epilepsy
Highlights
-
•
Occipital seizure localization and semiology should not dissuade the diagnosis of Rasmussen's encephalitis
-
•
Movement disorders, can accompany Rasmussen's encephalitis
-
•
Ipsilateral cerebellar atrophy has been described in Rasmussen's encephalitis
-
•
Children with Rasmussen's encephalitis may be predisposed to autoimmune disorders in the later stages of the disease
1. Introduction
Rasmussen encephalitis (RE) is classically characterized by progressive, frontally predominant, unilateral hemispheric atrophy with corresponding focal neurological deficits and drug-resistant focal motor seizures [1,2]. Movement disorders and non-motor seizures as manifestations of RE have been reported, but only rarely [3,4]. Cerebellar atrophy contralateral to the affected cerebral hemisphere may occur, and ipsilateral cerebellar atrophy rarely has been reported [5].
Based on the pathological findings of a T-cell predominant encephalitis, various forms of immunotherapy have been used but with limited success, often leading to hemispherectomy for seizure control [[6], [7], [8], [9], [10]]. In addition to the pathological findings, the co-existence of autoimmune disorders is rare and has strengthened the autoimmune hypothesis [4,[11], [12], [13]].
Here, we present a patient with RE to highlight several noteworthy atypical features. These features include posterior predominance of the early seizure onset, slow progression to hemispheric atrophy, ipsilateral cerebellar atrophy, severe choreoathetosis, failure of immunotherapy (including rituximab) to control seizures, and the subsequent development of psoriasis and uveitis affecting the eye ipsilateral to the affected hemisphere.
2. Case report
Following normal birth and development, a 6-year-old boy had a focal to bilateral tonic-clonic seizure, which initially started with visual phenomena, leftward eye deviation, and preserved consciousness. Family history was significant for maternal inflammatory bowel disease, paternal psoriasis, and multiple sclerosis in the maternal great uncle. Physical exam showed left hemi-ataxia, which resolved by 48 h after the seizure. Electroencephalography (EEG) revealed right occipital and parietal slowing while a brain MRI and MRA were normal. Seizures recurred 15 months after the first seizure. His seizure semiology at that time consisted of visual phenomena of multicolored formed images (described by the patient as “beach balls”) lasting for 30 s to 1 min, at times longer, accompanied by nausea and followed by limpness and leftward eye deviation. Seizures were occurring at first weekly with waxing and waning periods of seizure control, but soon became daily after the first 6 months of treatment, at which point care was transferred to an epileptologist. Over the next 2 years, his seizures became drug-resistant to several anti-seizure medications (ASMs), including levetiracetam, oxcarbazepine, valproic acid, zonisamide, topiramate, and diazepam. Long-term EEG monitoring was performed 6 months after treatment started, initially showing 9 to 28 seizures per day. The semiology remained the same with stereotyped visual phenomena and remained focal. The seizures were mostly electroclinical with occasional exclusively electrographic seizures both with onset in the right posterior quadrant accompanied by right occipital slowing.
Three years after the initial presentation, the patient had almost continuous visual phenomena. His parents noted increasing left-sided clumsiness and gait abnormalities. Physical exam was notable for left-hand tremor. Despite treatment with valproic acid and topiramate, an EEG showed nearly continuous partial seizures with a maximum in the right posterior quadrant (Fig. 1). Accordingly, fosphenytoin was given acutely and added to the medication regimen temporarily with modest effect.
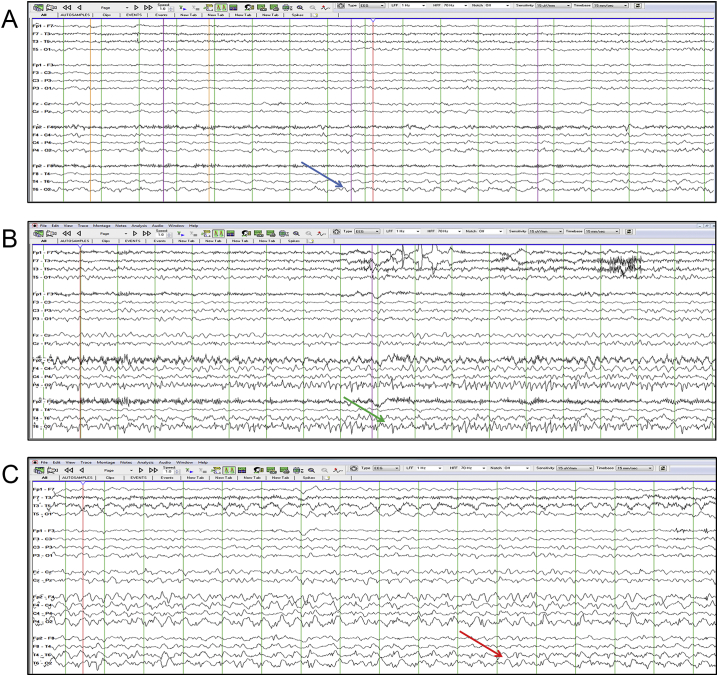
Fig. 1.
Continuous right posterior quadrant seizures.
This is an A-P longitudinal bipolar montage. The top Fig. 1a shows semi-rhythmic right posterior quadrant slowing depicted by the blue arrow. Fig. 1b shows a buildup of activity with fast spikes having a maximum negativity at O2 depicted by the green arrow. Fig. 1c shows spread to the posterior temporal region and continued evolution depicted by the red arrow.
EEG monitoring at age 11 years showed increased seizure frequency to approximately 4 to 5 seizures per hour, with 25% having clinical correlate. The patient subsequently developed left-sided high amplitude choreoathetoid movements, suppressed only by sleep, without clearly associated epileptiform changes on EEG. Brain MRI was again repeated and showed subcortical and gyral T2 prolongation with gyral swelling in the right temporal, parasagittal, parietal, occipital lobes, and right pulvinar without caudate involvement (Fig. 2). There was also right cerebral and cerebellar volume loss (Fig. 3). His ASMs were changed to lacosamide and phenobarbital. Repeat prolonged EEG revealed electrographic seizures arising more anteriorly in the right parasagittal and superior frontal regions, in addition to the right posterior region. There were now multiple right hemispheric spikes in all quadrants. Extensive diagnostic testing was notable for a positive antinuclear anti-body (titer 1:160); however, inflammatory markers (erythrocyte sedimentation rate [ESR] and C reactive protein [CRP]), complement levels, and other tested autoantibodies that included anti-double stranded DNA, anti-Smith, and endomysial and anti-deamidated gliadin IgA and IgG antibodies were normal. Cerebrospinal fluid (CSF) analysis showed 5 WBC/mm3 with normal glucose, protein, and lactate levels. Infectious studies included normal culture and gram stain, in addition to PCR for HSV, EBV, CMV, and enterovirus. The CSF IgG index was elevated at 2.46 (normal 0.28–0.66), and 12 CSF-restricted oligoclonal bands were noted. B cells comprised 15% of the lymphocyte gate on CSF flow cytometry. Serum and CSF autoimmune epilepsy panel were negative. This included CSF anti-neuronal nuclear antibody type 1 (anti-Hu), anti-glutamic acid decarboxylase 65 and anti-N-methyl-d-aspartate receptor antibodies. Targeted mitochondrial mutation analysis for mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) tRNA Leu gene and POLG-1 analysis were unrevealing.
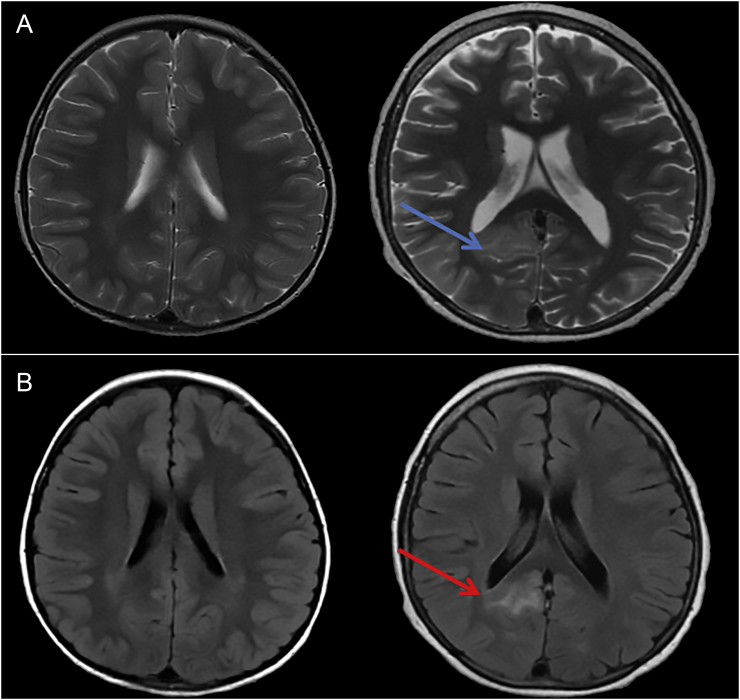
Fig. 2.
Right occipital lobe cortical and subcortical T2/FLAIR hyperintensity and gyral swelling.
The top figure (Fig. 2a) is a T2 weighted axial image comparing the initial MRI (left) to subsequent images (right). The blue arrow depicts T2 prolongation in the right posterior temporal and occipital region. The bottom figure (Fig. 2b) is the axial FLAIR image with the red arrow pointing to the same area of abnormality.
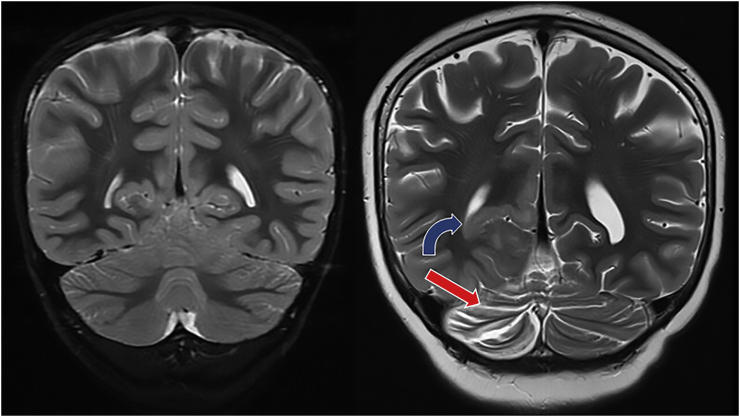
Fig. 3.
Right occipital lobe gyral swelling and T2 hyperintensity (blue curved arrow) and ipsilateral cerebellar volume loss (red straight arrow).
This is a coronal T2 weighted image comparing the initial MRI (left) to subsequent images (right). The blue curved arrow depicts right occipital lobe gyral swelling and T2 hyperintensity while the red arrow depicts ipsilateral cerebellar volume loss.
Based on the presence of drug-resistant epilepsy, progressive course, and progressive MRI findings, the patient's differential diagnoses included RE, small vessel CNS vasculitis, and a systemic inflammatory condition with CNS involvement. He was given intravenous immunoglobulin (IVIG) 2 g/kg over 2 days, followed 1 week later by 5 days of intravenous methylprednisolone 1 g daily. Starting two days after completion of the steroid course, 5 sessions of plasmapheresis were performed without improvement. To clarify the nature of the patient's condition, biopsy of the right occipital cortex and subcortical white matter and overlying dura was performed. The biopsy was consistent with meningoencephalitis with diffuse lymphoid and macrophage infiltrates, rare microglial nodules, and gliosis. There was a predominance of CD3 + T cells (CD8 > CD4) with admixed CD 20 + B cells. Immunostains for cytomegalovirus, Epstein Barr virus, herpes simplex virus, and varicella zoster virus were negative.
The patient was diagnosed with RE based on the constellation of clinical, electrographic, and radiographic components of Part A and B of the European consensus statement on RE [2,6,8]. This diagnosis was also supported by the pathological findings. Following the biopsy, the patient was treated with methylprednisolone 30 mg/kg/day for 5 days followed by a prednisone taper starting at 40 mg once per day. Based on a prior report and evidence of a CD20 + B cell population on the biopsy, rituximab 375 mg/m2 weekly for four weeks was given [8]. Despite these treatments, the patient's seizures and choreiform movements continued.
Functional hemispherectomy with deafferentation of the right hemisphere was performed three months after the final dose of rituximab. The pathologic specimen consisted of right frontal, lateral temporal, hippocampus, and corpus callosum, and showed meningoencephalitis with scattered microglial nodules, perivascular lymphocytes, and wide-spread gliosis with no major differences compared to the prior biopsy. Three months after hemispherectomy, there was no evidence of clinical seizures or hemichorea. At 6 months following hemispherectomy, all of the patient's ASMs were discontinued. EEG at that time showed numerous right-sided electrographic seizures with frequent sharp waves over the right hemisphere, without propagation to the left hemisphere or clinical correlate. Examination was significant for the expected left homonymous hemianopsia and left hemiparesis with ability to bear weight and walk independently.
Prior to the procedure, a neuropsychological assessment was performed and demonstrated poor nonverbal skills, problems with visual perception and motor integration, difficulty with left-hand dexterity, and social challenges consistent with non-dominant hemisphere dysfunction. Additionally, neurobehavioral deficits were seen in executive function and impulse regulation, while he was found to struggle with anxiety. The assessment was repeated 18 months after surgery, showing continued struggles with executive function and working memory but improved impulse control. The patient showed some improvements across many language-based areas with stable neuropsychological function.
One year after hemispherectomy, the patient developed psoriasis involving the ears, popliteal fossa, gluteal clefts, and umbilicus. One year later, the patient was found to have a right-sided, non-reactive pupil with gray discoloration of the iris during a routine neurologic examination. Ophthalmologic evaluation showed evidence of chronic anterior uveitis with 2 + cells and flare in the anterior chamber, posterior synechiae, cataract, and decreased visual acuity in the right eye. Rheumatology was consulted and no other clinical manifestations of autoimmunity such as arthritis were noted. Laboratory studies were notable for an elevated ESR of 60 mm/h and CRP of 3.3 mg/dL. His anti-nuclear antibody (ANA) titer continued to be positive at 1:80 while antineutrophilic cytoplasmic antibody (ANCA), lysozyme, angiotensin converting enzyme (ACE level), and HLAB27 antigen testing were negative. Infectious evaluation, including assessment for toxoplasmosis, Toxocara, Bartonella, Lyme disease, and syphilis was unrevealing. Given the patient's multiple manifestations of autoimmunity, there was concern for an underlying immunodeficiency and a specific evaluation of immune function was conducted. The patient had normal T cell subsets, T cell proliferation to mitogens and antigens, and immunoglobulin levels; however, his switched and unswitched memory B cells were low, and his response to pneumococcal vaccines and tetanus was marginal. Despite this extensive workup, the patient was not thought to have a known rheumatologic or immunologic condition.
The uveitis initially responded to topical steroid drops applied every 2 h; however, the inflammation recurred when the frequency of the eye drops decreased. Ultimately, remission of the patient's uveitis was attained with oral prednisone 20 mg daily and mycophenolate mofetil 1000 mg twice daily.
The patient underwent cataract extraction and intraocular lens implantation at age 12 years. Two months after surgery, recurrent inflammation in the right eye was noted and mycophenolate 1000 mg twice daily and oral prednisone was continued. The inflammatory markers remained persistently elevated with a CRP of 4.32 mg/dL and ESR of 63 mm/h. He was subsequently admitted and received 1 g of IV methylprednisolone daily for a 3 day course. A brain MRI was stable. Infliximab was added to the treatment regimen and titrated up 15 mg/kg every 3 weeks with good response. He remains on prednisone 5 mg daily, mycophenolate 1500 mg twice daily, and prednisolone eye drops. The patient continues to be followed closely by multiple services including Epilepsy, Ophthalmology, and Rheumatology and continues to remain seizure-free.
3. Discussion
Although the patient presented here ultimately met established diagnostic criteria for RE as the clinical course evolved, he also manifested several unique and atypical features [2,6,8]. In this case report, we highlight three main features. First, posterior seizures and a long prodrome of focal epilepsy can be seen in RE. Second, movement disorders, although rare, can be part of RE. Finally, patients with RE are at risk for autoimmune disorders. We highlight these features to facilitate early diagnosis subsequently leading to early treatment of future cases in hopes of ameliorating neuropsychological sequelae of frequent seizures and failed antiseizure medication trials.
In this patient, the seizure semiology and electrographic and radiographic findings were all consistent with a process that initially predominated in the occipital lobe. Occipital seizures are noted in atypical adolescent- and adult-onset RE, but rarely in childhood-onset RE [14]. Radiographically, parieto-occipital atrophy may be seen in younger patients with more widespread hemispheric involvement, but frontal and insular regions remain predominantly affected [15]. Pathologic changes in RE are sparse in the occipital lobe compared to other regions [16]. Thus, while most cases of RE show a frontal predilection, occipital localization and semiology should not necessarily dissuade clinicians from the diagnosis of RE. Our patient developed cerebellar atrophy ipsilateral to the affected cerebral hemisphere. Most previously reported cases of cerebellar atrophy in RE describe its occurrence contralateral to the affected cerebral hemisphere, which is explained by crossed cerebral to cerebellar afferent inputs; however, rare cases of ipsilateral cerebellar atrophy have been described [5,17,18]. Finally, this patient had a long prodrome of focal epilepsy before his case declared itself as RE. Prolonged time from disease onset to meeting criteria for RE has been described, but is more typical in adolescents and adults than in young children [11]. We highlight the early features in particular because prior to developing seizures, he was not suspected to have the neurobehavioral profile or deficits documented through standardized testing. While an early diagnosis initially may not have led directly to hemispherectomy, it might have been performed sooner, increasing the chances of improved neurobehavioral outcomes.
In addition to the several atypical cranial manifestations of RE highlighted, this patient also developed several unique extracranial features. He developed a severe choreoathetoid movement disorder at the time when seizures had become drug-resistant and the seizure onset zone had spread from the occipital region to involve a larger region of the right hemisphere. Our case adds to the small existing literature documenting the occurrence of movement disorders in RE and further supports the involvement of the basal ganglia [3,4]. Finally, he developed uveitis ipsilateral to the cerebral hemisphere involved in addition to diffuse psoriasis. This comorbid autoimmunity has been rarely described [12].
Taken together, the early, unusual features in our case made the diagnosis of RE challenging until more typical features evolved years later. Nonetheless, in the setting of drug-resistant epilepsy and unihemispheric MRI abnormalities suggestive of an inflammatory process, RE should be a leading consideration, being mindful of these previously reported albeit rare features.
Despite a prior report and open-label trial that suggested potential benefit of rituximab in RE, our patient showed no appreciable improvement following its use [8,19]. While our case adds to the disappointing record for immunotherapy in RE, it is possible that rituximab might have been more effective if it had been used earlier in the disease course or more time was given following its use. Alternatively, although rituximab may have secondary effects on T cell function. Therapies directly targeting T cells or the innate immune system, which have been shown to be key effector mechanisms in RE, may be more effective. Seizure frequency was decreased with natalizumab in one case, and adalimunab in 5 out of 11 patients in an open-label pilot study [13,20]. However, as is more often the case, immunotherapy may slow the progression of cortical atrophy or motor weakness without significant effect on seizure burden, delaying definitive treatment with hemispherectomy despite ongoing, drug-resistant seizures. This was noted in the only randomized RE trial to date, comparing IVIG with tacrolimus to historical controls in which patients experienced delayed deterioration with either agent without appreciable seizure effect [21]. Overall, future multicenter immunotherapy trials in RE with novel, early targets are needed.
Finally, in the setting of a family history of multiple autoimmune disorders, our patient subsequently developed psoriasis and uveitis affecting the eye ipsilateral to the affected hemisphere. Family history of autoimmune disorders has been described in RE [22]. Uveitis has been rarely described, with ipsilateral, contralateral, and bilateral involvement [11,12]. We have also recently shown an association between numerous systemic autoimmune conditions, including psoriasis, and epilepsy suggesting potential shared mechanisms [23].
Autoimmune disorders with similar pathophysiologic processes are known to affect different organ systems within individual family members. This has been described in numerous conditions, specifically multiple sclerosis, a demyelinating condition with a presumed autoimmune mechanism leading to T cell mediated inflammation. These observations support the concept of a shared genetic diathesis to autoimmunity with additional “second hits” leading to specific disease expression. Unlike most other presumed autoimmune disorders, RE is strikingly unilateral in its effects on the cerebrum. The occurrence of cerebellar atrophy and uveitis ipsilateral to the affected cerebral hemisphere in our patient highlights the unilateral processes involved in RE. In some cases of RE such as ours, patients may inherit a genetic predisposition to autoimmunity with a “second hit” leading to the peculiar unilateral disease manifestations. This theory is supported further by our patient's development of uveitis well after the brain involvement. This is in comparison to other reported cases of uveitis associated with this disorder in pediatrics that occurred at the time of the progressive phase of RE [12]. Further research is needed to identify both the underlying genetic architecture of RE as well as potential “second hit” mechanisms (trauma, viral infection, somatic mutations) underlying this phenomenon. Awareness of atypical features of RE may lead to early immune-based therapy and seizure-alleviating surgery when appropriate.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Data statement
The data that support the findings of this case report are available on request from the corresponding author, A.S. The data are not publicly available due to their containing information that could compromise the privacy of the patient in this case report.
Contributor Information
Arnold J. Sansevere, Email: arnold.sansevere@childrens.harvard.edu.
Lauren A. Henderson, Email: lauren.henderson@childrens.harvard.edu.
Coral M. Stredny, Email: coral.stredny@childrens.harvard.edu.
Sanjay P. Prabhu, Email: sanjay.prabhu@childrens.harvard.edu.
Ankoor Shah, Email: ankoor.shah@childrens.harvard.edu.
Robert Sundel, Email: robert.sundel@childrens.harvard.edu.
Joseph Madsen, Email: joseph.madsen@childrens.harvard.edu.
Chantal Dufreney, Email: chantal.dufreny@hms.harvard.edu.
Annapurna Poduri, Email: annapurna.poduri@childrens.harvard.edu.
Mark P. Gorman, Email: mark.gorman@childrens.harvard.edu.
References
- 1.Rasmussen T., Olszewski J., Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/wnl.8.6.435. [DOI] [PubMed] [Google Scholar]
- 2.Olson H.E., Lechpammer M., Prabhu S.P., Ciarlini P.D., Poduri A., Gooty V.D. Clinical application and evaluation of the Bien diagnostic criteria for Rasmussen encephalitis. Epilepsia. 2013;54:1753–1760. doi: 10.1111/epi.12334. [DOI] [PubMed] [Google Scholar]
- 3.Frucht S. Dystonia, athetosis, and epilepsia partialis continua in a patient with late-onset Rasmussen’s encephalitis. Mov Disord. 2002;17:609–612. doi: 10.1002/mds.10131. [DOI] [PubMed] [Google Scholar]
- 4.Kankirawatana P., Dure Lst, Bebin E.M. Chorea as manifestation of epilepsia partialis continua in a child. Pediatr Neurol. 2004;31:126–129. doi: 10.1016/j.pediatrneurol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Chiapparini L., Granata T., Farina L., Ciceri E., Erbetta A., Ragona F. Diagnostic imaging in 13 cases of Rasmussen’s encephalitis: can early MRI suggest the diagnosis? Neuroradiology. 2003;45:171–183. doi: 10.1007/s00234-002-0923-7. [DOI] [PubMed] [Google Scholar]
- 6.Bien C.G., Granata T., Antozzi C., Cross J.H., Dulac O., Kurthen M. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 7.Bien C.G., Schramm J. Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res. 2009;86:101–112. doi: 10.1016/j.eplepsyres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Thilo B., Stingele R., Knudsen K., Boor R., Bien C.G., Deuschl G. A case of Rasmussen encephalitis treated with rituximab. Nat Rev Neurol. 2009;5:458–462. doi: 10.1038/nrneurol.2009.98. [DOI] [PubMed] [Google Scholar]
- 9.Schwab N., Bien C.G., Waschbisch A., Becker A., Vince G.H., Dornmair K. CD8 + T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain. 2009;132:1236–1246. doi: 10.1093/brain/awp003. [DOI] [PubMed] [Google Scholar]
- 10.Papetti L., Nicita F., Granata T., Guerrini R., Ursitti F., Properzi E. Early add-on immunoglobulin administration in Rasmussen encephalitis: the hypothesis of neuroimmunomodulation. Med Hypotheses. 2011;77:917–920. doi: 10.1016/j.mehy.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Kashihara K., Ohno M., Takahashi Y. Twenty-one-year course of adult-onset Rasmussen’s encephalitis and bilateral uveitis: case report. J Neurol Sci. 2010;294:127–130. doi: 10.1016/j.jns.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Harvey A.S., Andermann F., Hopkins I.J., Kirkham T.H., Berkovic S.F. Chronic encephalitis (Rasmussen’s syndrome) and ipsilateral uveitis. Ann Neurol. 1992;32:826–829. doi: 10.1002/ana.410320621. [DOI] [PubMed] [Google Scholar]
- 13.Bittner S., Simon O.J., Gobel K., Bien C.G., Meuth S.G., Wiendl H. Rasmussen encephalitis treated with natalizumab. Neurology. 2013;81:395–397. doi: 10.1212/WNL.0b013e31829c5ceb. [DOI] [PubMed] [Google Scholar]
- 14.Hart Y.M., Andermann F., Fish D.R., Dubeau F., Robitaille Y., Rasmussen T. Chronic encephalitis and epilepsy in adults and adolescents: a variant of Rasmussen’s syndrome? Neurology. 1997;48:418–424. doi: 10.1212/wnl.48.2.418. [DOI] [PubMed] [Google Scholar]
- 15.Wagner J., Schoene-Bake J.C., Bien C.G., Urbach H., Elger C.E., Weber B. Automated 3D MRI volumetry reveals regional atrophy differences in Rasmussen encephalitis. Epilepsia. 2012;53:613–621. doi: 10.1111/j.1528-1167.2011.03396.x. [DOI] [PubMed] [Google Scholar]
- 16.Pardo C.A., Vining E.P., Guo L., Skolasky R.L., Carson B.S., Freeman J.M. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45:516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- 17.Quesada C.M., Urbach H., Elger C.E., Bien C.G. Rasmussen encephalitis with ipsilateral brain stem involvement in an adult patient. J Neurol Neurosurg Psychiatry. 2007;78:200–201. doi: 10.1136/jnnp.2006.097816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira G.P., Arantes P., Ono C.R., Passarelli V., Castro L.H.M. S132: ipsilateral cerebellar atrophy and crossed ictal cerebellar diaschisis in a case of Rasmussen encephalitis. Clin Neurophysiol. 2018;129(Suppl. 1):e191. doi: 10.1016/j.clinph.2018.04.492. [DOI] [Google Scholar]
- 19.Laxer K.D., Wilfong A., Morris G.L., III, Andermann F. 1.277: Pilot study of rituximab to treat chronic focal encephalitis. Epilepsia. 2008;49(Suppl. 7):121. doi: 10.1111/j.1528-1167.2008.01871_1.x. [DOI] [Google Scholar]
- 20.Lagarde S., Villeneuve N., Trebuchon A., Kaphan E., Lepine A., McGonigal A. Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussen’s encephalitis: an open pilot study. Epilepsia. 2016;57:956–966. doi: 10.1111/epi.13387. [DOI] [PubMed] [Google Scholar]
- 21.Bien C.G., Tiemeier H., Sassen R., Kuczaty S., Urbach H., von Lehe M. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 2013;54:543–550. doi: 10.1111/epi.12042. [DOI] [PubMed] [Google Scholar]
- 22.Amrom D., Kinay D., Hart Y., Berkovic S.F., Laxer K., Andermann F. Rasmussen encephalitis and comorbid autoimmune diseases: a window into disease mechanism? Neurology. 2014;83:1049–1055. doi: 10.1212/WNL.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong M., Kohane I.S., Cai T., Gorman M.P., Mandl K.D. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014;71:569–574. doi: 10.1001/jamaneurol.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]